Shaping and Focusing Magnetic Field in the Human Body: State-of-the Art and Promising Technologies
Abstract
1. Introduction
2. Biomedical Applications Exploiting Magnetic Field
2.1. Hyperthermia and Thermoablation
2.2. Transcranial Magnetic Stimulation
2.3. Magnetic Resonance Imaging
2.4. Critical Analysis and Comparison
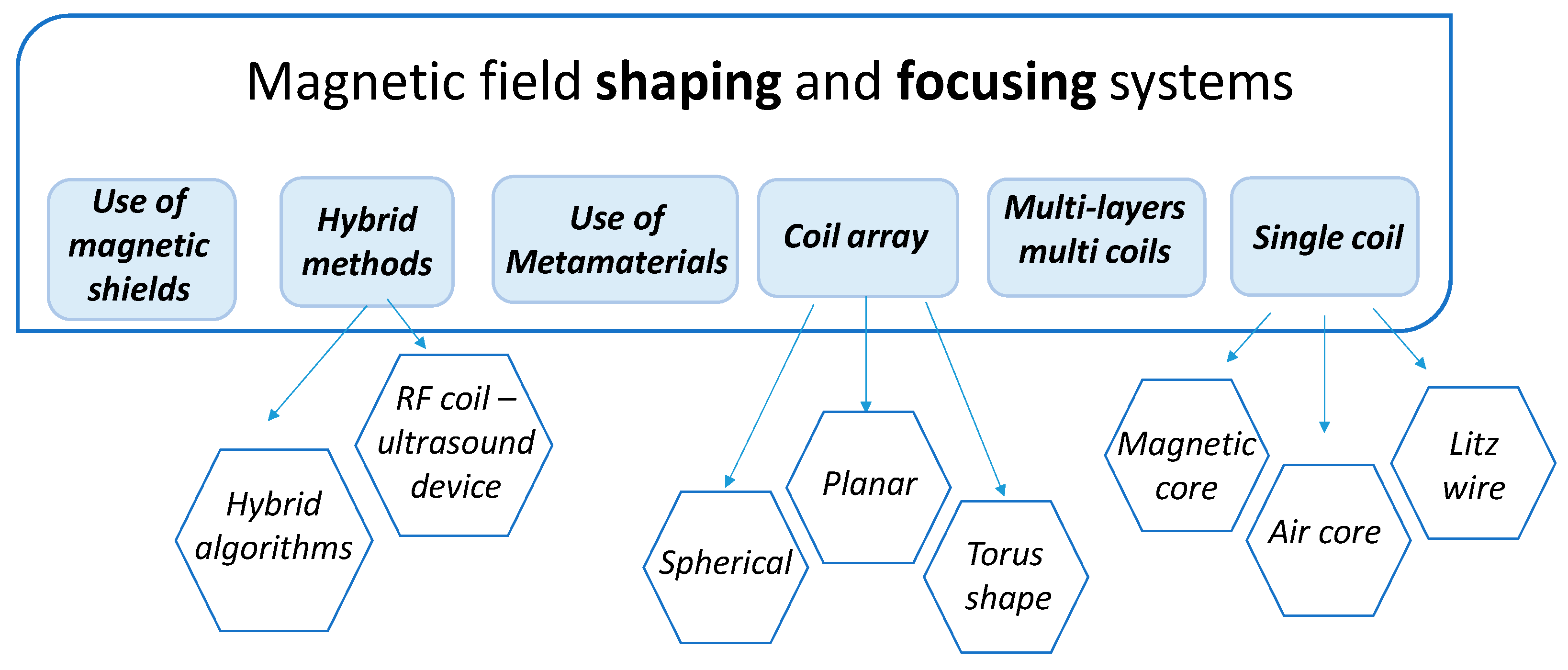
3. Technical Approaches for Magnetic Field Shaping and Focusing
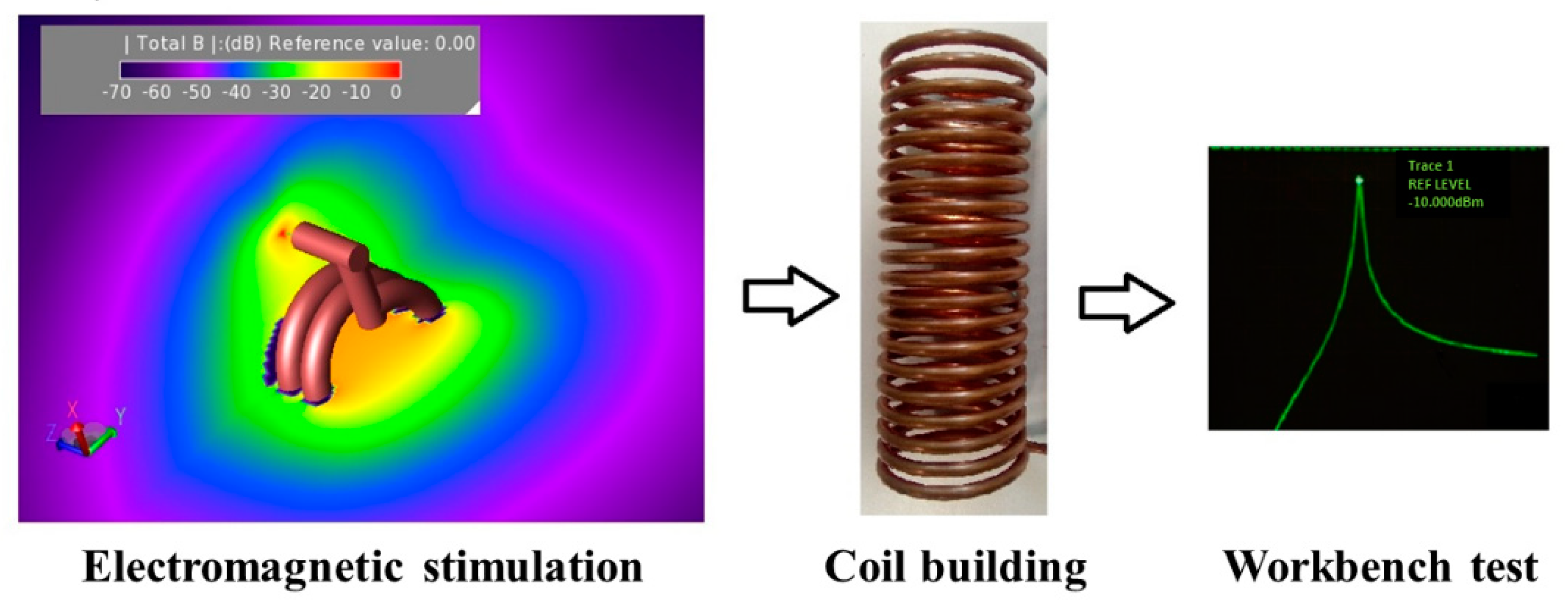
3.1. Applicators for Magnetic Hyperthermia
3.1.1. Volume Inductors
3.1.2. Surface Inductors
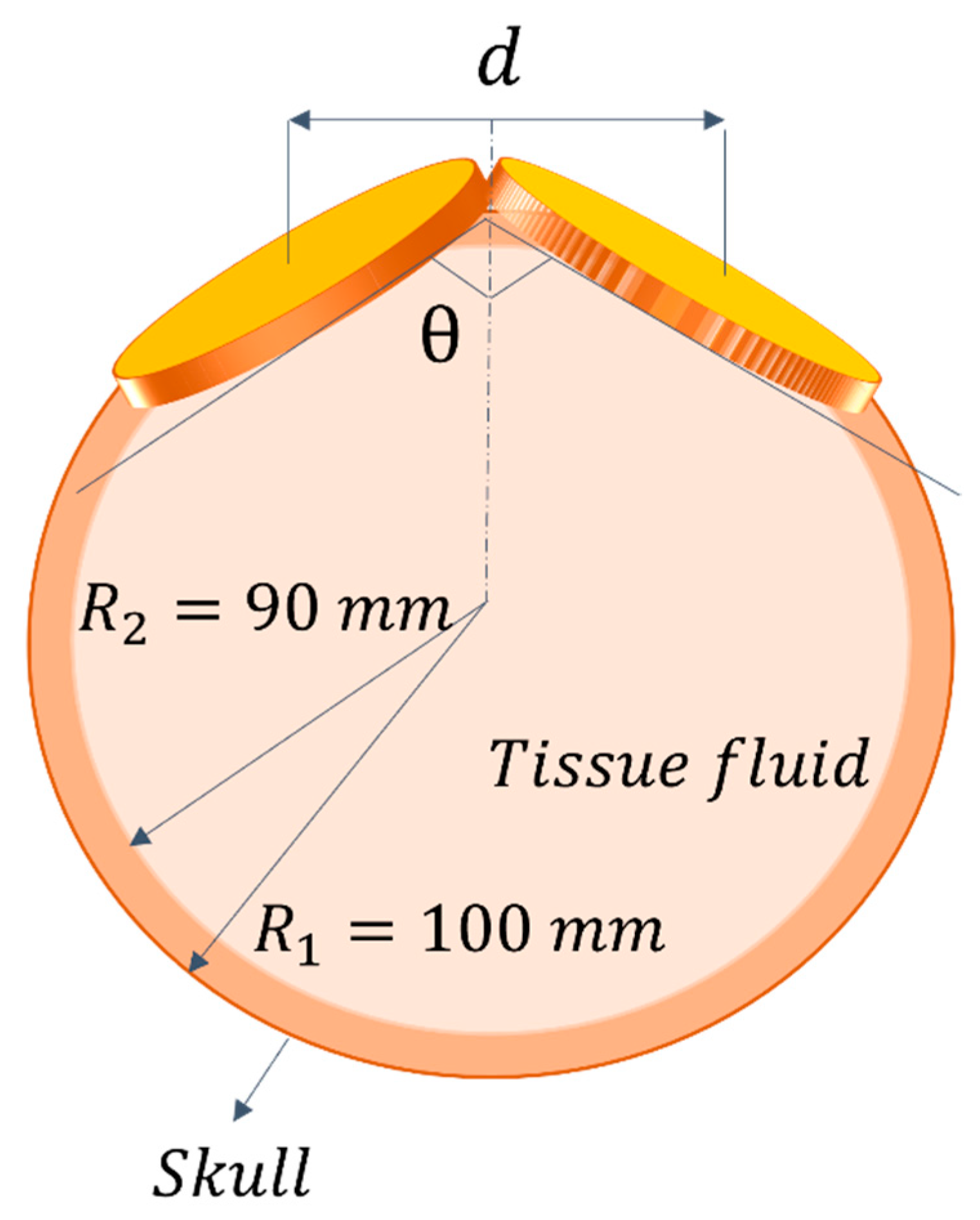
3.2. Applicators for Transcranical Magnetic Stimulation
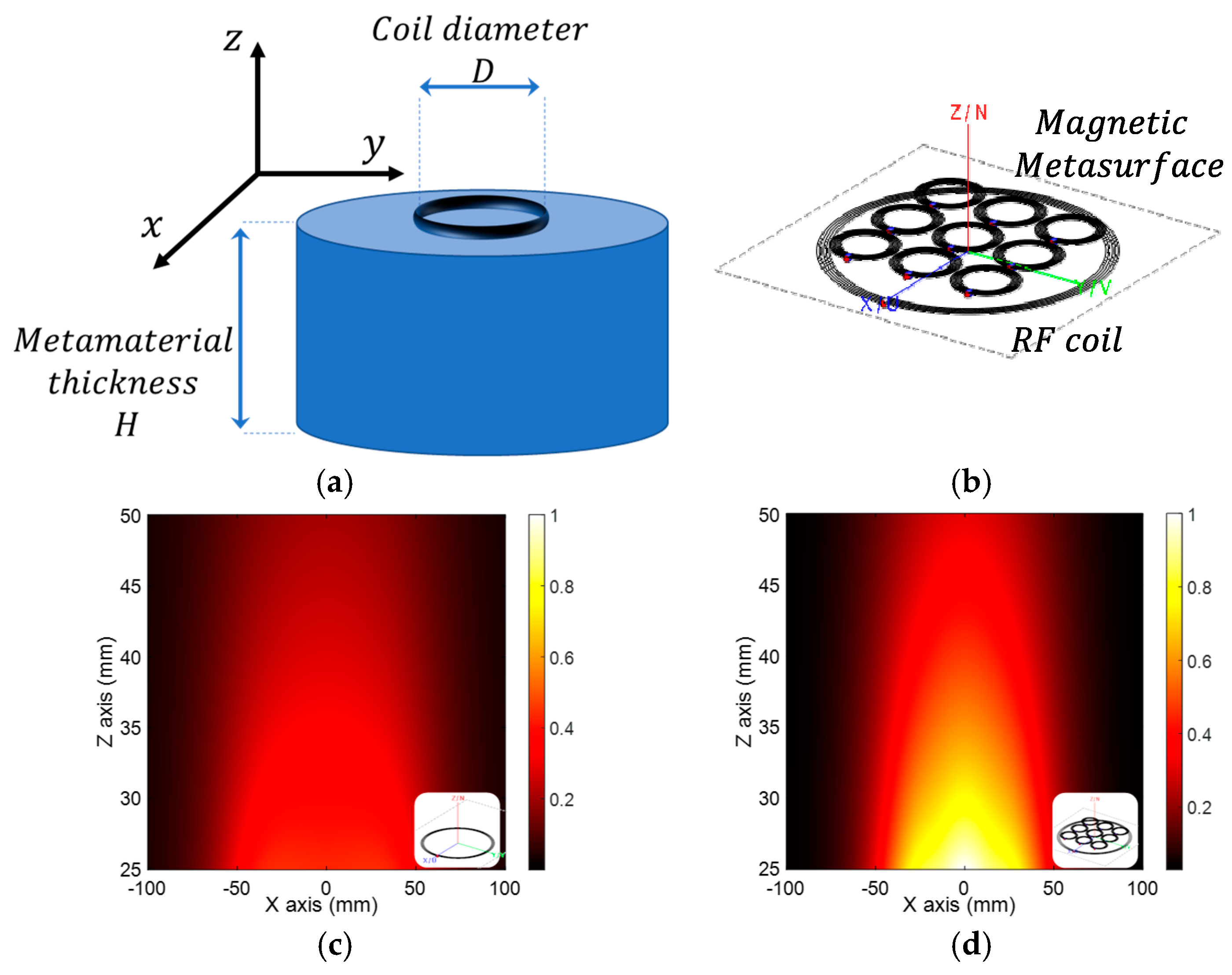
3.3. The Frontiers of Magnetic Field Shaping: Use of Metamaterials
3.4. Critical Comparison between the Different Radiating Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obaidat, I.M.; Narayanaswamy, V.; Alaabed, S.; Sambasivam, S.; Muralee Gopi, C.V. Principles of magnetic hyperthermia: A focus on using multifunctional hybrid magnetic nanoparticles. Magnetochemistry 2019, 5, 67. [Google Scholar] [CrossRef]
- Etlï, E.E.; Akar, A. Magnetic nanoparticles for diagnosis and treatment. Medicine 2022, 11, 934–941. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective inductive heating of lymph nodes. Ann. Surg. 1957, 146, 596. [Google Scholar] [CrossRef]
- Hilger, I. In vivo applications of magnetic nanoparticle hyperthermia. Int. J. Hyperth. 2013, 29, 828–834. [Google Scholar] [CrossRef]
- Piao, D.; Le, K.; Saunders, D.; Smith, N.; Goddard, J.; Figueroa, D.; Krasinski, J.S.; Chen, W.R.; Towner, R.A. Development of a vertically and horizontally applicable multi-frequency alternating-magnetic-field device for hyperthermia of glioma in rodent model using iron oxide based nanoparticles. In Proceedings of the Biomedical Optics Optical Society of America, Miami, FL, USA, 28 April–2 May 2012; p. BSu3A-2. [Google Scholar]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, L.; Cheng, R.; Mao, L.; Arnold, R.D.; Howerth, E.W.; Chen, Z.G.; Platt, S. Magnetic nanoparticle-based hyperthermia for head & neck cancer in mouse models. Theranostics 2012, 2, 113. [Google Scholar]
- Kumar, C.S.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef]
- Stapf, M.; Pömpner, N.; Kettering, M.; Hilger, I. Magnetic thermoablation stimuli alter BCL2 and FGF-R1 but not HSP70 expression profiles in BT474 breast tumors. Int. J. Nanomed. 2015, 10, 1931. [Google Scholar] [CrossRef]
- Skumiel, A.; Leszczyński, B.; Molcan, M.; Timko, M. The comparison of magnetic circuits used in magnetic hyperthermia. J. Magn. Magn. Mater. 2016, 420, 177–184. [Google Scholar] [CrossRef]
- Lu, Y.; Rivera-Rodriguez, A.; Tay, Z.W.; Hensley, D.; Fung, K.B.; Colson, C.; Saayujya, C.; Huynh, Q.; Kabuli, L.; Fellows, B. Combining magnetic particle imaging and magnetic fluid hyperthermia for localized and image-guided treatment. Int. J. Hyperth. 2020, 37, 141–154. [Google Scholar] [CrossRef]
