Investigation of Formulations on Pyrene-Based Anodized-Aluminum Pressure-Sensitive Paints for Supersonic Phenomena
Abstract
:1. Introduction
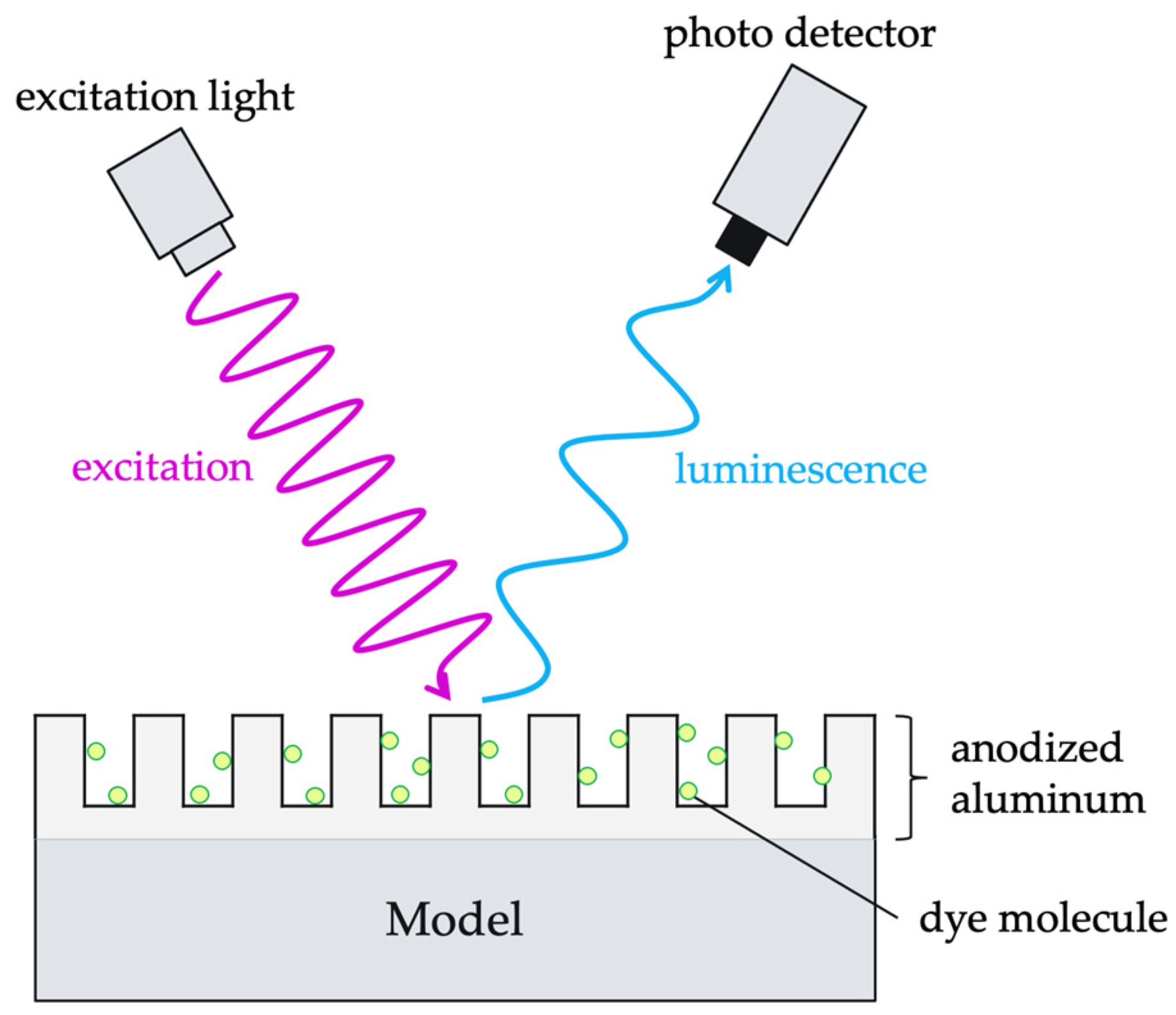
2. Principle of AA-PSP
3. Preparation of Test Piece
3.1. Formulations of PSP
3.2. Anodization
3.3. Dipping
4. Experimental and Analytical Methods
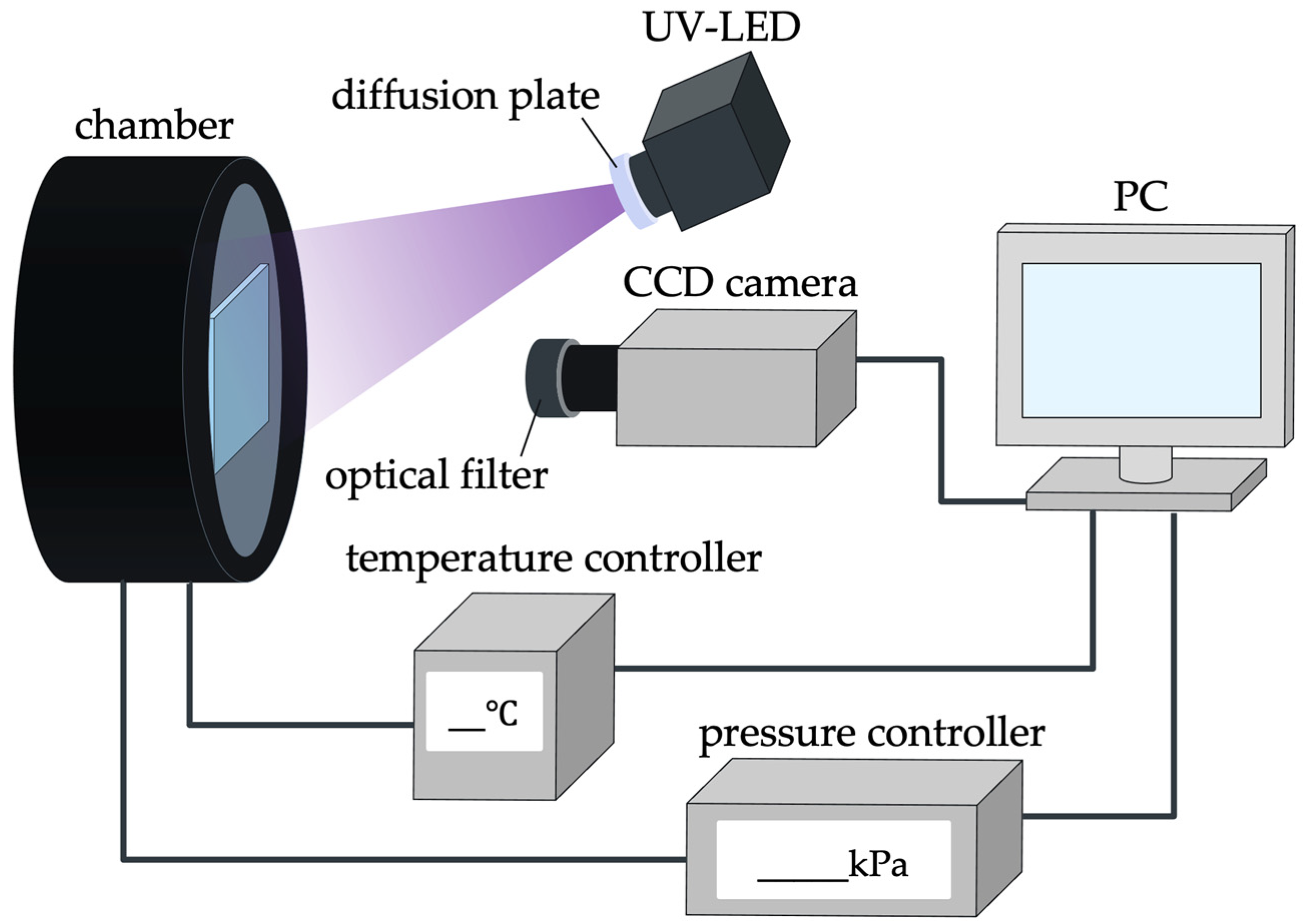
4.1. Calibration System
4.2. Calibration Test
4.3. Measurement of Luminescence Intensity
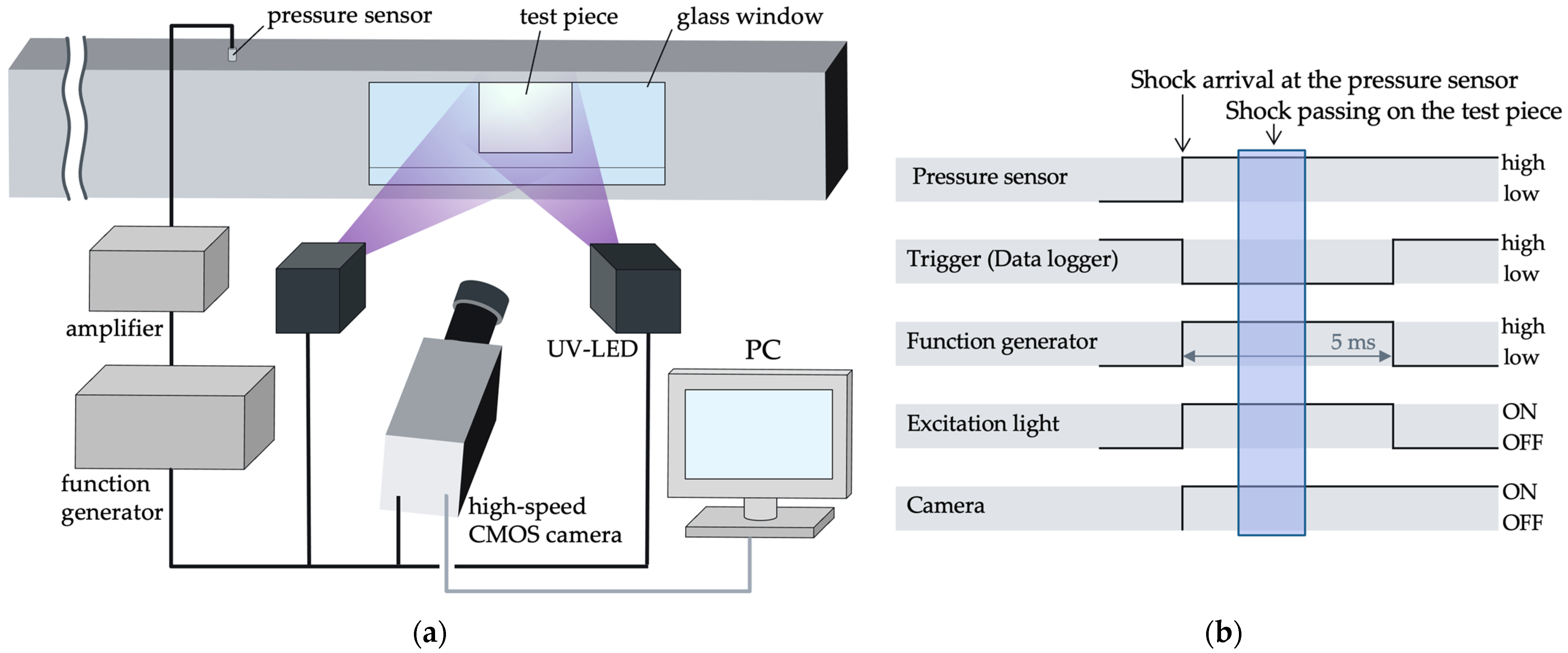
4.4. Shock Tube
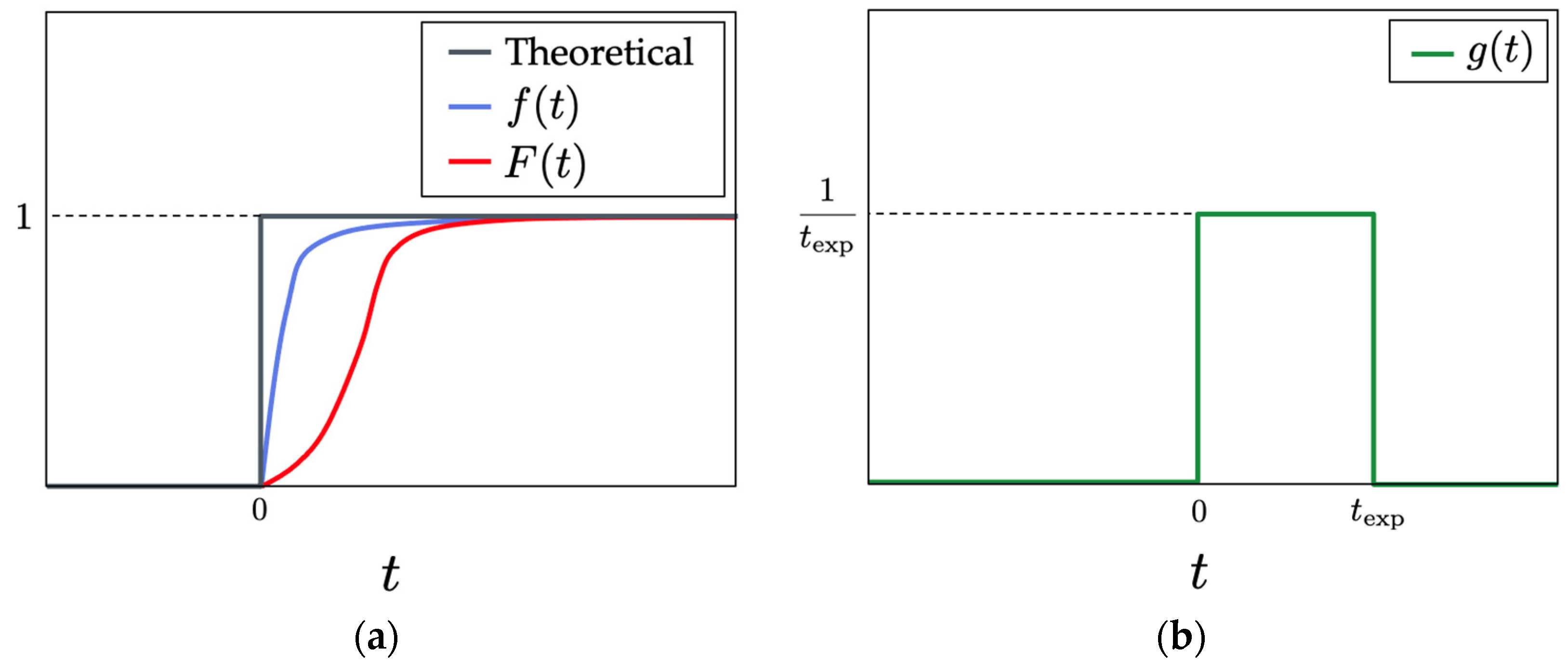
4.5. Measurement of Time Response
5. Results and Discussion
5.1. Pressure Sensitivity
5.1.1. Different Dipping Solvents
5.1.2. Different Anodization Time and Different Dipping Concentrations
5.2. Luminescence Intensity
5.3. Time Response
6. Conclusions
- Pressure sensitivity
- Luminescence intensity
- Time-response test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, T.; Sullivan, J.P.; Asai, K.; Klein, C.; Egami, Y. Pressure and Temperature Sensitive Paints, 2nd ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Gregory, J.W.; Asai, K.; Kameda, M.; Liu, T.; Sullivan, J.P. A Review of Pressure-Sensitive Paint for High-Speed and Unsteady Aerodynamics. