Dynamic Connectivity Analysis Using Adaptive Window Size
Abstract
:1. Introduction
2. Methods
| Algorithm 1: The RICI algorithm used to estimate the time-varying FC. |
|
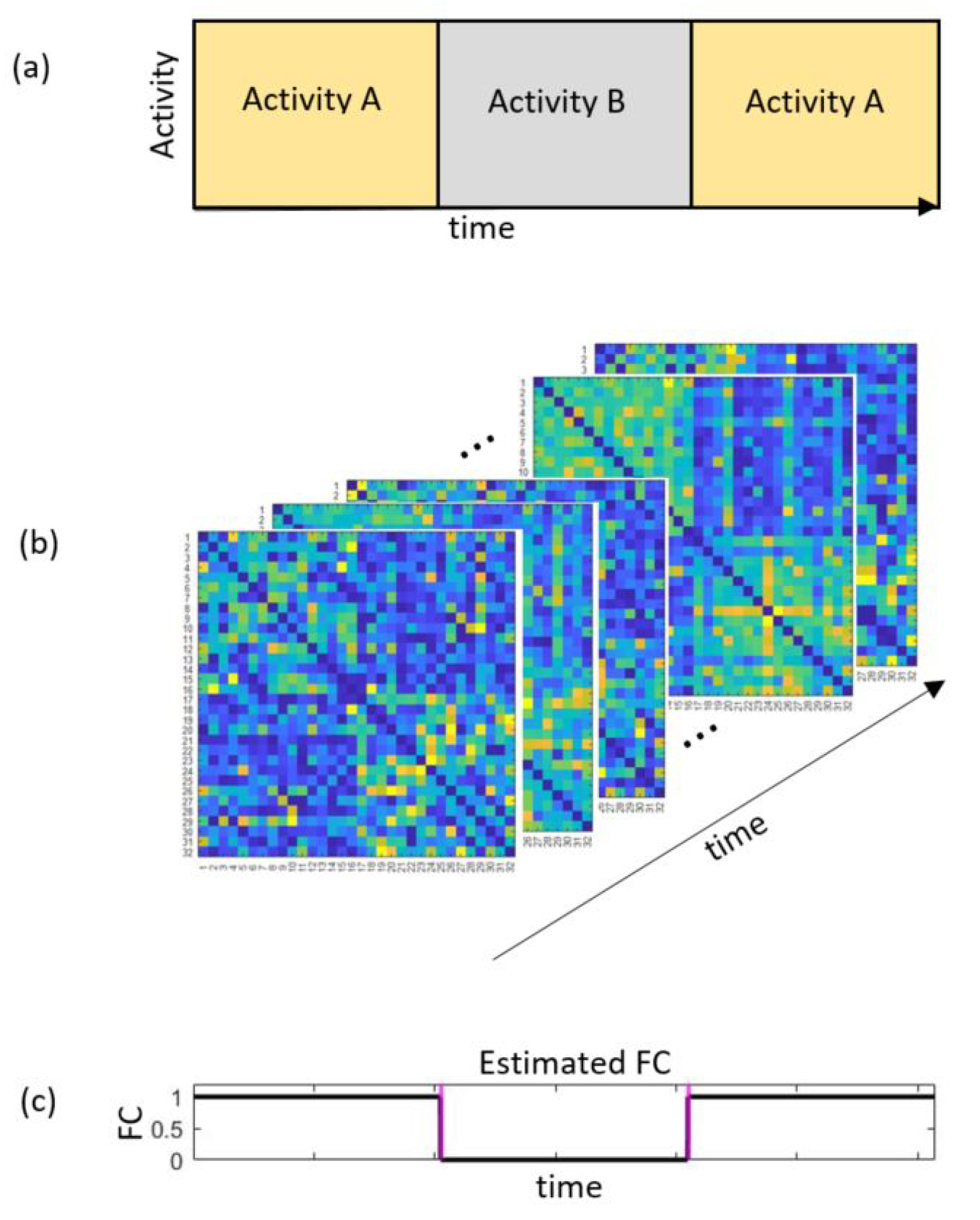
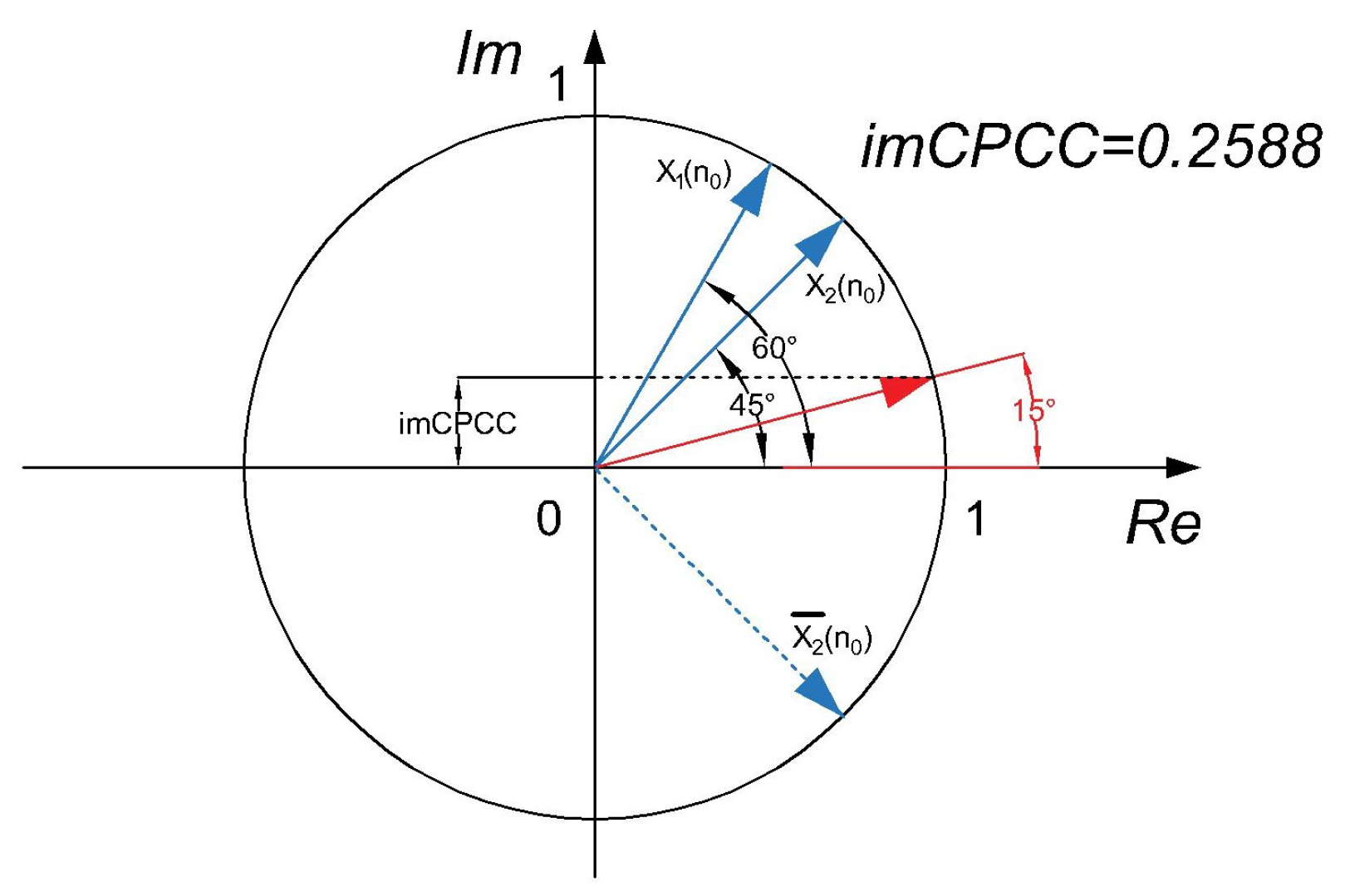
2.1. Measuring Temporal Functional Connectivity
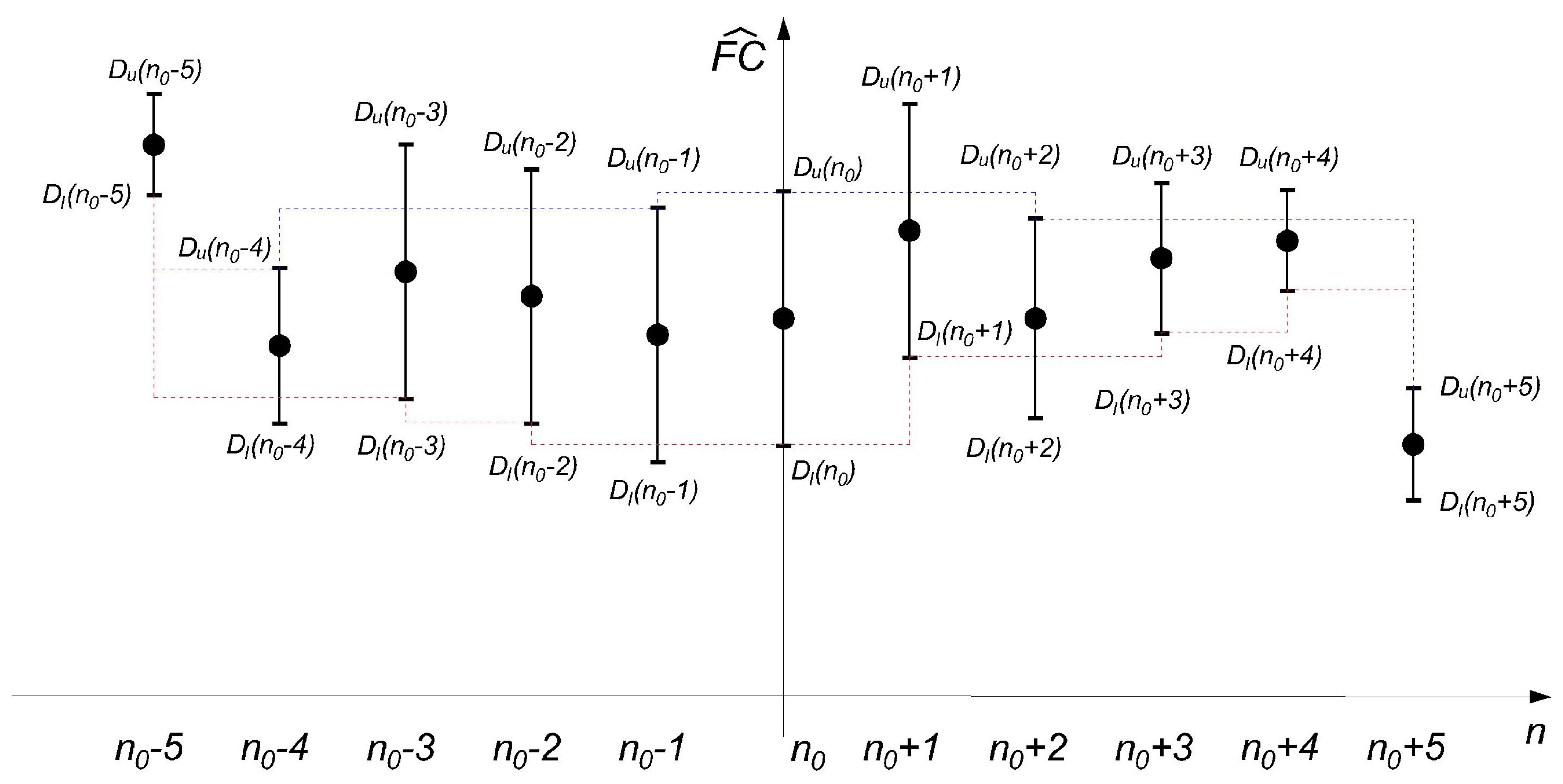
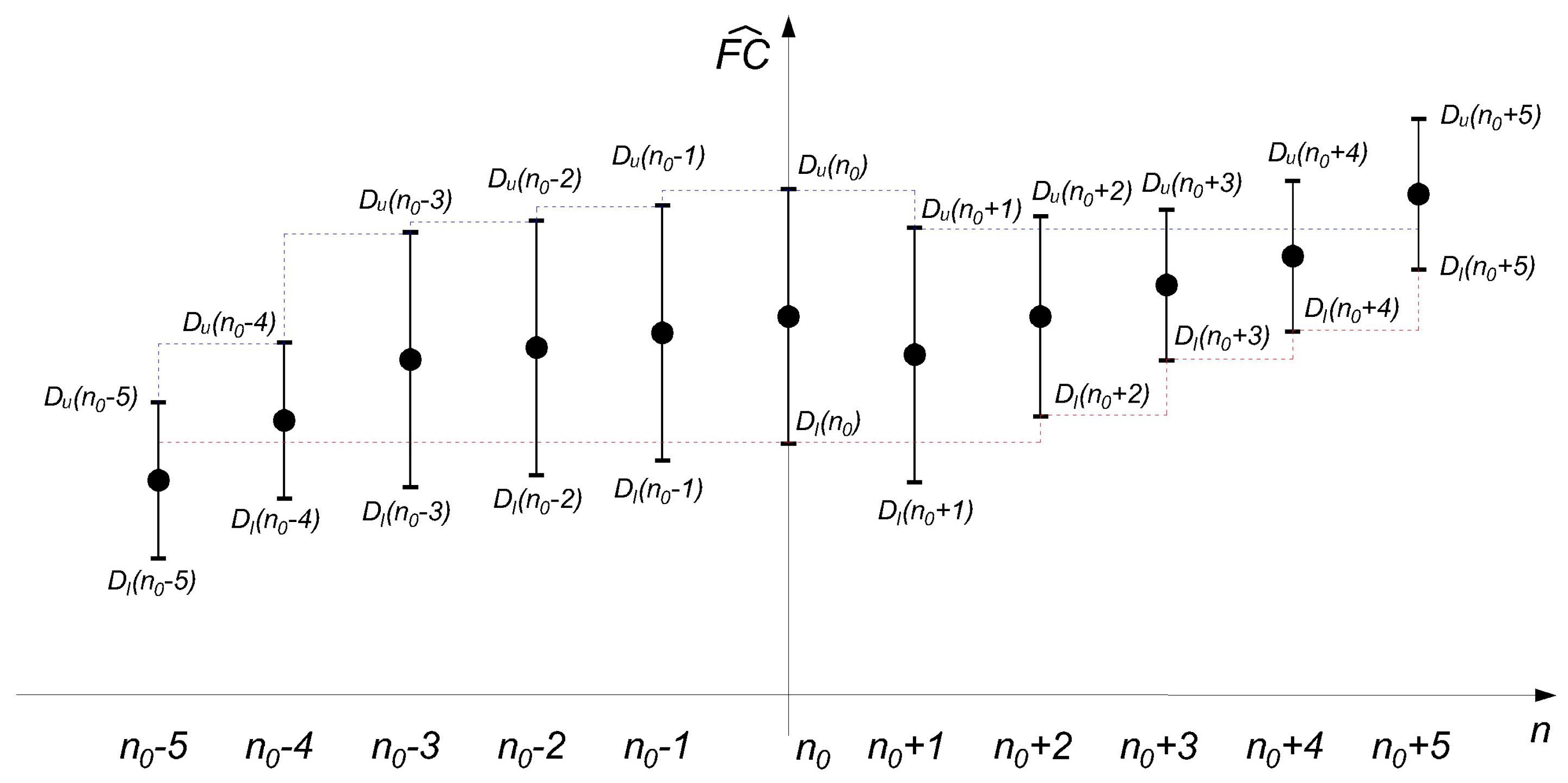
2.2. Using the RICI Method to Determine the Optimum Window Width
2.3. Description of Datasets
2.3.1. Description of Synthetic Signals
2.3.2. Description of the Auditory Oddball Dataset
2.3.3. Description of the Motor Imagery Dataset
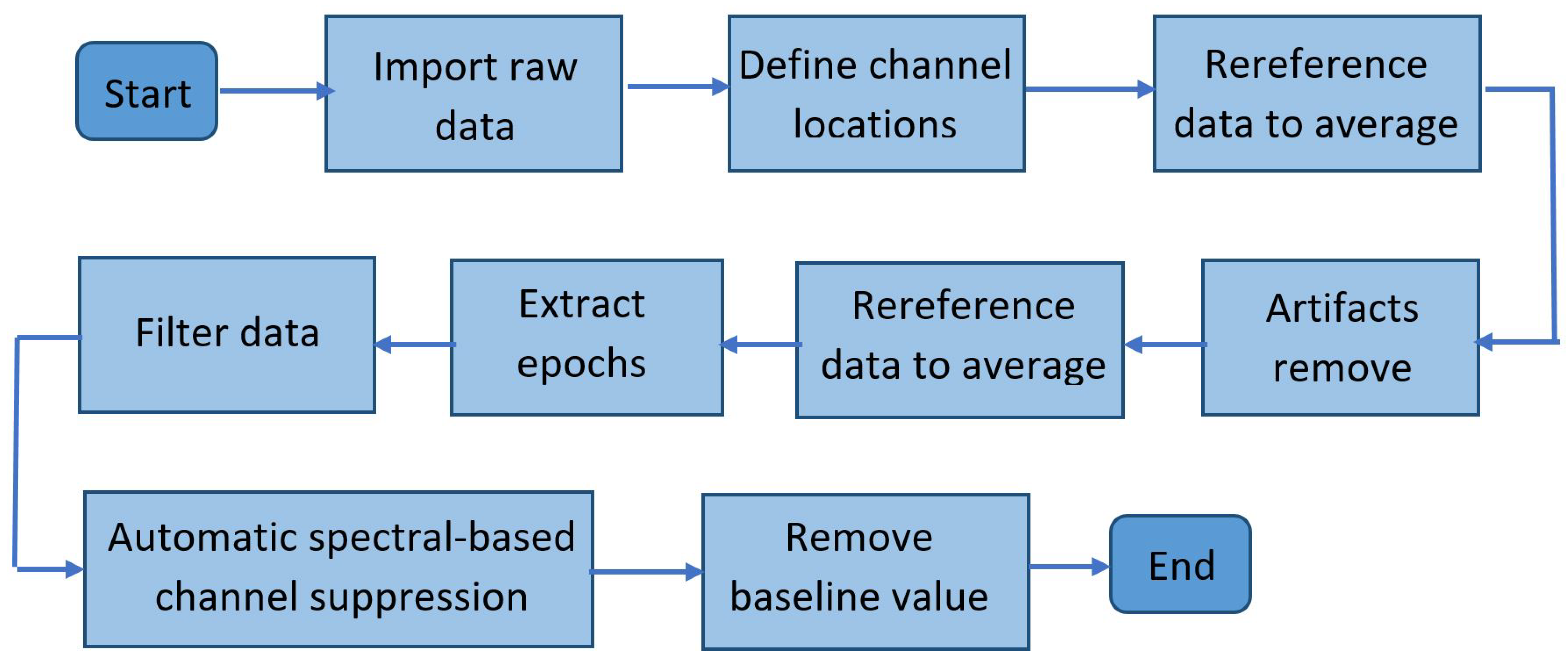
2.4. Offline Preprocessing
3. Results
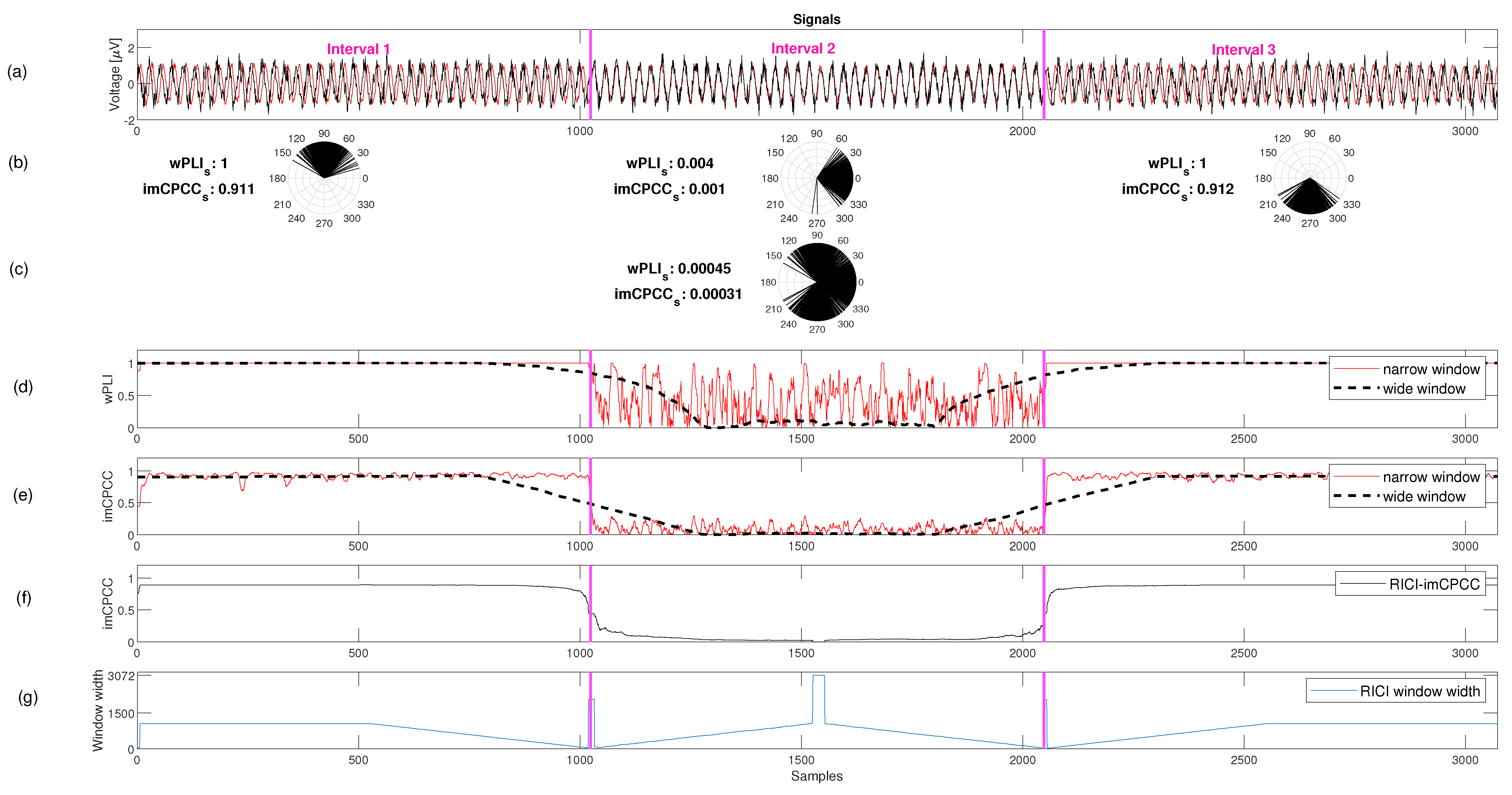
3.1. Synthetic Signals
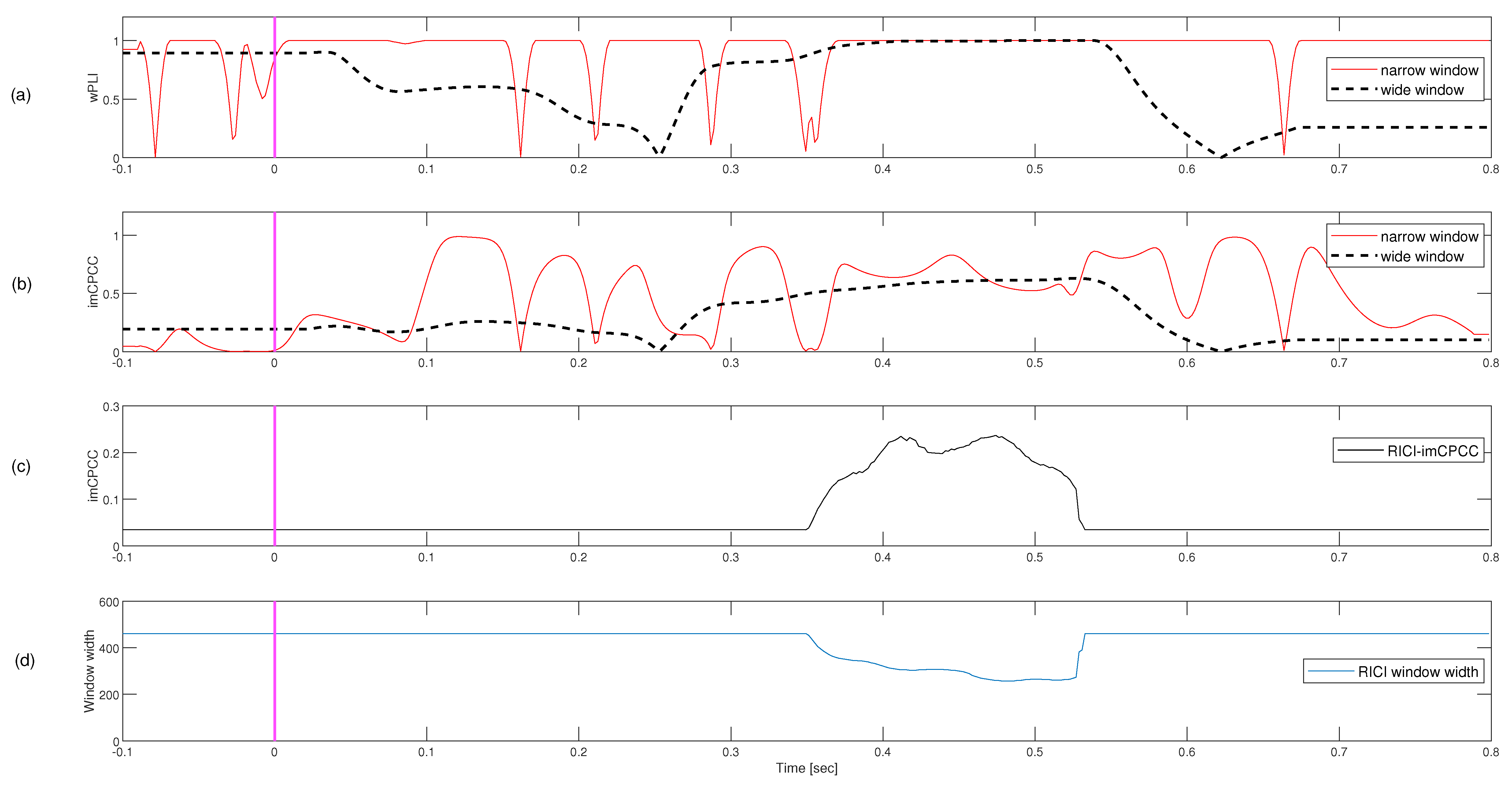
3.2. Auditory Oddball Real-Life Signals
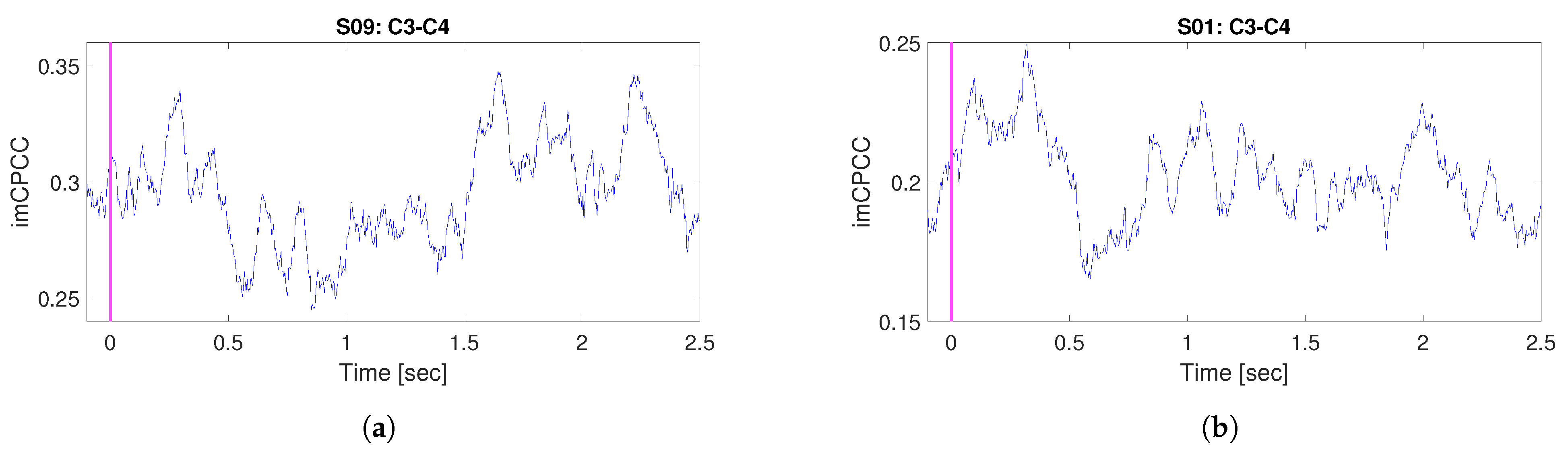
3.3. Motor Imagery Real-Life Signals
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| absCPCC | absolute value of complex Pearson correlation coefficient |
| BOLD-fMRI | blood-oxygenation-level-dependent functional magnetic resonance imaging |
| CPCC | Complex Pearson correlation coefficient |
| DTI | Difussion Tensor Imaging |
| EC | eyes closed |
| EEG | Electroencephalography |
| EO | eyes open |
| ERP | event-related potential |
| FC | Functional Connectivity |
| FICI | Fast Intersection of Confidence Intervals |
| fMRI | Functional Magnetic Resonance Imaging |
| ICI | Intersection of Confidence Intervals |
| imCPCC | imaginary component of complex Pearson correlation coefficient |
| MEG | Magnetoencephalography |
| MRI | Magnetic Resonance Imaging |
| PDFs | Probability Density Functions |
