MiWEndo: Evaluation of a Microwave Colonoscopy Algorithm for Early Colorectal Cancer Detection in Ex Vivo Human Colon Models
Abstract
:1. Introduction
2. Materials and Methods
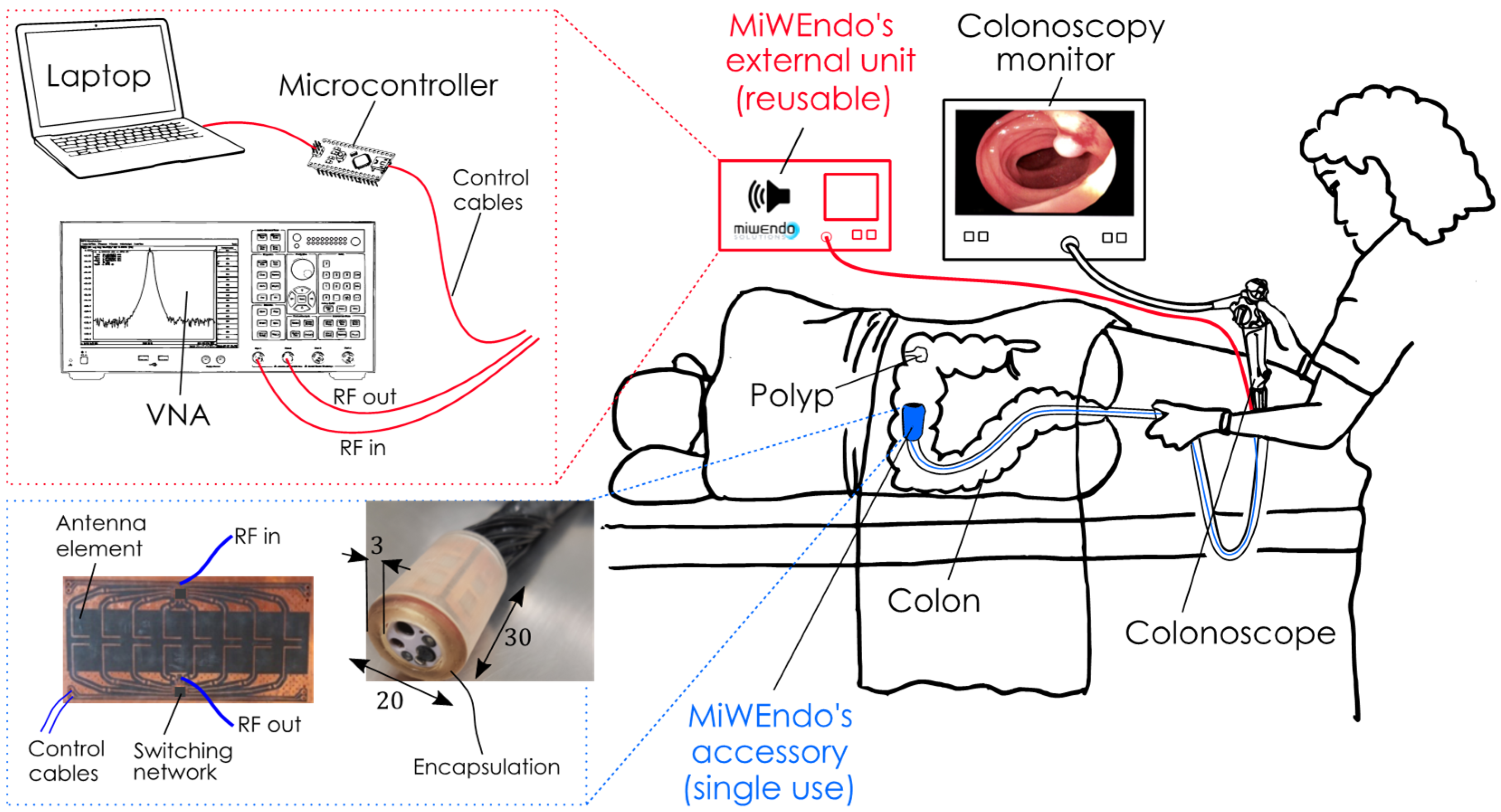
2.1. Microwave-Based Colonoscopy System
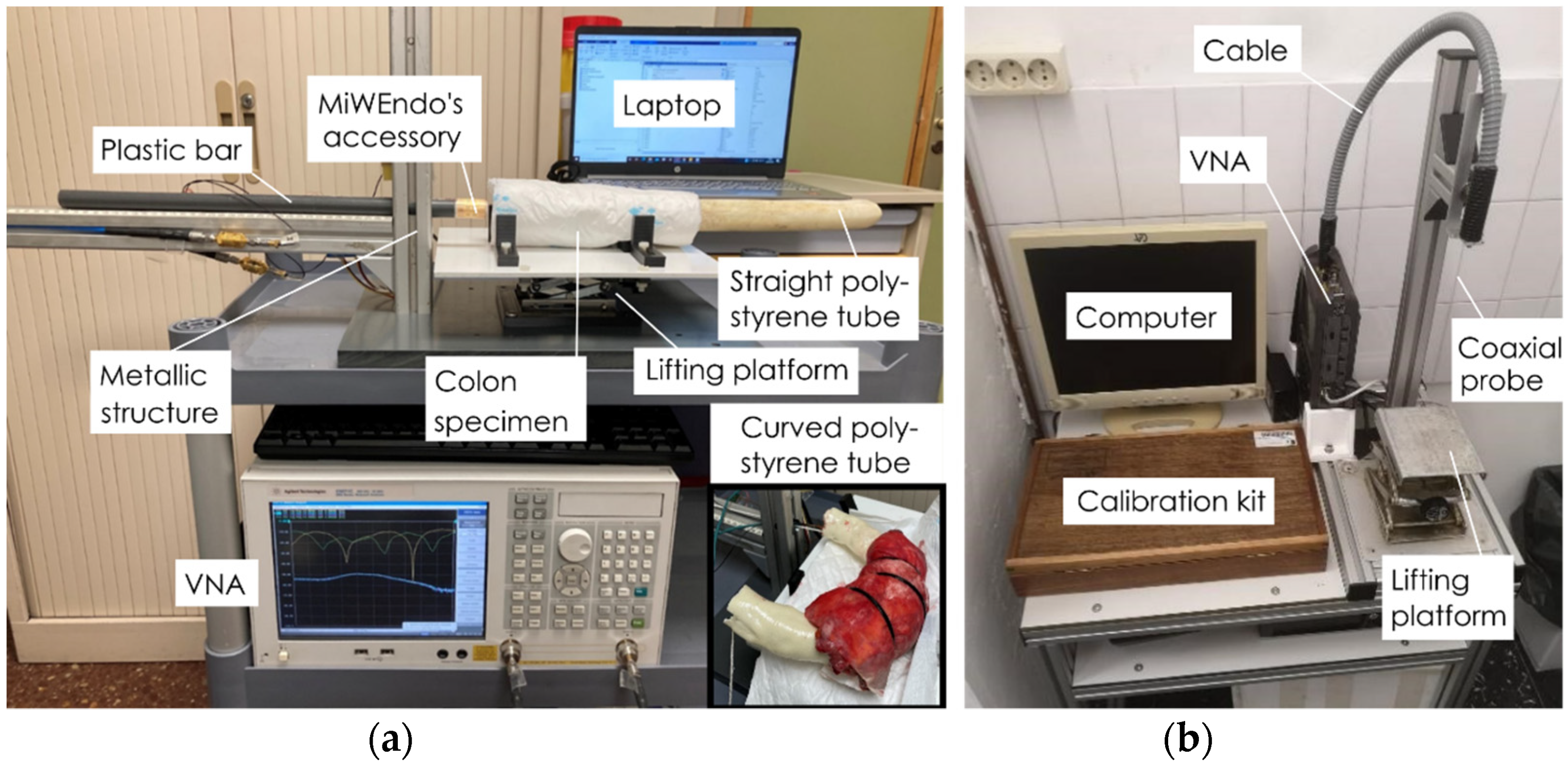
