Muscle Co-Contraction Detection in the Time–Frequency Domain
Abstract
:1. Introduction
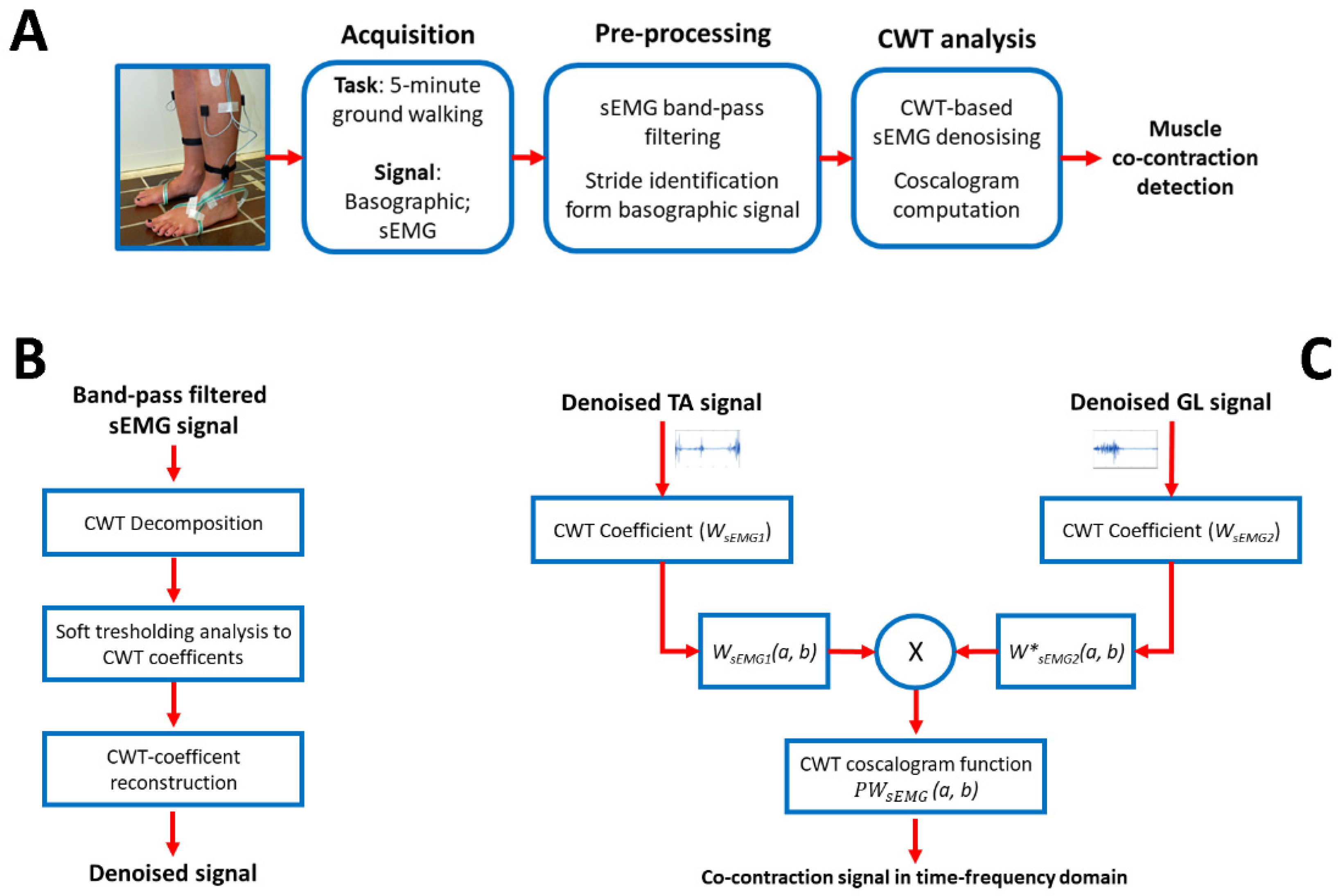
2. Materials and Methods
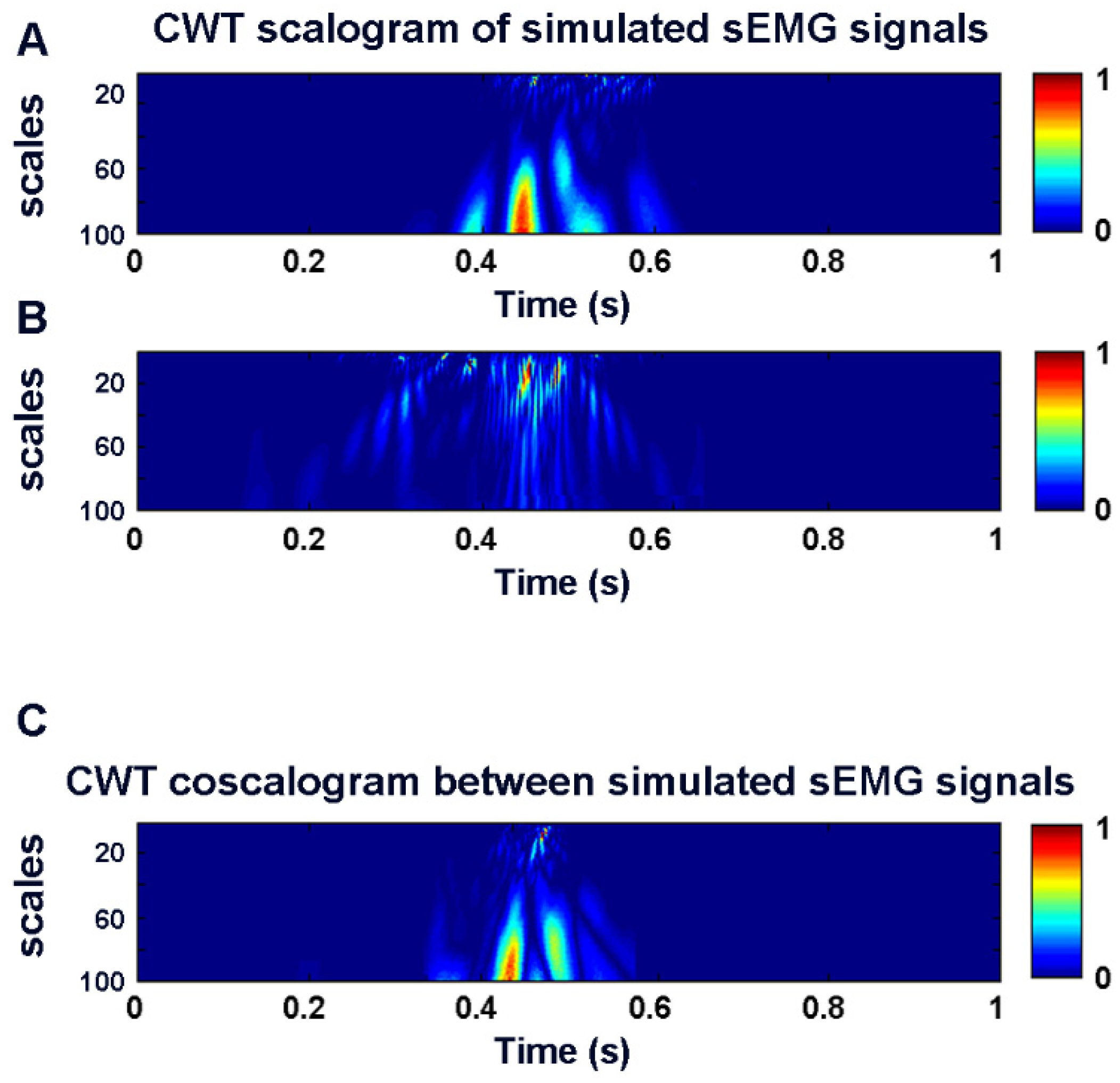
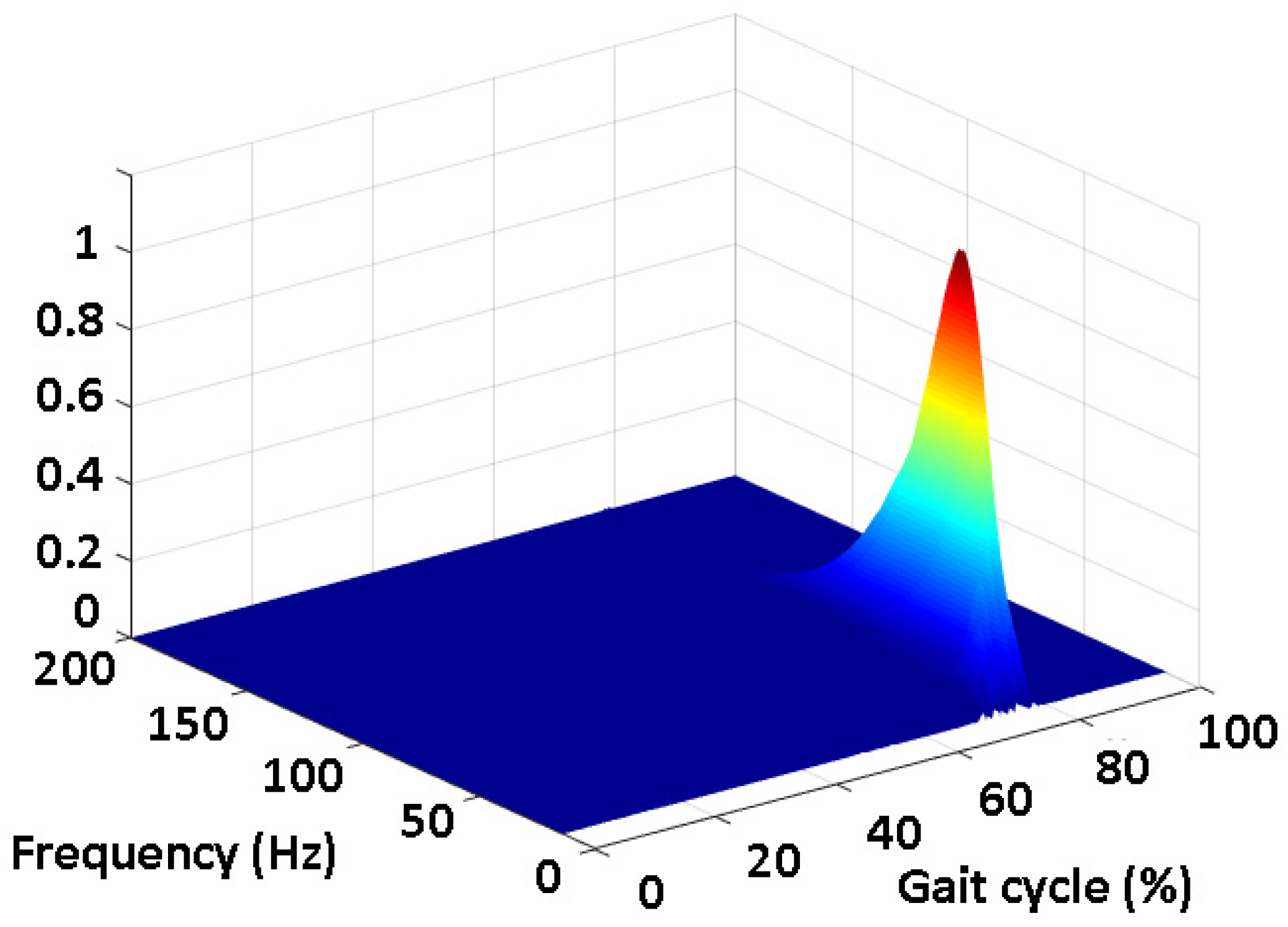
2.1. Co-Contraction Detection
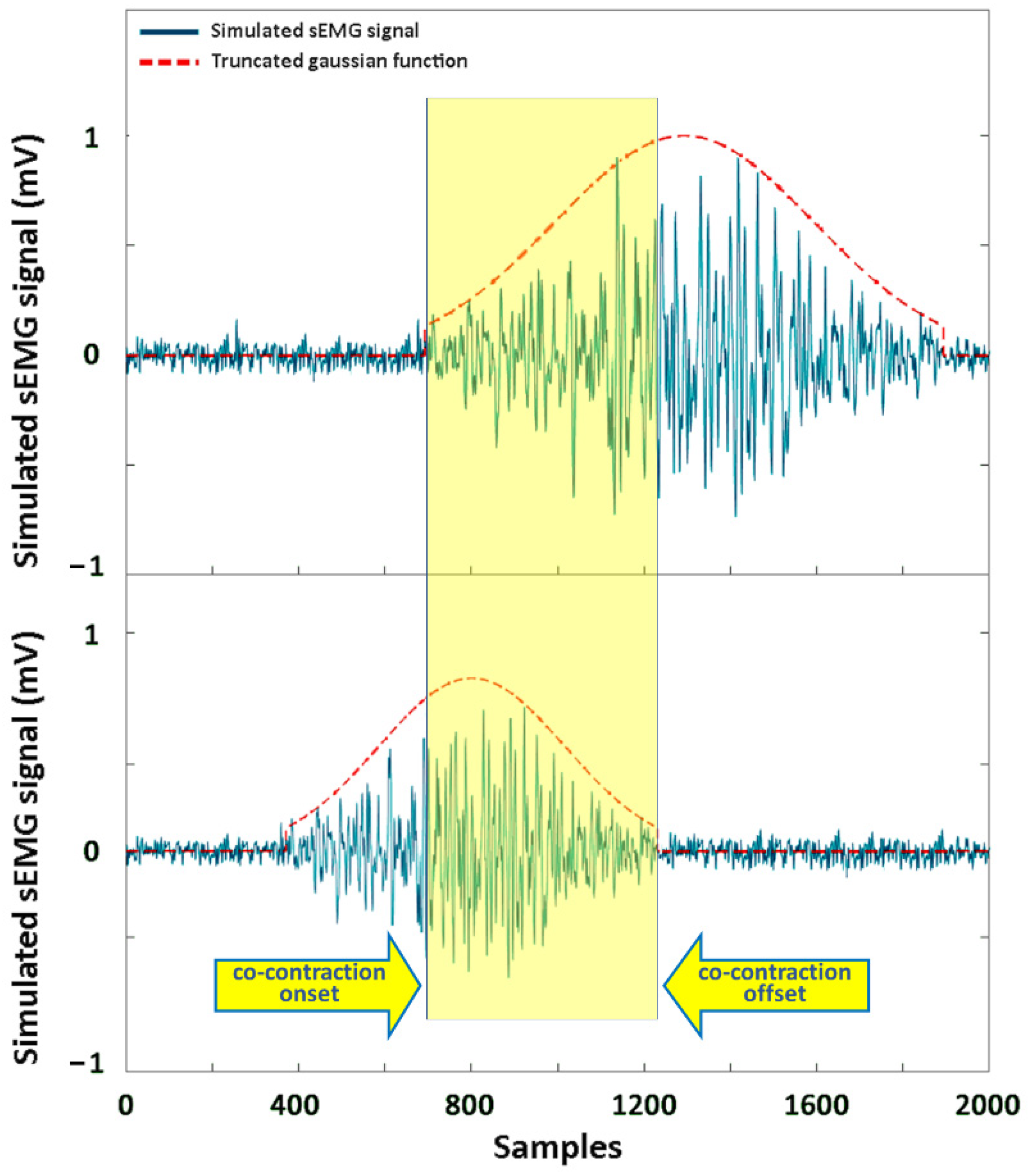
2.2. Simulation Study
2.3. Experimental Study
2.4. Statistics
3. Results
3.1. Simulation Study
3.2. Experimental Study
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piche, E.; Chorin, F.; Zory, R.; Duarte Freitas, P.; Guerin, O.; Gerus, P. Metabolic cost and co-contraction during walking at different speeds in young and old adults. Gait Posture 2022, 91, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Babadi, S.; Vahdat, S.; Milner, T.E. Neural Substrates of Muscle Co-contraction during Dynamic Motor Adaptation. J. Neurosci. 2021, 41, 5667–5676. [Google Scholar] [CrossRef] [PubMed]
- Milner, T.E. Adaptation to destabilizing dynamics by means of muscle cocontraction. Exp. Brain Res. 2002, 143, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Yamada, M.; Mori, S.; Tanaka, B.; Uemura, K.; Aoyama, T.; Ichihashi, N.; Tsuboyama, T. Effect of the muscle coactivation during quiet standing on dynamic postural control in older adults. Arch. Gerontol. Geriatr. 2013, 56, 129–133. [Google Scholar] [CrossRef]
- Rosa, M.C.; Marques, A.; Demain, S.; Metcalf, C.D.; Rodrigues, J. Methodologies to assess muscle co-contraction during gait in people with neurological impairment. A systematic literature review. J. Electromyogr. Kinesiol. 2014, 24, 179–191. [Google Scholar] [CrossRef]