| PLI | Phase Lag Index |
| PLV | Phase Locking Value |
| RICI | relative Intersection of Confidence Intervals |
| RICI-imCPCC | relative Intersection of Confidence Intervals for imaginary component |
| of complex Pearson correlation coefficient | |
| TFC | Temporal functional connectivity |
| wPLI | Weighted Phase Lag Index |
References
- Rossini, P.; Di Iorio, R.; Bentivoglio, M.; Bertini, G.; Ferreri, F.; Gerloff, C.; Ilmoniemi, R.; Miraglia, F.; Nitsche, M.; Pestilli, F.; et al. Methods for analysis of brain connectivity: An IFCN-sponsored review. Clin. Neurophysiol. 2019, 130, 1833–1858. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M.; de Haan, W.; Deco, G.; Dengler, R.; Di Iorio, R.; Gallea, C.; Gerloff, C.; Grefkes, C.; Helmich, R.C.; Kringelbach, M.L.; et al. Human brain connectivity: Clinical applications for clinical neurophysiology. Clin. Neurophysiol. 2020, 131, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.A.; Damaraju, E.; Plis, S.M.; Erhardt, E.B.; Eichele, T.; Calhoun, V.D. Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex 2014, 24, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Glover, G.H. Time–frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 2010, 50, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Di, X.; Biswal, B.B. Dynamic brain functional connectivity modulated by resting-state networks. Brain Struct. Funct. 2015, 220, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Handwerker, D.A.; Roopchansingh, V.; Gonzalez-Castillo, J.; Bandettini, P.A. Periodic changes in fMRI connectivity. Neuroimage 2012, 63, 1712–1719. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.L.; Zahneisen, B.; Hugger, T.; LeVan, P.; Hennig, J. Tracking dynamic resting-state networks at higher frequencies using MR-encephalography. Neuroimage 2013, 65, 216–222. [Google Scholar] [CrossRef]