- Pucci, C.; Degl’Innocenti, A.; Gümüş, M.B.; Ciofani, G. Superparamagnetic iron oxide nanoparticles for magnetic hyperthermia: Recent advancements, molecular effects, and future directions in the omics era. Biomater. Sci. 2022, 10, 2103–2121. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, H.F.; Capistrano, G.; Bakuzis, A.F. In vivo magnetic nanoparticle hyperthermia: A review on preclinical studies, low-field nano-heaters, noninvasive thermometry and computer simulations for treatment planning. Int. J. Hyperth. 2020, 37, 76–99. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Kaur, P.; Aliru, M.L.; Chadha, A.S.; Asea, A.; Krishnan, S. Hyperthermia using nanoparticles–promises and pitfalls. Int. J. Hyperth. 2016, 32, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Zhang, T.; Peng, M.; Yi, J.; He, Y.; Fan, H. Design of Magnetic Nanoplatforms for Cancer Theranostics. Biosensors 2022, 12, 38. [Google Scholar] [CrossRef]
- Connord, V.; Mehdaoui, B.; Tan, R.P.; Carrey, J.; Respaud, M. An air-cooled Litz wire coil for measuring the high frequency hysteresis loops of magnetic samples—A useful setup for magnetic hyperthermia applications. Rev. Sci. Instrum. 2014, 85, 093904. [Google Scholar] [CrossRef]
- Garaio, E.; Collantes, J.M.; Plazaola, F.; Garcia, J.A.; Castellanos-Rubio, I. A multifrequency eletromagnetic applicator with an integrated AC magnetometer for magnetic hyperthermia experiments. Meas. Sci. Technol. 2014, 25, 115702. [Google Scholar] [CrossRef]
- Tasci, T.O.; Vargel, I.; Arat, A.; Guzel, E.; Korkusuz, P.; Atalar, E. Focused RF hyperthermia using magnetic fluids. Med. Phys. 2009, 36, 1906–1912. [Google Scholar] [CrossRef]
- Iszály, Z.; Márián, I.G.; Szabó, I.A.; Trombettoni, A.; Nándori, I. Theory of superlocalized magnetic nanoparticle hyperthermia: Rotating versus oscillating fields. J. Magn. Magn. Mater. 2022, 541, 168528. [Google Scholar] [CrossRef]
- Gresits, I.; Thuróczy, G.; Sági, O.; Gyüre-Garami, B.; Márkus, B.G.; Simon, F. Non-calorimetric determination of absorbed power during magnetic nanoparticle based hyperthermia. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abu-Bakr, A.F.; Zubarev, A.Y. Hyperthermia in a system of interacting ferromagnetic particles under rotating magnetic field. J. Magn. Magn. Mater. 2019, 477, 404–407. [Google Scholar] [CrossRef]
- Konopacki, M.; Jędrzejczak-Silicka, M.; Szymańska, K.; Mijowska, E.; Rakoczy, R. Effect of rotating magnetic field on ferromagnetic structures used in hyperthermia. J. Magn. Magn. Mater. 2021, 518, 167418. [Google Scholar] [CrossRef]
- Beković, M.; Trlep, M.; Jesenik, M.; Hamler, A. A comparison of the heating effect of magnetic fluid between the alternating and rotating magnetic field. J. Magn. Magn. Mater. 2014, 355, 12–17. [Google Scholar] [CrossRef]
- Ivkov, R.; DeNardo, S.J.; Daum, W.; Foreman, A.R.; Goldstein, R.C.; Nemkov, V.S.; DeNardo, G.L. Application of high amplitude alternating magnetic fields for heat induction of nanoparticles localized in cancer. Clin. Cancer Res. 2005, 11, 7093s–7103s. [Google Scholar] [CrossRef]