- Lamontagne, A.; Richards, C.L.; Malouin, F. Coactivation during gait as an adaptive behavior after stroke. J. Electromyogr. Kinesiol. 2000, 10, 407–415. [Google Scholar] [CrossRef]
- McGinnis, K.; Snyder-Mackler, L.; Flowers, P.; Zeni, J. Dynamic joint stiffness and co-contraction in subjects after total knee arthroplasty. Clin. Biomech. 2013, 28, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Souissi, H.; Zory, R.; Bredin, J.; Gerus, P. Comparison of methodologies to assess muscle co-contraction during gait. J. Biomech. 2017, 24, 141–145. [Google Scholar] [CrossRef]
- Frost, G.; Dowling, J.; Dyson, K.; Bar-Or, O. Cocontraction in three age groups of children during treadmill locomotion. J. Electromyogr. Kinesiol. 1997, 7, 179–186. [Google Scholar] [CrossRef]
- Hesse, S.; Brandl-Hesse, B.; Seidel, U.; Doll, B.; Gregoric, M. Lower limb muscle activity in ambulatory children with cerebral palsy before and after the treatment with Botulinum toxin A. Restor. Neurol. Neurosci. 2000, 17, 1–8. [Google Scholar]
- Rudolph, K.S.; Axe, M.J.; Snyder-Mackler, L. Dynamic stability after ACL injury: Who can hop? Knee Surg Sports Traumatol. Arthrosc. 2000, 8, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Marin-Pardo, O.; Laine, C.M.; Rennie, M.; Ito, K.L.; Finley, J.; Liew, S.-L. A Virtual Reality Muscle–Computer Interface for Neurorehabilitation in Chronic Stroke: A Pilot Study. Sensors 2020, 20, 3754. [Google Scholar] [CrossRef] [PubMed]
- Falconer, K.; Winter, D.A. Quantitative assessment of co-contraction at the ankle joint in walking. Electromyogr. Clin. Neurophysiol. 1985, 25, 135–149. [Google Scholar] [PubMed]