- Ward, P.G.; Orchard, E.R.; Oldham, S.; Arnatkevičiūtė, A.; Sforazzini, F.; Fornito, A.; Storey, E.; Egan, G.F.; Jamadar, S.D. Individual differences in haemoglobin concentration influence BOLD fMRI functional connectivity and its correlation with cognition. NeuroImage 2020, 221, 117196. [Google Scholar] [CrossRef]
- Hutchison, R.M.; Womelsdorf, T.; Gati, J.S.; Everling, S.; Menon, R.S. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum. Brain Mapp. 2013, 34, 2154–2177. [Google Scholar] [CrossRef]
- De Pasquale, F.; Della Penna, S.; Snyder, A.Z.; Lewis, C.; Mantini, D.; Marzetti, L.; Belardinelli, P.; Ciancetta, L.; Pizzella, V.; Romani, G.L.; et al. Temporal dynamics of spontaneous MEG activity in brain networks. Proc. Natl. Acad. Sci. USA 2010, 107, 6040–6045. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Wu, G.R.; Xu, Q.; Ji, G.J.; Zhang, Z.; Zang, Y.F.; Lu, G. DynamicBC: A MATLAB toolbox for dynamic brain connectome analysis. Brain Connect. 2014, 4, 780–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detti, P.; de Lara, G.Z.M.; Bruni, R.; Pranzo, M.; Sarnari, F.; Vatti, G. A patient-specific approach for short-term epileptic seizures prediction through the analysis of EEG synchronization. IEEE Trans. Biomed. Eng. 2018, 66, 1494–1504. [Google Scholar] [CrossRef]
- Detti, P.; Vatti, G.; Zabalo Manrique de Lara, G. Eeg synchronization analysis for seizure prediction: A study on data of noninvasive recordings. Processes 2020, 8, 846. [Google Scholar] [CrossRef]