- Ashikbayeva, Z.; Tosi, D.; Balmassov, D.; Schena, E.; Saccomandi, P.; Inglezakis, V. Application of nanoparticles and nanomaterials in thermal ablation therapy of cancer. Nanomaterials 2019, 9, 1195. [Google Scholar] [CrossRef]
- Hilger, I.; Hiergeist, R.; Hergt, R.; Winnefeld, K.; Schubert, H.; Kaiser, W.A. Thermal ablation of tumors using magnetic nanoparticles: An in vivo feasibility study. Investig. Radiol. 2002, 37, 580–586. [Google Scholar] [CrossRef]
- Johannsen, M.; Thiesen, B.; Wust, P.; Jordan, A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth. 2010, 26, 790–795. [Google Scholar] [CrossRef]
- Bredlau, A.-L.; McCrackin, M.A.; Motamarry, A.; Helke, K.; Chen, C.; Broome, A.-M.; Haemmerich, D. Thermal therapy approaches for treatment of brain tumors in animals and humans. Crit. Rev. Biomed. Eng. 2016, 44, 443–457. [Google Scholar] [CrossRef]
- Bruners, P.; Braunschweig, T.; Hodenius, M.; Pietsch, H.; Penzkofer, T.; Baumann, M.; Günther, R.W.; Schmitz-Rode, T.; Mahnken, A.H. Thermoablation of malignant kidney tumors using magnetic nanoparticles: An in vivo feasibility study in a rabbit model. Cardiovasc. Interv. Radiol. 2010, 33, 127–134. [Google Scholar] [CrossRef]
- Moore, J.; Xu, S.; Wood, B.J.; Ren, H.; Tse, Z.T.H. Radiofrequency tumor ablation system with a wireless or implantable probe. Wirel. Power Transf. 2020, 7, 95–105. [Google Scholar] [CrossRef]
- Matsui, H.; Hamuro, M.; Nakamura, K.; Kayahara, H.; Murano, K.; Kotsuka, Y.; Miki, Y. Development of a highly efficient implanted thermal ablation device: In vivo experiment in rat liver. Br. J. Radiol. 2012, 85, e734–e739. [Google Scholar] [CrossRef] [PubMed]
- Jin, J. Electromagnetic Analysis and Design in Magnetic Resonance Imaging; Routledge: London, UK, 2018. [Google Scholar]
- Haase, A.; Odoj, F.; Von Kienlin, M.; Warnking, J.; Fidler, F.; Weisser, A.; Nittka, M.; Rommel, E.; Lanz, T.; Kalusche, B. NMR probeheads for in vivo applications. Concepts Magn. Reson. 2000, 12, 361–388. [Google Scholar] [CrossRef]
- Roemer, P.B.; Edelstein, W.A.; Hayes, C.E.; Souza, S.P.; Mueller, O.M. The NMR phased array. Magn. Reson. Med. 1990, 16, 192–225. [Google Scholar] [CrossRef]
- Giovannetti, G. Comparison between circular and square loops for low-frequency magnetic resonance applications: Theoretical performance estimation. Concepts Magn. Reson. Part B: Magn. Reson. Eng. 2016, 46, 146–155. [Google Scholar] [CrossRef]
- Giovannetti, G.; Hartwig, V.; Positano, V.; Vanello, N. Radiofrequency coils for magnetic resonance applications: Theory, design, and evaluation. Crit. Rev. Biomed. Eng. 2014, 42, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Collick, B.D.; Behzadnezhad, B.; Hurley, S.A.; Mathew, N.K.; Behdad, N.; Lindsay, S.A.; Robb, F.; Stormont, R.S.; McMillan, A.B. Rapid development of application-specific flexible MRI receive coils. Phys. Med. Biol. 2020, 65, 19NT01. [Google Scholar] [CrossRef]