2.2. Setup for Ex Vivo Human Colon Measurements
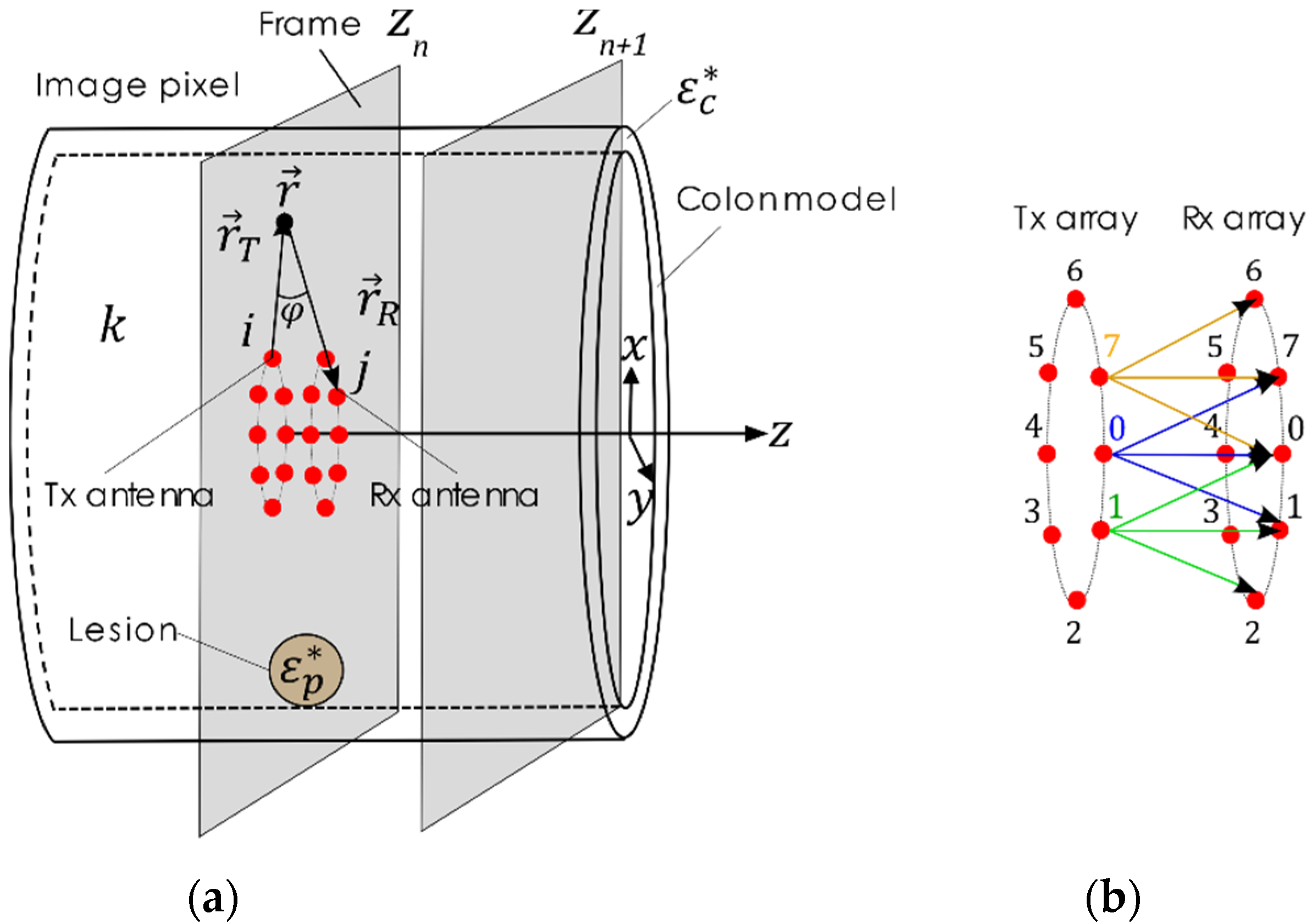
2.3. Polyp Detection Algorithm for Microwave-Assisted Colonoscopy
2.3.1. Calibration
2.3.2. Focuser
2.3.3. Detector
3. Results
3.1. Sample Characterization Results
3.2. Polyp Detection Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today. Int. Agency Res. Cancer 2018, 3, 2019. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Surveillance Research Program, National Cancer Institute SEER*Stat Software. Available online: Seer.cancer.gov/seerstat (accessed on 8 October 2021).