- Ghumare, E.G.; Schrooten, M.; Vandenberghe, R.; Dupont, P. A time-varying connectivity analysis from distributed EEG sources: A simulation study. Brain Topogr. 2018, 31, 721–737. [Google Scholar] [CrossRef] [Green Version]
- Yi, C.; Qiu, Y.; Chen, W.; Chen, C.; Wang, Y.; Li, P.; Yang, L.; Zhang, X.; Jiang, L.; Yao, D.; et al. Constructing Time-varying Directed EEG network by Multivariate Nonparametric Dynamical Granger Causality. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, Y.; Li, H.; Yu, X.; Wang, C.; Liu, M.; Jiang, L.; Feng, C.; Li, J.; Wang, D.; et al. Time-Varying Effective Connectivity for Describing the Dynamic Brain Networks of Post-stroke Rehabilitation. Front. Aging Neurosci. 2022, 14, 911513. [Google Scholar] [CrossRef]
- Sanei, S.; Chambers, J.A. EEG Signal Processing; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Tadić, B.; Andjelković, M.; Boshkoska, B.M.; Levnajić, Z. Algebraic topology of multi-brain connectivity networks reveals dissimilarity in functional patterns during spoken communications. PLoS ONE 2016, 11, e0166787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sporns, O.; Tononi, G.; Kötter, R. The human connectome: A structural description of the human brain. PLoS Comput. Biol. 2005, 1, e42. [Google Scholar] [CrossRef]
- Rose, S.; Pannek, K.; Bell, C.; Baumann, F.; Hutchinson, N.; Coulthard, A.; McCombe, P.; Henderson, R. Direct evidence of intra-and interhemispheric corticomotor network degeneration in amyotrophic lateral sclerosis: An automated MRI structural connectivity study. Neuroimage 2012, 59, 2661–2669. [Google Scholar] [CrossRef]
- Raichle, M.E. The restless brain. Brain Connect. 2011, 1, 3–12. [Google Scholar] [CrossRef]