- Zamarayeva, A.M.; Gopalan, K.; Corea, J.R.; Liu, M.Z.; Pang, K.; Lustig, M.; Arias, A.C. Custom, spray coated receive coils for magnetic resonance imaging. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Brizi, D.; Fontana, N.; Giovannetti, G.; Menichetti, L.; Cappiello, L.; Doumett, S.; Ravagli, C.; Baldi, G.; Monorchio, A. A Radiating System for Low-Frequency Highly Focused Hyperthermia with Magnetic Nanoparticles. IEEE J. Electromagn. RF Microw. Med. Biol. 2019, 4, 109–116. [Google Scholar] [CrossRef]
- Stauffer, P.R.; Sneed, P.K.; Hashemi, H.; Phillips, T.L. Practical induction heating coil designs for clinical hyperthermia with ferromagnetic implants. IEEE Trans. Biomed. Eng. 1994, 41, 17–28. [Google Scholar] [CrossRef]
- Nemkov, V.; Ruffini, R.; Goldstein, R.; Jackowski, J.; DeWeese, T.L.; Ivkov, R. Magnetic field generating inductor for cancer hyperthermia research. COMPEL-Int. J. Comput. Math. Electr. Electron. Eng. 2011, 30, 5. [Google Scholar] [CrossRef]
- Nieskoski, M.D.; Trembly, B.S. Comparison of a single optimized coil and a Helmholtz pair for magnetic nanoparticle hyperthermia. IEEE Trans. Biomed. Eng. 2014, 61, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Andujar, C.; Ortega, D.; Southern, P.; Nesbitt, S.A.; Thanh, N.T.K.; Pankhurst, Q.A. Real-time tracking of delayed-onset cellular apoptosis induced by intracellular magnetic hyperthermia. Nanomedicine 2016, 11, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Hadadian, Y.; Azimbagirad, M.; Navas, E.A.; Pavan, T.Z. A versatile induction heating system for magnetic hyperthermia studies under different experimental conditions. Rev. Sci. Instrum. 2019, 90, 074701. [Google Scholar] [CrossRef]
- Tang, Y.; Jin, T.; Flesch, R.C.; Gao, Y. Improvement of solenoid magnetic field and its influence on therapeutic effect during magnetic hyperthermia. J. Phys. D Appl. Phys. 2020, 53, 305003. [Google Scholar] [CrossRef]
- Cano, M.E.; Barrera, A.; Estrada, J.C.; Hernandez, A.; Cordova, T. An induction heater device for studies of magnetic hyperthermia and specific absorption ratio measurements. Rev. Sci. Instrum. 2011, 82, 114904. [Google Scholar] [CrossRef]
- Mazon, E.E.; Sámano, A.H.; Calleja, H.; Quintero, L.H.; Paz, J.A.; Cano, M.E. A frequency tuner for resonant inverters suitable for magnetic hyperthermia applications. Meas. Sci. Technol. 2017, 28, 095901. [Google Scholar] [CrossRef]
- Brizi, D.; Fontana, N.; Giovannetti, G.; Flori, A.; Menichetti, L.; Doumett, S.; Baldi, G.; Monorchio, A. A novel approach for determining the electromagnetic properties of a colloidal fluid with magnetic nanoparticles for hyperthermia applications. IEEE J. Electromagn. RF Microw. Med. Biol. 2018, 2, 70–77. [Google Scholar] [CrossRef]
- Di Barba, P.; Dughiero, F.; Sieni, E. Magnetic field synthesis in the design of inductors for magnetic fluid hyperthermia. IEEE Trans. Magn. 2010, 46, 931–2934. [Google Scholar] [CrossRef]