- Müller, A.D.; Sonnenberg, A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veterans. Ann. Intern. Med. 1995, 123, 904–910. Available online: http://www.ncbi.nlm.nih.gov/pubmed/7486484 (accessed on 12 May 2017). [CrossRef]
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Jerome, D.W.; Melvin, S.; John, H.B.; Joel, F.P.; et al. Prevention of Colorectal Cancer by Colonoscopic Polypectomy. N. Engl. J. Med. 1993, 329, 1977–1981. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8247072 (accessed on 12 May 2017). [CrossRef]
- van Rijn, J.C.; Reitsma, J.B.; Stoker, J.; Bossuyt, P.M.; van Deventer, S.J.; Dekker, E. Polyp miss rate determined by tandem colonoscopy: A systematic review. Am. J. Gastroenterol. 2006, 101, 343–350. [Google Scholar] [CrossRef]
- Samadder, N.J.; Curtin, K.; Tuohy, T.M.; Pappas, L.; Boucher, K.; Provenzale, D.; Rowe, K.G.; Mineau, G.P.; Smith, K.; Pimentel, R.; et al. Characteristics of missed or interval colorectal cancer and patient survival: A population-based study. Gastroenterology. 2014, 146, 950–960. [Google Scholar] [CrossRef]
- Lee, T.; Rees, C.; Blanks, R.; Moss, S.; Nickerson, C.; Wright, K.; James, P.W.; McNally, R.J.Q.; Patnick, J. Colonoscopic factors associated with adenoma detection in a national colorectal cancer screening program. Endoscopy 2014, 46, 203–211. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24473907 (accessed on 12 May 2017). [CrossRef] [Green Version]
- East, J.E.; Saunders, B.P.; Burling, D.; Boone, D.; Halligan, S.; Taylor, S.A. Surface visualization at CT colonography simulated colonoscopy: Effect of varying field of view and retrograde view. Am. J. Gastroenterol. 2007, 102, 2529–2535. [Google Scholar] [CrossRef]
- Corley, A.C.; Jensen, C.D.; Marks, A.R.; Zhao, W.K.; Lee, J.K.; Doubeni, C.A.; Zauber, A.G.; de Boer, J.; Fireman, B.H.; Schottinger, J.E.; et al. Adenoma Detection Rate and Risk of Colorectal Cancer and Death. N. Engl. J. Med. 2014, 370, 1298–1306. [Google Scholar] [CrossRef] [Green Version]
- Ngu, W.S.; Bevan, R.; Tsiamoulos, Z.P.; Bassett, P.; Hoare, Z.; Rutter, M.D.; Clifford, G.; Totton, N.; Lee, T.J.; Ramadas, A.; et al. Improved adenoma detection with Endocuff Vision: The ADENOMA randomised controlled trial. Gut 2019, 68, 280–288. [Google Scholar] [CrossRef]
- Dik, V.K.; Gralnek, I.M.; Segol, O.; Suissa, A.; Belderbos, T.D.G.; Moons, L.M.G.; Segev, M.; Domanov, S.; Rex, D.K.; Siersema, P.D. Multicenter, randomized, tandem evaluation of EndoRings colonoscopy—Results of the CLEVER study. Endoscopy 2015, 47, 1151–1158. [Google Scholar] [CrossRef] [Green Version]
- Ngu, W.S.; Rees, C. Can technology increase adenoma detection rate? Ther. Adv. Gastroenterol. 2018, 11, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, O.F.; Soares, A.S.; Mazomenos, E.; Brandao, P.; Vega, R.; Seward, E.; Stoyanov, P.D.; Chand, M.; Lovat, L.B. Artificial intelligence and computer-aided diagnosis in colonoscopy: Current evidence and future directions. Lancet Gastroenterol. Hepatol. 2019, 4, 71–80. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30527583 (accessed on 14 December 2018). [CrossRef] [Green Version]
- Repici, A.; Badalamenti, M.; Maselli, R.; Correale, L.; Radaelli, F.; Rondonotti, E.; Ferrara, E.; Spadaccini, M.; Alkandari, A.; Fugazza, A. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 2020, 159, 512–520. [Google Scholar] [CrossRef]
- Kumashiro, R.; Konishi, K.; Chiba, T.; Akahoshi, T.; Nakamura, S.; Murata, M.; Tomikawa, M.; Matsumoto, T.; Maehara, Y.; Hashizume, M. Integrated endoscopic system based on optical imaging and hyperspectral data analysis for colorectal cancer detection. Anticancer Res. 2016, 36, 3925–3932. Available online: https://ar.iiarjournals.org/content/36/8/3925 (accessed on 10 October 2021).