- Lim, S.; Han, C.E.; Uhlhaas, P.J.; Kaiser, M. Preferential detachment during human brain development: Age-and sex-specific structural connectivity in diffusion tensor imaging (DTI) data. Cereb. Cortex 2015, 25, 1477–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Daducci, A.; He, Y.; Schiavi, S.; Seguin, C.; Smith, R.; Yeh, C.H.; Zhao, T.; O’Donnell, L.J. Quantitative mapping of the brain’s structural connectivity using diffusion MRI tractography: A review. Neuroimage 2022, 249, 118870. [Google Scholar] [CrossRef] [PubMed]
- Lachaux, J.P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef] [Green Version]
- Stam, C.J.; Nolte, G.; Daffertshofer, A. Phase lag index: Assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007, 28, 1178–1193. [Google Scholar] [CrossRef] [PubMed]
- Vinck, M.; Oostenveld, R.; Van Wingerden, M.; Battaglia, F.; Pennartz, C.M. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 2011, 55, 1548–1565. [Google Scholar] [CrossRef] [PubMed]
- Šverko, Z.; Vrankić, M.; Vlahinić, S.; Rogelj, P. Complex Pearson Correlation Coefficient for EEG Connectivity Analysis. Sensors 2022, 22, 1477. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, R.M.; Womelsdorf, T.; Allen, E.A.; Bandettini, P.A.; Calhoun, V.D.; Corbetta, M.; Della Penna, S.; Duyn, J.H.; Glover, G.H.; Gonzalez-Castillo, J.; et al. Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage 2013, 80, 360–378. [Google Scholar] [CrossRef] [Green Version]
- Yi, G.; Wang, L.; Chu, C.; Liu, C.; Zhu, X.; Shen, X.; Li, Z.; Wang, F.; Yang, M.; Wang, J. Analysis of complexity and dynamic functional connectivity based on resting-state EEG in early Parkinson’s disease patients with mild cognitive impairment. Cogn. Neurodynamics 2022, 16, 309–323. [Google Scholar] [CrossRef]