- Bertani, R.; Ceretta, F.; Di Barba, P.; Dughiero, F.; Forzan, M.; Michelin, R.A.; Sgarbossa, P.; Sieni, E.; Spizzo, F. Optimal inductor design for nanofluid heating characterisation. Eng. Comput. 2015, 32, 1870–1892. [Google Scholar] [CrossRef]
- Subramanian, M.; Miaskowski, A.; Pearce, G.; Dobson, J. A coil system for real-time magnetic fluid hyperthermia microscopy studies. Int. J. Hyperth. 2016, 32, 112–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frijia, F.; Flori, A.; Giovannetti, G. Design, simulation, and test of surface and volume radio frequency coils for 13C magnetic resonance imaging and spectroscopy. Rev. Sci. Instrum. 2021, 92, 081402. [Google Scholar] [CrossRef] [PubMed]
- Bordelon, D.E.; Goldstein, R.C.; Nemkov, V.S.; Kumar, A.; Jackowski, J.K.; DeWeese, T.L.; Ivkov, R. Modified solenoid coil that efficiently produces high amplitude AC magnetic fields with enhanced uniformity for biomedical applications. IEEE Trans. Magn. 2011, 48, 47–52. [Google Scholar] [CrossRef]
- Attaluri, A.; Jackowski, J.; Sharma, A.; Kandala, S.K.; Nemkov, V.; Yakey, C.; DeWeese, T.L.; Kumar, A.; Goldstein, R.C.; Ivkov, R. Design and construction of a Maxwell-type induction coil for magnetic nanoparticle hyperthermia. Int. J. Hyperth. 2020, 37, 1–14. [Google Scholar] [CrossRef]
- Giovannetti, G.; Landini, L.; Santarelli, M.F.; Positano, V. A fast and accurate simulator for the design of birdcage coils in MRI. Magn. Reson. Mater. Phys. Biol. Med. 2002, 15, 36–44. [Google Scholar] [CrossRef]
- Giovannetti, G.; Francesconi, R.; Landini, L.; Viti, V.; Santarelli, M.F.; Positano, V.; Benassi, A. A quadrature lowpass birdcage coil for a vertical low field MRI scanner. Concepts Magn. Reson. Part B Magn. Reson. Eng. Educ. J. 2004, 22, 1–6. [Google Scholar] [CrossRef]
- Wu, Z.; Zhuo, Z.; Cai, D.; Wu, J.; Wang, J.; Tang, J. An induction heating device using planar coil with high amplitude alternating magnetic fields for magnetic hyperthermia. Technol. Health Care 2015, 23, S203–S209. [Google Scholar] [CrossRef] [PubMed]
- Dürr, S.; Schmidt, W.; Janko, C.; Kraemer, H.P.; Tripal, P.; Eiermann, F.; Tietze, R.; Lyer, S.; Alexiou, C. A novel magnetic field device for inducing hyperthermia using magnetic nanoparticles. Biomed. Eng. Biomed. Tech. 2013, 58, 000010151520134129. [Google Scholar] [CrossRef]
- Giovannetti, G.; Menichetti, L. Litz wire RF coils for low frequency NMR applications. Measurement 2017, 110, 116–120. [Google Scholar] [CrossRef]
- Lacroix, L.-M.; Carrey, J.; Respaud, M. A frequency-adjustable electromagnet for hyperthermia measurements on magnetic nanoparticles. Rev. Sci. Instrum. 2008, 79, 093909. [Google Scholar] [CrossRef]
- Nieminen, J.O.; Sinisalo, H.; Souza, V.H.; Malmi, M.; Yuryev, M.; Tervo, A.E.; Stenroos, M.; Milardovich, D.; Korhonen, J.T.; Koponen, L.M.; et al. Multi-locus transcranial magnetic stimulation system for electronically targeted brain stimulation. Brain Stimul. 2022, 15, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, Y.; Zhang, W.; Luo, F.; Han, R. Simulation Research on Focusing Characteristics of 8-shaped Magnetic Coil. In Proceedings of the 2020 IEEE International Conference on High Voltage Engineering and Application (ICHVE), Beijing, China, 6–10 September 2020; IEEE: Beijing, China, 2020; pp. 1–4. [Google Scholar]