- Nikolova, N.K. Introduction to Microwave Imaging; Cambridge University Press: Cambridge, UK, 2017; p. 366. Available online: http://ebooks.cambridge.org/ref/id/CBO9781316084267 (accessed on 24 October 2017).
- Shere, M.; Lyburn, I.; Sidebottom, R.; Massey, H.; Gillett, C.; Jones, L. MARIA® M5: A multicentre clinical study to evaluate the ability of the Micrima radio-wave radar breast imaging system (MARIA®) to detect lesions in the symptomatic breast. Eur. J. Radiol. 2019, 116, 61–67. Available online: https://www.sciencedirect.com/science/article/pii/S0720048X19301512?via%3Dihub (accessed on 26 March 2020). [CrossRef]
- O’Loughlin, D.; O’Halloran, M.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave breast imaging: Clinical advances and remaining challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2580–2590. [Google Scholar] [CrossRef]
- Toaha Mobashsher, A.; Abbosh, A.M. On-site Rapid Diagnosis of Intracranial Hematoma using Portable Multi-slice Microwave Imaging System. Auto Moto Patras.Gr 2016, 6, 1–17. Available online: https://www.nature.com/articles/srep37620.pdf (accessed on 8 December 2021). [CrossRef]
- Meaney, P.M.; Goodwin, D.; Golnabi, A.H.; Zhou, T.; Pallone, M.; Geimer, S.D.; Burke, G.; Paulsen, K.D. Clinical microwave tomographic imaging of the calcaneus: A first-in-human case study of two subjects. IEEE Trans. Biomed. Eng. 2012, 59, 3304–3313. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22829363 (accessed on 24 October 2017). [CrossRef] [Green Version]
- Semenov, S.Y.; Posukh, V.G.; Bulyshev, A.E.; Williams, T.C.; Sizov, Y.E.; Repin, P.N.; Souvorov, A.; Nazarov, A. Microwave Tomographic Imaging of the Heart in Intact Swine. J. Electromagn. Waves Appl. J. Electromagn. Waves Appl. 2006, 207, 873–890. Available online: http://www.tandfonline.com/action/journalInformation?journalCode=tewa20 (accessed on 24 October 2017). [CrossRef]
- Guardiola, M.; Djafri, K.; Challal, M.; Gonzalez Ballester, M.A.; Fernandez-Esparrach, G.; Camara, O.; Romeu, J. Design and evaluation of an antenna applicator for a microwave colonoscopy system. IEEE Trans. Antennas Propag. 2019, 67, 4968–4977. [Google Scholar] [CrossRef]
- Garrido, A.; Sont, R.; Dghoughi, W.; Marcoval, S.; Romeu, J.; Fernández-Esparrach, G.; Belda, I.; Guardiola, M. Polyp Automatic Detection using Microwave Endoscopy for Colorectal Cancer Prevention and Early Detection. Phantom Validation. IEEE Access 2021, 9, 148048–148059. [Google Scholar] [CrossRef]
- Fernández-Esparrach, G.; Garrido, A.; Sont, R.; Dghoughi, W.; Marcoval, S.; Cuatrecasas, M.; López-Prades, S.; de Lacy, F.B.; Pellisé, M.; Belda, I.; et al. Microwave-Based Colonoscopy: Preclinical Evaluation in an Ex Vivo Human Colon Model. Gastroenterol. Res. Pract. 2022, 2022, 9522737. [Google Scholar] [CrossRef]
- Garrido-atienza, A.; Dghoughi, W.; Robert, J.R.; Garcia, M.G. Preliminary phantom-based dynamic calibration techniques assessment for microwave colonoscopy systems. In Proceedings of the 2022 16th European Conference on Antennas and Propagation (EuCAP), Madrid, Spain, 27 March 2022–1 April 2022; pp. 6–9. [Google Scholar]
- Guardiola, M.; Buitrago, S.; Fernández-Esparrach, G.; O’Callaghan, J.M.; Romeu, J.; Cuatrecasas, M.; Córdova, H.; Ballester, M.Á.G.; Camara, O. Dielectric properties of colon polyps, cancer, and normal mucosa: Ex vivo measurements from 0.5 to 20 GHz. Med. Phys. 2018, 45, 3768–3782. [Google Scholar] [CrossRef] [Green Version]