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2008, 222, 249–290. [Google Scholar] [CrossRef]
- Peng, D.; Liu, Y. Fast Pressure-Sensitive Paint for Understanding Complex Flows: From Regular to Harsh Environments. Exp. Fluids 2020, 61, 8. [Google Scholar] [CrossRef]
- Gregory, J.W.; Sakaue, H.; Liu, T.; Sullivan, J. P Fast Pressure-Sensitive Paint for Flow and Acoustic Diagnostics. Annu. Rev. Fluid Mech. 2014, 46, 303–330. [Google Scholar] [CrossRef]
- Asai, K.; Kanda, H.; Cunningham, C.T.; Erausquin, R.; Sullivan, J.P. Surface Pressure Measurement in a Cryogenic Wind Tunnel by Using Luminescent Coating. In Proceedings of the ICIASF’97 Record. International Congress on Instrumentation in Aerospace Simulation Facilities, Pacific Grove, CA, USA, 29 September–2 October 1997; pp. 105–114. [Google Scholar] [CrossRef]
- Asai, K.; Amao, Y.; Iijima, Y.; Okura, I.; Nishide, H. Novel Pressure-Sensitive Paint for Cryogenic and Unsteady Wind-Tunnel Testing. J. Thermophys. Heat Transf. 2002, 16, 109–115. [Google Scholar] [CrossRef]