- Liu, C.; Ding, H.; Fang, X.; Wang, Z. Optimal Design of Transcranial Magnetic Stimulation Thin Core Coil With Trade-Off Between Stimulation Effect and Heat Energy. IEEE Trans. Appl. Supercond. 2020, 30, 1–6. [Google Scholar] [CrossRef]
- Li, Y.; Lee, J.; Long, X.; Qiao, Y.; Ma, T.; He, Q.; Cao, P.; Zhang, X.; Zheng, H. A Magnetic Resonance-Guided Focused Ultrasound Neuromodulation System With a Whole Brain Coil Array for Nonhuman Primates at 3 T. IEEE Trans. Med. Imaging 2020, 39, 4401–4412. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Hou, W.; Zhao, M.; Xu, G. Design of a new low-intensity focused ultrasound stimulation system with homogeneous magnetic field. In Proceedings of the 2016 Asia-Pacific International Symposium on Electromagnetic Compatibility (APEMC), Shenzhen, China, 17–21 May 2016; pp. 738–740. [Google Scholar] [CrossRef]
- Wu, Y.X.; Yu, H.Y.; Liu, Z.W. Numerical Investigation of the Magnetic and Electric Field Distributions Produced by Biconical Transcranial Magnetic Stimulation Coil for Optimal Design. IEEE Trans. Magn. 2018, 54, 1–5. [Google Scholar] [CrossRef]
- Meng, Q.; Cherry, M.; Refai, A.; Du, X.; Lu, H.; Hong, E.; Yang, Y.; Choa, F.-S. Development of Focused Transcranial Magnetic Stimulation for Rodents by Copper-Array Shields. IEEE Trans. Magn. 2018, 54, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Tang, Y.; Zhang, E.; Lee, G.; Hadimani, R.L.; Jiles, D.C. Quadruple Butterfly Coil With Passive Magnetic Shielding for Focused Transcranial Magnetic Stimulation. IEEE Trans. Magn. 2017, 53, 1–5. [Google Scholar] [CrossRef]
- Xiong, H.; Shi, J.H.; Hu, X.-W.; Li, J.-Z. The focusing Optimization of transcranial magnetic stimulation system. Prog. Electromagn. Res. M 2016, 48, 145–154. [Google Scholar] [CrossRef][Green Version]
- Hasan, M.M.; Sufian, S.M.A.; Mehdi, H.; Siddique-e-Rabbani, K. Designing a transcranial magnetic stimulator coil for Deep Brain Stimulation. In Proceedings of the 2016 9th International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 20–22 December 2016; IEEE: Dhaka, Bangladesh, 2016; pp. 303–306. [Google Scholar]
- March, S.D.; McAtee, S.; Senter, M.; Spoth, K.; Stiner, D.R.; Crowther, L.J.; Hadimani, R.L.; Jiles, D.C. Focused and deep brain magnetic stimulation using new coil design in mice. In Proceedings of the 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER), San Diego, CA, USA, 6–8 November 2013; pp. 125–128. [Google Scholar] [CrossRef]
- Yang, S.; Xu, G.; Wang, L.; Geng, Y.; Yu, H.; Yang, Q. Circular Coil Array Model for Tran-scranial Magnetic Stimulation. IEEE Trans. Appl. Supercond. 2010, 20, 829–833. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.; Liu, C.; Hu, Y. Coil Arrays Modeling and Optimization for Transcranial Magnetic Stimulation. In Proceedings of the 2009 2nd International Conference on Biomedical Engineering and Informatics, Tianjin, China, 17–19 October 2009; pp. 1–5. [Google Scholar] [CrossRef]