- Iwamoto, Y.; Takahashi, M.; Shinkoda, K. Differences of muscle co-contraction of the ankle joint between young and elderly adults during dynamic postural control at different speeds. J. Physiol. Anthropol. 2017, 36, 32. [Google Scholar] [CrossRef] [Green Version]
- Nam, C.; Zhang, B.; Chow, T.; Ye, F.; Huang, Y.; Guo, Z.; Li, W.; Rong, W.; Hu, X.; Poon, W. Home-based self-help telerehabilitation of the upper limb assisted by an electromyography-driven wrist/hand exoneuromusculoskeleton after stroke. J. Neuroeng. Rehabil. 2021, 18, 137. [Google Scholar] [CrossRef]
- Peterson, D.S.; Martin, P.E. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture 2018, 31, 355–359. [Google Scholar] [CrossRef]
- Mohr, M.; Lorenzen, K.; Palacios-Derflingher, L.; Emery, C.; Nigg, B.M. Reliability of the knee muscle co-contraction index during gait in young adults with and without knee injury history. J. Electromyogr. Kinesiol. 2018, 38, 17–27. [Google Scholar] [CrossRef]
- Strazza, A.; Mengarelli, A.; Fioretti, A.; Burattini, L.; Agostini, V.; Knaflitz, M.; Di Nardo, F. Surface-EMG analysis for the quantification of thigh muscle dynamic co-contractions during normal gait. Gait Posture 2017, 51, 228–233. [Google Scholar] [CrossRef]
- Di Nardo, F.; Mengarelli, A.; Maranesi, E.; Burattini, L.; Fioretti, S. Assessment of the ankle muscle co-contraction during normal gait: A surface electromyography study. J. Electromyogr. Kinesiol. 2015, 25, 347–354. [Google Scholar] [CrossRef]
- Vannozzi, G.; Conforto, S.; D’Alessio, T. Automatic detection of surface EMG activation timing using a wavelet transform based method. J. Electromyogr. Kinesiol. 2010, 20, 767–772. [Google Scholar] [CrossRef]