- Lamoš, M.; Mareček, R.; Slavíček, T.; Mikl, M.; Rektor, I.; Jan, J. Spatial-temporal-spectral EEG patterns of BOLD functional network connectivity dynamics. J. Neural Eng. 2018, 15, 036025. [Google Scholar] [CrossRef]
- Panwar, S.; Joshi, S.D.; Gupta, A.; Kunnatur, S.; Agarwal, P. Recursive dynamic functional connectivity reveals a characteristic correlation structure in human scalp EEG. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Tabarelli, D.; Brancaccio, A.; Zrenner, C.; Belardinelli, P. Functional Connectivity States of Alpha Rhythm Sources in the Human Cortex at Rest: Implications for Real-Time Brain State Dependent EEG-TMS. Brain Sci. 2022, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Damaraju, E.; Tagliazucchi, E.; Laufs, H.; Calhoun, V.D. Connectivity dynamics from wakefulness to sleep. NeuroImage 2020, 220, 117047. [Google Scholar] [CrossRef] [PubMed]
- Goldenshluger, A.; Nemirovski, A. Adaptive de-noising of signals satisfying differential inequalities. IEEE Trans. Inf. Theory 1997, 43, 872–889. [Google Scholar] [CrossRef] [Green Version]
- Katkovnik, V.; Stankovic, L. Instantaneous frequency estimation using the Wigner distribution with varying and data-driven window length. IEEE Trans. Signal Process. 1998, 46, 2315–2325. [Google Scholar] [CrossRef] [Green Version]
- Stanković, L.J.; Katkovnik, V. Algorithm for the instantaneous frequency estimation using time-frequency distributions with adaptive window width. In Proceedings of the 9th European Signal Processing Conference (EUSIPCO 1998), Rhodes, Greece, 8–11 September 1998; pp. 1–4. [Google Scholar]