- Al-Mutawaly, N.; de Bruin, H.; Findlay, D. Magnetic nerve stimulation: Field focality and depth of penetration. In Proceedings of the 2001 Conference Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Istanbul, Turkey, 25–28 October 2001; Volume 1, pp. 877–880. [Google Scholar] [CrossRef]
- Xiong, H.; Li, Q.; Liu, J. Performance Optimization and Simulation Research of New Coil for Transcranial Magnetic Stimulation Based on Improved Particle Swarm Optimizer. IEEE Trans. Magn. 2021, 57, 1–11. [Google Scholar] [CrossRef]
- Zhang, T.; Edrich, J. Excentric coils for focused neuromagnetic stimulation and biomagnetic detection. Proceedings of 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Amsterdam, The Netherlands, 31 October–3 November 1996; Volume 1, pp. 391–392. [Google Scholar] [CrossRef]
- Sorkhabi, M.M.; Wendt, K.; Denison, T. Temporally Interfering TMS: Focal and Dynamic Stimulation Location. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 3537–3543. [Google Scholar] [CrossRef]
- Tsuyama, S.; Katayama, Y.; Hyodo, A.; Hayami, T.; Ueno, S.; Iramina, K. Effects of Coil Parameters on the Stimulated Area by Transcranial Magnetic Stimulation. IEEE Trans. Magn. 2009, 45, 4845–4848. [Google Scholar] [CrossRef]
- Rotundo, S.; Brizi, D.; Monorchio, A. Bessel Beam Radiating System for Focused Transcranial Magnetic Stimulation. In Proceedings of the 2022 16th European Conference on Antennas and Propagation (EuCAP), Madrid, Spain, 27 March–1 April 2022; pp. 1–4. [Google Scholar] [CrossRef]
- Pendry, J.B.; Holden, A.J.; Robbins, D.J.; Stewart, W.J. Magnetism from conductors and enhanced nonlinear phenomena. IEEE Trans. Microw. Theory Tech. 1999, 47, 2075–2084. [Google Scholar] [CrossRef]
- Choi, B.H.; Kim, J.H.; Cheon, J.P.; Rim, C.T. Synthesized magnetic field focusing using a current-controlled coil array. IEEE Magn. Lett. 2016, 7, 1–4. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, B.H.; Kim, H.R.; Rim, C.T. 2-D Synthesized Magnetic Field Focusing Technology With Loop Coils Distributed in a Rectangular Formation. IEEE Trans. Ind. Electron. 2019, 66, 5558–5566. [Google Scholar] [CrossRef]
- Falchi, M.; Rotundo, S.; Brizi, D.; Monorchio, A. A Design Methodology for Response-controlled Passive Magnetic Metasurfaces. In Proceedings of the 2022 16th European Conference on Antennas and Propagation (EuCAP), Madrid, Spain, 27 March–1 April 2022. [Google Scholar]
- Brizi, D.; Monorchio, A. An Analytical Approach for the Arbitrary Control of Magnetic Metasurfaces Frequency Response. IEEE Antennas Wirel. Propag. Lett. 2021, 20, 1003–1007. [Google Scholar] [CrossRef]