- Erausquin, R.; Cunningham, C.T.; Sullivan, J.P.; Asai, K.; Kanda, H.; Kunimasu, T.; Iijima, Y. Cryogenic Pressure Sensitive Fluorescent Paint Systems. In Proceedings of the 36th AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 12–15 January 1998. [Google Scholar] [CrossRef]
- Egami, Y.; Fey, U.; Engler, R.H. Appropriate selection of pressure-sensitive paint for cryogenic wind tunnels. In Proceedings of the 44th AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 9–12 January 2006. [Google Scholar] [CrossRef]
- Sakaue, H.; Kakisako, T.; Ishikawa, H. Characterization and Optimization of Polymer-Ceramic Pressure-Sensitive Paint by Controlling Polymer Content. Sensors 2011, 11, 6967–6977. [Google Scholar] [CrossRef]
- Sakaue, H.; Hayashi, T.; Ishikawa, H. Luminophore Application Study of Polymer-Ceramic Pressure-Sensitive Paint. Sensors 2013, 13, 7053–7064. [Google Scholar] [CrossRef]
- Kameda, M.; Seki, H.; Makoshi, T.; Amao, Y.; Nakakita, K. A Fast-Response Pressure Sensor Based on a Dye-Adsorbed Silica Nanoparticle Film. Sens. Actuators B 2012, 171–172, 343–349. [Google Scholar] [CrossRef]
- Egami, Y.; Konishi, S.; Sato, Y.; Matsuda, Y. Effects of Solvents for Luminophore on Dynamic and Static Characteristics of Sprayable Polymer/Ceramic Pressure-Sensitive Paint. Sens. Actuators A 2019, 286, 188–194. [Google Scholar] [CrossRef]
- Egami, Y.; Sato, Y.; Konishi, S. Development of Sprayable Pressure-Sensitive Paint with a Response Time of Less than 10 μs. AIAA J. 2019, 57, 2198–2203. [Google Scholar] [CrossRef]
- Egami, T.; Hasegawa, Y.; Matsuda, Y.; Ikami, T.; Nagai, H. Ruthenium-Based Fast-Responding Pressure-Sensitive Paint for Measuring Small Pressure Fluctuation in Low-Speed Flow Field. Meas. Sci. Technol. 2021, 32, 024003. [Google Scholar] [CrossRef]
- Sakaue, H.; Sullivan, J.P.; Asai, K.; Iijima, Y.; Kunimasu, T. Anodized Aluminum Pressure Sensitive Paint in a Cryogenic Wind Tunnel. In Proceedings of the 45th International Instrumentation Symposium, Instrument Society of America, Albuquerque, NM, USA, 2–6 May 1999; pp. 345–354. [Google Scholar]