- Lerga, J.; Vrankic, M.; Sucic, V. A signal denoising method based on the improved ICI rule. IEEE Signal Process. Lett. 2008, 15, 601–604. [Google Scholar] [CrossRef]
- Katkovnik, V. A new method for varying adaptive bandwidth selection. IEEE Trans. Signal Process. 1999, 47, 2567–2571. [Google Scholar] [CrossRef] [Green Version]
- Stankovic, L. Performance analysis of the adaptive algorithm for bias-to-variance tradeoff. IEEE Trans. Signal Process. 2004, 52, 1228–1234. [Google Scholar] [CrossRef]
- Kübler, P.D.A. Auditory Oddball during Hypnosis (005-2014). Available online: http://bnci-horizon-2020.eu/database/data-sets (accessed on 2 July 2022).
- Leeb, R.; Lee, F.; Keinrath, C.; Scherer, R.; Bischof, H.; Pfurtscheller, G. Brain–Computer Communication: Motivation, Aim, and Impact of Exploring a Virtual Apartment. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 473–482. [Google Scholar] [CrossRef]
- Sameiro-Barbosa, C.M.; Geiser, E. Sensory entrainment mechanisms in auditory perception: Neural synchronization cortico-striatal activation. Front. Neurosci. 2016, 10, 361. [Google Scholar] [CrossRef] [Green Version]
- Lakatos, P.; Shah, A.S.; Knuth, K.H.; Ulbert, I.; Karmos, G.; Schroeder, C.E. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J. Neurophysiol. 2005, 94, 1904–1911. [Google Scholar] [CrossRef] [Green Version]
- Lakatos, P.; Musacchia, G.; O’Connel, M.N.; Falchier, A.Y.; Javitt, D.C.; Schroeder, C.E. The spectrotemporal filter mechanism of auditory selective attention. Neuron 2013, 77, 750–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talairach. EEG: Electrode Positions and Broadmann Atlas. Available online: http://www.brainm.com/software/pubs/dg/BA_10-20_ROI_Talairach/nearesteeg.htm (accessed on 2 July 2022).