- Severini, G.; Conforto, S.; Schmid, M.; D’Alessio, T. Novel formulation of a double threshold algorithm for the estimation of muscle activation intervals designed for variable SNR environments. J. Electromyogr. Kinesiol. 2012, 22, 878–885. [Google Scholar] [CrossRef] [PubMed]
- González-Izal, M.; Malanda, A.; Gorostiaga, E.; Izquierdo, M. Electromyographic models to assess muscle fatigue. J. Electromyogr. Kinesiol. 2012, 22, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Sacco, I.C.; Hamamoto, A.N.; Onodera, A.N.; Gomes, A.A.; Weiderpass, H.A.; Pachi, C.G.; Yamamoto, J.F.; von Tscharner, V. Motor strategy patterns study of diabetic neuropathic individuals while walking—A wavelet approach. J. Biomech. 2014, 47, 2475–2482. [Google Scholar] [CrossRef]
- Mohr, M.; von Tscharner, V.; Emery, C.A.; Nigg, B.M. Classification of gait muscle activation patterns according to knee injury history using a support vector machine approach. Hum. Mov. Sci. 2019, 66, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Sukiennik, P.; Białasiewicz, J.T. Cross-correlation of bio-signals using continuous wavelet transform and genetic algorithm. J. Neurosci. Methods 2015, 247, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Strazza, A.; Verdini, F.; Mengarelli, A.; Cardarelli, S.; Burattini, L.; Fioretti, S.; Di Nardo, F. A time-frequency approach for the assessment of dynamic muscle co-contractions. IFMBE Proc. 2018, 68, 223–226. [Google Scholar] [CrossRef]
- Rafiee, J.; Rafiee, M.A.; Prause, N.; Schoen, M.P. Wavelet basis functions in biomedical signal processing. Expert Syst. Appl. 2011, 38, 6190–6201. [Google Scholar] [CrossRef]