- Sakaue, H.; Sullivan, L.P. Time Response of Anodized Aluminum Pressure-Sensitive Paint. AIAA J. 2001, 39, 1944–1949. [Google Scholar] [CrossRef]
- Sakaue, H. Luminophore Application Method of Anodized Aluminum Pressure Sensitive Paint as a Fast Responding Global Sensor. Rev. Sci. Instrum. 2005, 76, 084101. [Google Scholar] [CrossRef]
- Numata, D.; Fujii, S.; Nagai, H.; Asai, K. Ultrafast-Response Anodized-Aluminum Pressure-Sensitive Paints for Unsteady Flow Measurement. AIAA J. 2017, 55, 1118–1125. [Google Scholar] [CrossRef]
- Kameda, M.; Tezuka, N.; Hangai, T.; Asai, K.; Nakakita, K.; Amao, Y. Adsorptive Pressure-Sensitive Coatings on Porous Anodized Aluminium. Meas. Sci. Technol. 2004, 15, 489. [Google Scholar] [CrossRef]
- Merienne, M.-C.; Coponet, D.; Luyssen, J.-M. Transient Pressure-Sensitive-Paint Investigation in a Nozzle. AIAA J. 2012, 50, 7. [Google Scholar] [CrossRef]
- Yang, L.; Zare-Behtash, H.; Erdem, E.; Kontis, K. Application of AA-PSP to Hypersonic Flows: The Double Ramp Model. Sens. Actuators B 2012, 161, 100–107. [Google Scholar] [CrossRef]