- Messé, A.; Rudrauf, D.; Benali, H.; Marrelec, G. Relating structure and function in the human brain: Relative contributions of anatomy, stationary dynamics, and non-stationarities. PLoS Comput. Biol. 2014, 10, e1003530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kropotov, J. Quantitative EEG, Event-Related Potentials and Neurotherapy; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Volaric, I.; Lerga, J.; Sucic, V. A fast signal denoising algorithm based on the LPA-ICI method for real-time applications. Circuits Syst. Signal Process. 2017, 36, 4653–4669. [Google Scholar] [CrossRef]


| Methods | |
|---|---|
| Narrow window size-wPLI | |
| Wide window size-wPLI | |
| Narrow window size-imCPCC | |
| Wide window size-imCPCC | |
| RICI-imCPCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šverko, Z.; Vrankic, M.; Vlahinić, S.; Rogelj, P. Dynamic Connectivity Analysis Using Adaptive Window Size. Sensors 2022, 22, 5162. https://doi.org/10.3390/s22145162
Šverko Z, Vrankic M, Vlahinić S, Rogelj P. Dynamic Connectivity Analysis Using Adaptive Window Size. Sensors. 2022; 22(14):5162. https://doi.org/10.3390/s22145162
Chicago/Turabian StyleŠverko, Zoran, Miroslav Vrankic, Saša Vlahinić, and Peter Rogelj. 2022. "Dynamic Connectivity Analysis Using Adaptive Window Size" Sensors 22, no. 14: 5162. https://doi.org/10.3390/s22145162
APA StyleŠverko, Z., Vrankic, M., Vlahinić, S., & Rogelj, P. (2022). Dynamic Connectivity Analysis Using Adaptive Window Size. Sensors, 22(14), 5162. https://doi.org/10.3390/s22145162