- Reaz, M.B.I.; Hussain, M.S.; Mohd-Yasin, F. Techniques of EMG signal analysis: Detection, processing, classification and applications (Correction). Biol. Proced. Online 2006, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Di Nardo, F.; Basili, T.; Meletani, S.; Scaradozzi, D. Wavelet-Based Assessment of the Muscle-Activation Frequency Range by EMG Analysis. IEEE Access 2022, 10, 9793–9805. [Google Scholar] [CrossRef]
- Ghislieri, M.; Cerone, G.L.; Knaflitz, M.; Agostini, V. Long short-term memory (LSTM) recurrent neural network for muscle activity detection. J. Neuroeng. Rehabil. 2021, 18, 153. [Google Scholar] [CrossRef]
- Di Nardo, F.; Morbidoni, C.; Fioretti, S. Surface electromyographic signals collected during long-lasting ground walking of young able-bodied subjects (version 1.0.0). PhysioNet 2022. [Google Scholar] [CrossRef]
- Goldberger, A.; Amaral, L.; Glass, L.; Hausdorff, J.; Ivanov, P.C.; Mark, R.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [Green Version]
- Di Nardo, F.; Morbidoni, C.; Cucchiarelli, A.; Fioretti, S. Influence of EMG-Signal Processing and Experimental Set-up on Prediction of Gait Events by Neural Network. Biomed. Signal Process. Control 2021, 63, 102232. [Google Scholar] [CrossRef]
- Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for sEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Agostini, V.; Balestra, G.; Knaflitz, M. Segmentation and Classification of Gait Cycles. IEEE Trans. Neural. Syst. Rehabil. Eng. 2013, 22, 946–952. [Google Scholar] [CrossRef] [Green Version]