- Kurihara, D.; Gonzales, J.; Claucherty, S.; Kiritani, H.; Fujita, K.; Jemcov, A.; Nagai, H.; Sakaue, H. Sub-millimeter Resolution Pressure Measurement on Free Flight Model at Mach 1.5 using Novel Non-intrusive Optical Technique. Exp. Therm. Fluid Sci. 2021, 120, 110243. [Google Scholar] [CrossRef]
- Running, C.; Sakaue, H.; Juliano, T. Hypersonic Boundary-layer Separation Detection with Pressure-sensitive Paint for a Cone at High Angle of Attack. Exp. Fluids 2019, 60, 23. [Google Scholar] [CrossRef]
- Gonzales, J.; Suzuki, K.; Sakaue, H. Temporally and Spatially Resolved Pressure and Temperature Maps in Hypersonic Flow. Int. J. Heat Mass Transf. 2020, 156, 119782. [Google Scholar] [CrossRef]
- Gan, J.; Watanabe, T.; Himeno, T. Effect of Shock Wave Behavior on Unsteady Aerodynamic Characteristics of Oscillating Transonic Compressor Cascade. J. Eng. Gas Turbines Power 2022, 144, 011025. [Google Scholar] [CrossRef]
- Hangai, T.; Kameda, M.; Nakakita, K.; Asai, K. Time Response Characteristics of Pyrene-based Pressure-sensitive Coatings on Anodic Porous Alumina. In Proceedings of the 10th International Symposium on Flow Visualization, Kyoto, Japan, 26–29 August 2002. [Google Scholar]