- Brizi, D.; Monorchio, A. Magnetic metasurfaces properties in the near field regions. Sci. Rep. 2022, 12, 3258. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Hernandez, L.; Grbic, A.; Michielssen, E. A simulation of focal brain stimulation using metamaterial lenses. In Proceedings of the 2010 IEEE Antennas and Propagation Society International Symposium, Toronto, ON, Canada, 11–17 July 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Motovilova, E.; Huang, S.Y. Hilbert Curve-Based Metasurface to Enhance Sensitivity of Radio Frequency Coils for 7-T MRI. IEEE Trans. Microw. Theory Tech. 2019, 67, 615–625. [Google Scholar] [CrossRef]
- Kretov, E.I.; Shchelokova, A.V.; Slobozhanyuk, A.P. Control of the magnetic near-field pattern inside MRI machine with tunable metasurface. Appl. Phys. Lett. 2019, 115, 061604. [Google Scholar] [CrossRef]
- Stoja, E.; Konstandin, S.; Philipp, D.; Wilke, R.N.; Betancourt, D.; Bertuch, T.; Jenne, J.; Umathum, R.; Günther, M. Improving magnetic resonance imaging with smart and thin metasurfaces. Sci. Rep. 2021, 11, 16179. [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.-K.; Chen, Y.-S.; Zhao, Y. On-demand field shaping for enhanced magnetic resonance imaging using an ultrathin reconfigurable metasurface. VIEW 2021, 2, 20200099. [Google Scholar] [CrossRef]
- Saha, S.; Pricci, R.; Koutsoupidou, M.; Cano-Garcia, H.; Katana, D.; Rana, S.; Kosmas, P.; Palikaras, G.; Webb, A.; Kallos, E. A smart switching system to enable automatic tuning and detuning of metamaterial resonators in MRI scans. Sci. Rep. 2020, 10, 10042. [Google Scholar] [CrossRef]

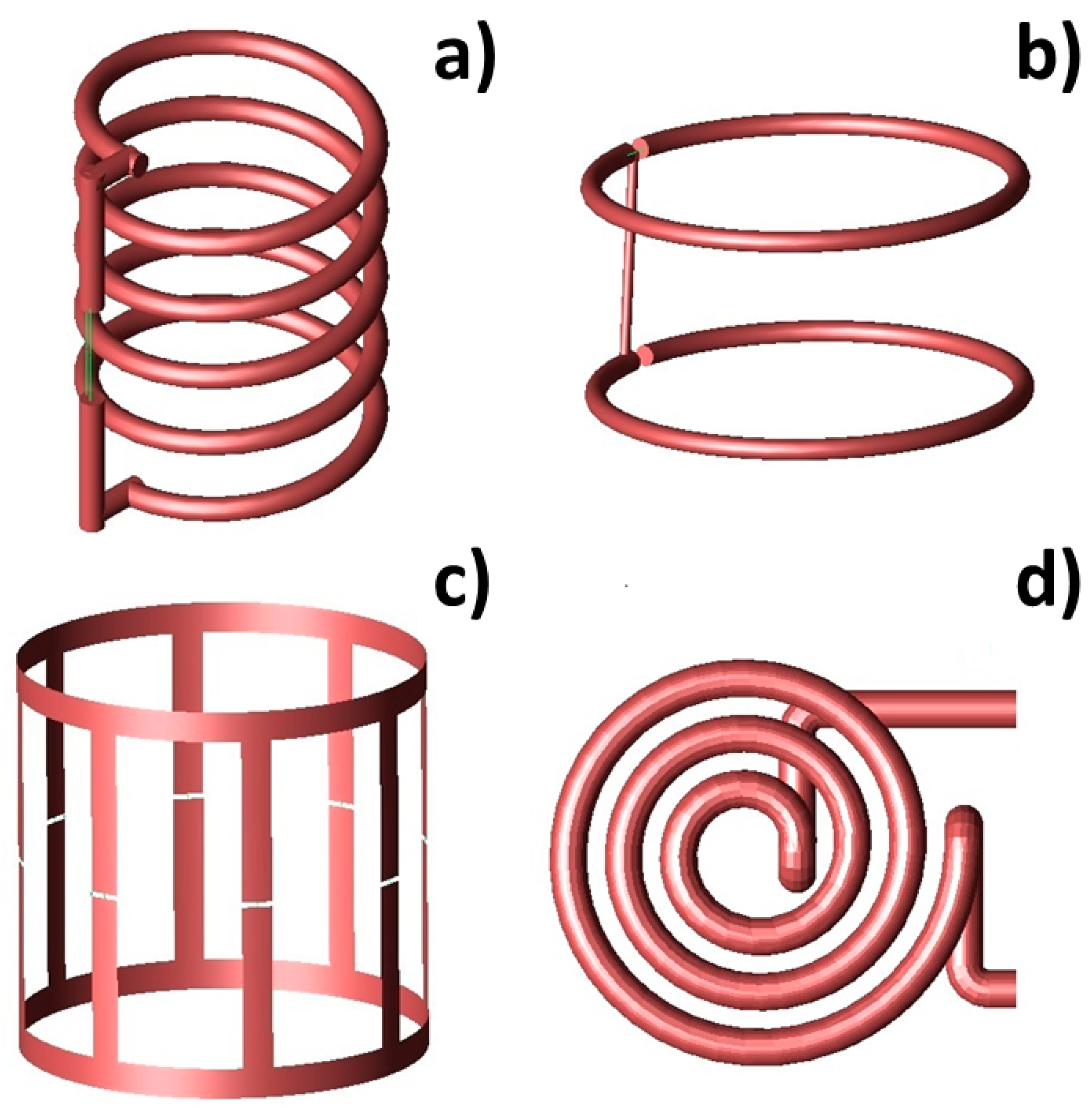



| Volume Coils | Pros | Cons | Refs. |
| Solenoid |
|
| [42,48,49,50,51,54,55] |
| Helmholtz |
|
| [43,44,46] |
| Birdcage |
|
| [22,57] |
| Surface Coils | Pros | Cons | Refs. |
| Pancake |
|
| [41,45,60] |
| Figure-of-eight |
|
| [65,69,70,72,74,76] |
| Bessel-beam |
|
| [81] |
| Arrays |
|
| [75,77,78] |
| Metamaterials and Metasurfaces | Pros | Cons | Refs. |
| Passive metasurface |
|
| [85,86,87,90,91] |
| Active metasurface |
|
| [83,84,92,93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotundo, S.; Brizi, D.; Flori, A.; Giovannetti, G.; Menichetti, L.; Monorchio, A. Shaping and Focusing Magnetic Field in the Human Body: State-of-the Art and Promising Technologies. Sensors 2022, 22, 5132. https://doi.org/10.3390/s22145132
Rotundo S, Brizi D, Flori A, Giovannetti G, Menichetti L, Monorchio A. Shaping and Focusing Magnetic Field in the Human Body: State-of-the Art and Promising Technologies. Sensors. 2022; 22(14):5132. https://doi.org/10.3390/s22145132
Chicago/Turabian StyleRotundo, Sabrina, Danilo Brizi, Alessandra Flori, Giulio Giovannetti, Luca Menichetti, and Agostino Monorchio. 2022. "Shaping and Focusing Magnetic Field in the Human Body: State-of-the Art and Promising Technologies" Sensors 22, no. 14: 5132. https://doi.org/10.3390/s22145132
APA StyleRotundo, S., Brizi, D., Flori, A., Giovannetti, G., Menichetti, L., & Monorchio, A. (2022). Shaping and Focusing Magnetic Field in the Human Body: State-of-the Art and Promising Technologies. Sensors, 22(14), 5132. https://doi.org/10.3390/s22145132