- Bonato, P.; D’Alessio, T.; Knaflitz, M. A statistical method for the measurement of muscle activation intervals from surface myoelectric signal during gait. IEEE Trans. Biomed. Eng. 1998, 45, 287–299. [Google Scholar] [CrossRef]
- Lee, S.S.M.; Lam, T.; Pauhl, K.; Wakeling, J.M. Quantifying muscle coactivation in individuals with incomplete spinal cord injury using wavelets. Clin. Biomech. 2020, 73, 101–107. [Google Scholar] [CrossRef]
- Du, W.; Li, H.; Omisore, O.M.; Wang, L.; Chen, W.; Sun, X. Co-contraction characteristics of lumbar muscles in patients with lumbar disc herniation during different types of movement. Biomed. Eng. Online 2018, 17, 8. [Google Scholar] [CrossRef] [Green Version]
- Frère, J.; Göpfert, B.; Slawinski, J.; Tourny-Chollet, C. Shoulder muscles recruitment during a power backward giant swing on high bar: A wavelet-EMG-analysis. Hum. Mov. Sci. 2012, 31, 472–485. [Google Scholar] [CrossRef]
- Xie, P.; Chang, Q.; Zhang, Y.; Dong, X.; Yu, J.; Chen, X. Estimation of Time-Frequency Muscle Synergy in Wrist Movements. Entropy 2022, 24, 707. [Google Scholar] [CrossRef]
- Merlo, A.; Farina, D.; Merletti, R. A fast and reliable technique for muscle activity detection from surface EMG signals. IEEE Trans. Biomed. Eng. 2003, 50, 316–323. [Google Scholar] [CrossRef]
- Sharma, T.; Veer, K. Comparative study of wavelet denoising in myoelectric control applications. J. Med. Eng. Technol. 2016, 40, 80–86. [Google Scholar] [CrossRef]
- Staude, G.; Flachenecker, C.; Daumer, M.; Wolf, W. Onset detection in surface electromyographic signals: A systematic comparison of methods. EURASIP J. Appl. Signal. Process. 2001, 2, 67–81. [Google Scholar] [CrossRef] [Green Version]