- La Gioia, A.; Porter, E.; Merunka, I.; Shahzad, A.; Salahuddin, S.; Jones, M.; O’Halloran, M. Open-Ended Coaxial Probe Technique for Dielectric Measurement of Biological Tissues: Challenges and Common Practices. Diagnostics 2018, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Shlepnev, Y. Evaluation of S-Parameters Similarity with Modified Hausdorff Distance. In Proceedings of the 30th IEEE Conference on Electrical Performance of Electronic Packaging and Systems, Austin, TX, USA, 17 October 2021. [Google Scholar]
- Zamani, A.; Abbosh, A. Hybrid Clutter Rejection Technique for Improved Microwave Head Imaging. IEEE Trans. Antennas Propag. 2015, 63, 4921–4931. [Google Scholar] [CrossRef]
- Brutlag, J.D. Aberrant behavior detection in time series for network monitoring. In Proceedings of the 14th Systems Administration Conference (LISA 2000), New Orleans, LA, USA, 3–8 December 2000; pp. 139–146. [Google Scholar]
- Ward, A.; Glynn, P.; Richardson, K. Internet service performance failure detection. Perform. Eval. Rev. 1998, 26, 38–43. [Google Scholar] [CrossRef]




| Patient | Age | Type of Neoplasm | Neoplasm Size (mm) | Sample Length (mm) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| 1 | 86 | Adenoma with HGD 1 | 10 | 200 | 100 | 80 |
| 2 | 64 | Adenocarcinoma 2 | 50 | 220 | 100 | 94.12 |
| 3 | 46 | Adenocarcinoma | 36 | 80 | 100 | 100 |
| 4 | 37 | Adenoma with HGD | 32 | 155 | 100 | 87.50 |
| 5 | 83 | Adenocarcinoma | 48 | 190 | 100 | 90.91 |
| 6 | 60 | Adenocarcinoma | 37 | 190 | 100 | 77.78 |
| 7 | 57 | Adenocarcinoma | 65 | 330 | 100 | 88.37 |
| 8 | 68 | Adenocarcinoma | 15 | 320 | 100 | 85.71 |
| 9 | 86 | Adenoma with HGD | 23 | 270 | 100 | 86.54 |
| 10 | 85 | Adenocarcinoma | 34 | 285 | 100 | 87.23 |
| 11 | 45 | Adenocarcinoma 3 | 32 | 260 | 100 | 83.33 |
| 12 | 75 | Adenocarcinoma 4 | 35 | 180 | 100 | 86.36 |
| 13 | 91 | Adenocarcinoma 3 | 40 | 160 | 100 | 84.21 |
| 14 | 62 | Adenocarcinoma 3 | 37 | 228 | 100 | 92.86 |
| 15 | 81 | Adenocarcinoma | 63 | 97 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guardiola, M.; Dghoughi, W.; Sont, R.; Garrido, A.; Marcoval, S.; Neira, L.M.; Belda, I.; Fernández-Esparrach, G. MiWEndo: Evaluation of a Microwave Colonoscopy Algorithm for Early Colorectal Cancer Detection in Ex Vivo Human Colon Models. Sensors 2022, 22, 4902. https://doi.org/10.3390/s22134902
Guardiola M, Dghoughi W, Sont R, Garrido A, Marcoval S, Neira LM, Belda I, Fernández-Esparrach G. MiWEndo: Evaluation of a Microwave Colonoscopy Algorithm for Early Colorectal Cancer Detection in Ex Vivo Human Colon Models. Sensors. 2022; 22(13):4902. https://doi.org/10.3390/s22134902
Chicago/Turabian StyleGuardiola, Marta, Walid Dghoughi, Roberto Sont, Alejandra Garrido, Sergi Marcoval, Luz María Neira, Ignasi Belda, and Glòria Fernández-Esparrach. 2022. "MiWEndo: Evaluation of a Microwave Colonoscopy Algorithm for Early Colorectal Cancer Detection in Ex Vivo Human Colon Models" Sensors 22, no. 13: 4902. https://doi.org/10.3390/s22134902
APA StyleGuardiola, M., Dghoughi, W., Sont, R., Garrido, A., Marcoval, S., Neira, L. M., Belda, I., & Fernández-Esparrach, G. (2022). MiWEndo: Evaluation of a Microwave Colonoscopy Algorithm for Early Colorectal Cancer Detection in Ex Vivo Human Colon Models. Sensors, 22(13), 4902. https://doi.org/10.3390/s22134902