- Angelova, S.; Ribagin, S.; Raikova, R.; Veneva, I. Power frequency spectrum analysis of surface EMG signals of upper limb muscles during elbow flexion-A comparison between healthy subjects and stroke survivors. J. Electromyogr. Kinesiol. 2018, 38, 7–16. [Google Scholar] [CrossRef]
- Preece, S.J.; Jones, R.K.; Brown, C.A.; Cacciatore, T.W.; Jones, A.K. Reductions in co-contraction following neuromuscular re-education in people with knee osteoarthritis. BMC Musculoskelet. Disord. 2016, 17, 372. [Google Scholar] [CrossRef]
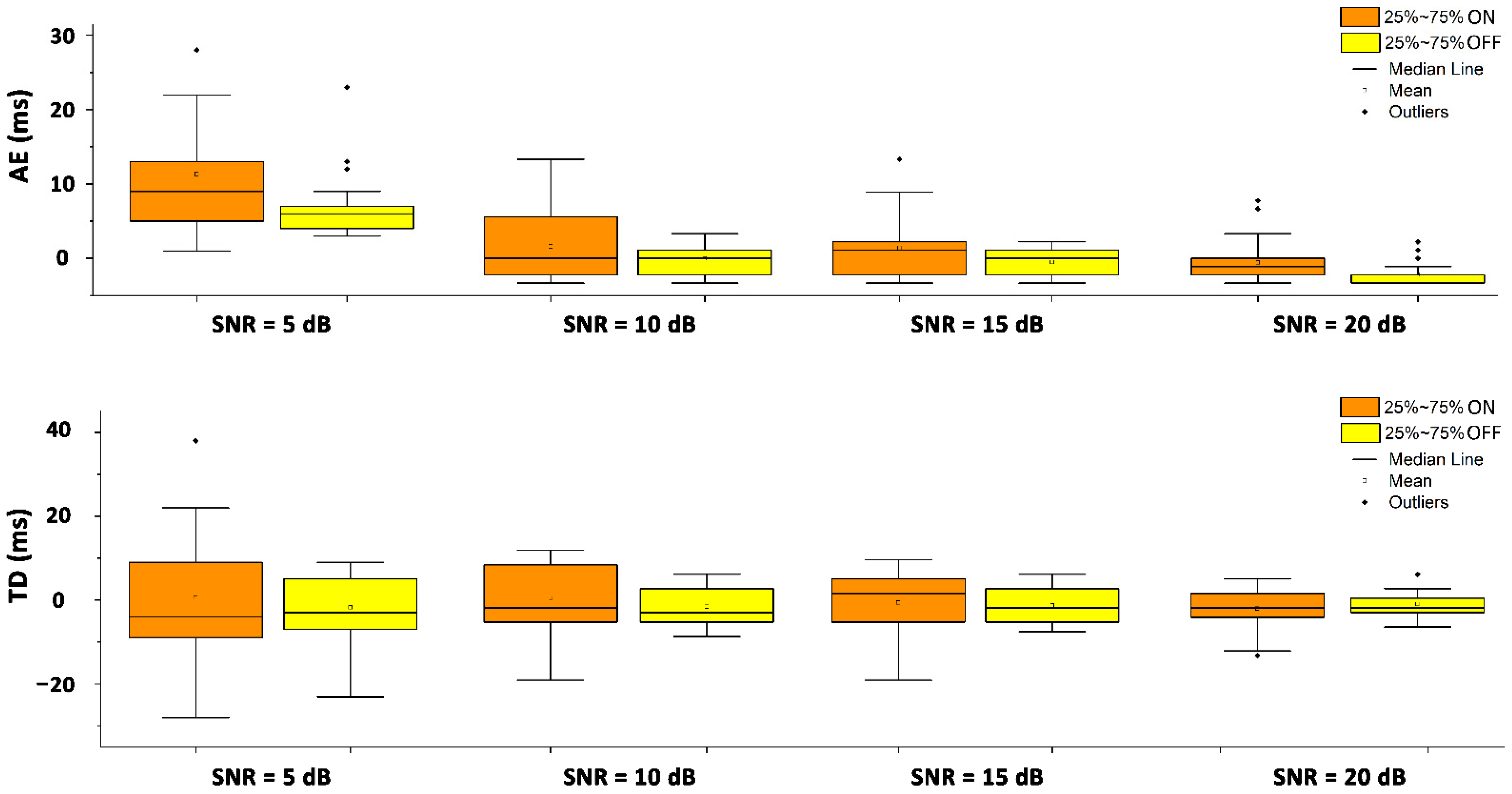





| SNR (dB) | Mean (ms) | SD (ms) | Median (ms) | 25th Percentile (ms) | 75th Percentile (ms) | |
|---|---|---|---|---|---|---|
| Onset | 5 | 0.52 | 14.6 | −4.0 | −9.1 | 9.2 |
| 10 | 0.96 | 6.9 | −1.2 | −4.0 | 8.1 | |
| 15 | 0.10 | 6.4 | 2.1 | −4.3 | 5.2 | |
| 20 | −1.16 | 4.3 | −1.4 | −3.2 | 2.4 | |
| Offset | 5 | −1.80 | 7.9 | −3.1 | −7.0 | 5.4 |
| 10 | 0.76 | 4.3 | −2.2 | −4.2 | 3.3 | |
| 15 | 0.52 | 4.0 | −1.2 | −4.1 | 3.0 | |
| 20 | 0.20 | 2.5 | −1.0 | −2.1 | 1.4 |
| Tibialis Anterior | Gastrocnemius Lateralis | |||
|---|---|---|---|---|
| Subject | SNR Raw | SNR Denoised | SNR Raw | SNR Denoised |
| 1 | 8.9 | 17.7 | 13.1 | 39.1 |
| 2 | 4.7 | 8.5 | 5.8 | 17.6 |
| 3 | 13.3 | 17.5 | 16.4 | 17.5 |
| 4 | 9.8 | 12.7 | 5.0 | 7.7 |
| 5 | 7.3 | 9.1 | 8.7 | 10.5 |
| 6 | 12.6 | 19.2 | 16.1 | 18.5 |
| 7 | 24.7 | 30.8 | 10.7 | 15.4 |
| 8 | 23.5 | 32.3 | 20.5 | 33.0 |
| 9 | 4.5 | 9.3 | 3.3 | 7.3 |
| 10 | 2.4 | 4.3 | 6.0 | 8.2 |
| 11 | 27.3 | 35.4 | 16.3 | 21.7 |
| 12 | 22.1 | 29.7 | 11.3 | 16.8 |
| 13 | 17.2 | 21.7 | 13.1 | 19.4 |
| 14 | 16.4 | 19.3 | 18.5 | 20.7 |
| 15 | 5.5 | 9.8 | 13.9 | 18.5 |
| 16 | 18.1 | 24.3 | 14.9 | 15.4 |
| 17 | 25.3 | 32.9 | 17.4 | 20.5 |
| 18 | 27.1 | 31.2 | 25.8 | 30.7 |
| 19 | 11.5 | 18.1 | 12.7 | 15.1 |
| 20 | 16.2 | 18.9 | 17.0 | 20.1 |
| 21 | 14.2 | 16.7 | 18.1 | 19.4 |
| 22 | 12.2 | 16.4 | 13.9 | 15.8 |
| 23 | 20.2 | 25.6 | 12.9 | 17.4 |
| 24 | 11.8 | 20.9 | 18.9 | 21.7 |
| 25 | 13.1 | 18.8 | 21.0 | 24.4 |
| 26 | 17.8 | 19.9 | 20.5 | 23.7 |
| 27 | 14.6 | 17.6 | 15.6 | 18.3 |
| 28 | 5.4 | 9.8 | 3.9 | 8.5 |
| 29 | 17.3 | 20.5 | 12.5 | 15.1 |
| 30 | 10.6 | 15.7 | 16.8 | 23.1 |
| Mean | 14.5 | 19.5 | 14.0 | 18.7 |
| SD | 7.0 | 8.0 | 5.5 | 7.1 |
| Median | 13.8 | 18.9 | 14.4 | 18.4 |
| 25th percentile | 9.8 | 15.7 | 11.3 | 15.4 |
| 75th percentile | 18.1 | 24.3 | 17.4 | 21.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nardo, F.; Morano, M.; Strazza, A.; Fioretti, S. Muscle Co-Contraction Detection in the Time–Frequency Domain. Sensors 2022, 22, 4886. https://doi.org/10.3390/s22134886
Di Nardo F, Morano M, Strazza A, Fioretti S. Muscle Co-Contraction Detection in the Time–Frequency Domain. Sensors. 2022; 22(13):4886. https://doi.org/10.3390/s22134886
Chicago/Turabian StyleDi Nardo, Francesco, Martina Morano, Annachiara Strazza, and Sandro Fioretti. 2022. "Muscle Co-Contraction Detection in the Time–Frequency Domain" Sensors 22, no. 13: 4886. https://doi.org/10.3390/s22134886
APA StyleDi Nardo, F., Morano, M., Strazza, A., & Fioretti, S. (2022). Muscle Co-Contraction Detection in the Time–Frequency Domain. Sensors, 22(13), 4886. https://doi.org/10.3390/s22134886