- Sakaue, H.; Ishii, K. A Dipping Duration Study for Optimization of Anodized-Aluminum Pressure-Sensitive Paint. Sensors 2010, 10, 9799–9807. [Google Scholar] [CrossRef] [PubMed]
- Sakaue, H.; Ishii, K. Optimization of Anodized-Aluminum Pressure-Sensitive Paint by Controlling Luminophore Concentration. Sensors 2010, 10, 6836–6847. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.K.; Yang, L.; Kontis, K. Pressure-Sensitive Paint: Effect of Substrate. Sensors 2011, 11, 11649–11663. [Google Scholar] [CrossRef] [PubMed]
- Sakaue, H.; Sullivan, J.P.; Egami, Y.; Iijima, Y.; Asai, K.; Engler, R.H.; Beifuss, U.; Doring, F. Open-System Pressure Sensitive Paint for Surface Pressure Measurements in a Cryogenic Wind Tunnel. In Proceedings of the ICIASF 2001 Record, 19th International Congress on Instrumentation in Aerospace Simulation Facilities (Cat. No.01CH37215), Cleveland, OH, USA, 27–30 August 2001; pp. 186–195. [Google Scholar] [CrossRef]
- Sakaue, H.; Kuriki, T.; Miyazaki, T. A Temperature-Cancellation Method of Pressure-Sensitive Paint on Porous Anodic Alumina Using 1-Pyrenesulfonic Acid. J. Lumin. 2012, 132, 256–260. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Peng, D. Suppression of Thermal Quenching in Fast-responding Pressure-sensitive Paint by Restricting Lattice Relaxation of Luminescent Molecules. J. Lumin. 2020, 217, 116803. [Google Scholar] [CrossRef]
- Claucherty, S.; Sakaue, H. An Optical-Chemical Sensor Using Pyrene-Sulfonic Acid for Unsteady Surface Pressure Measurements. Sens. Actuators A Phys. 2021, 317, 112359. [Google Scholar] [CrossRef]
- Mérienne, M.-C.; Le Sant, Y.; Ancelle, J.; Soulevant, D. Unsteady Pressure Measurement Instrumentation Using Anodized-Aluminium PSP Applied in a Transonic Wind Tunnel. Meas. Sci. Technol. 2004, 15, 2349–2360. [Google Scholar] [CrossRef]
- Ikami, T.; Fujita, K.; Nagai, H. Influence of Formulations on Characteristics of Ruthenium-Based Temperature-Sensitive Paints. Sensors 2022, 22, 901. [Google Scholar] [CrossRef]
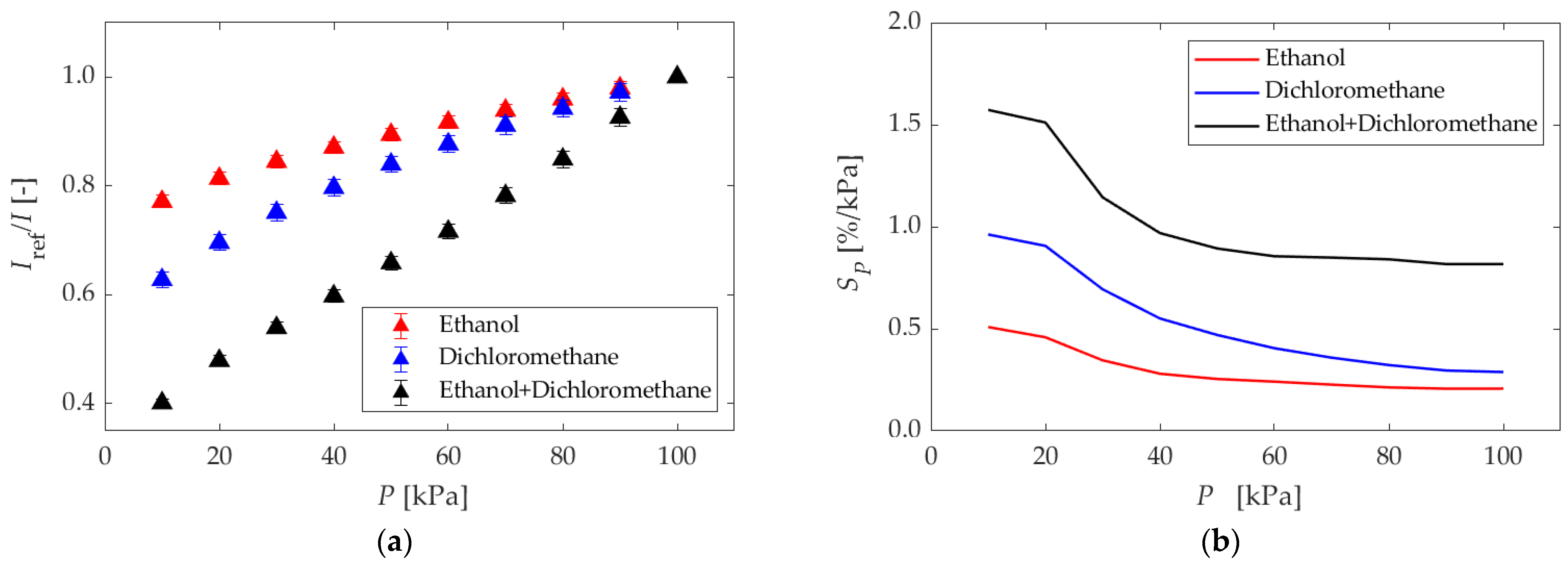
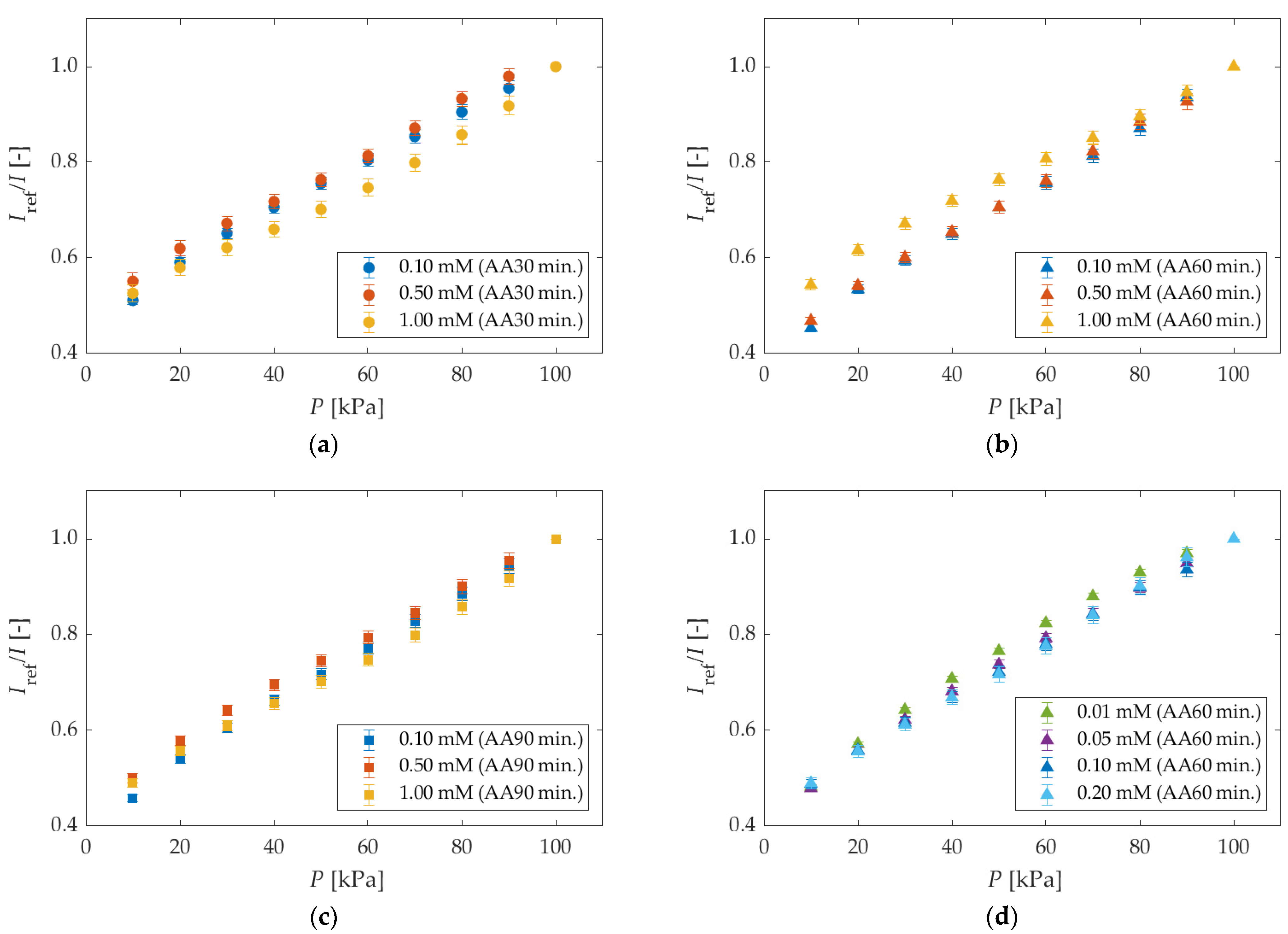



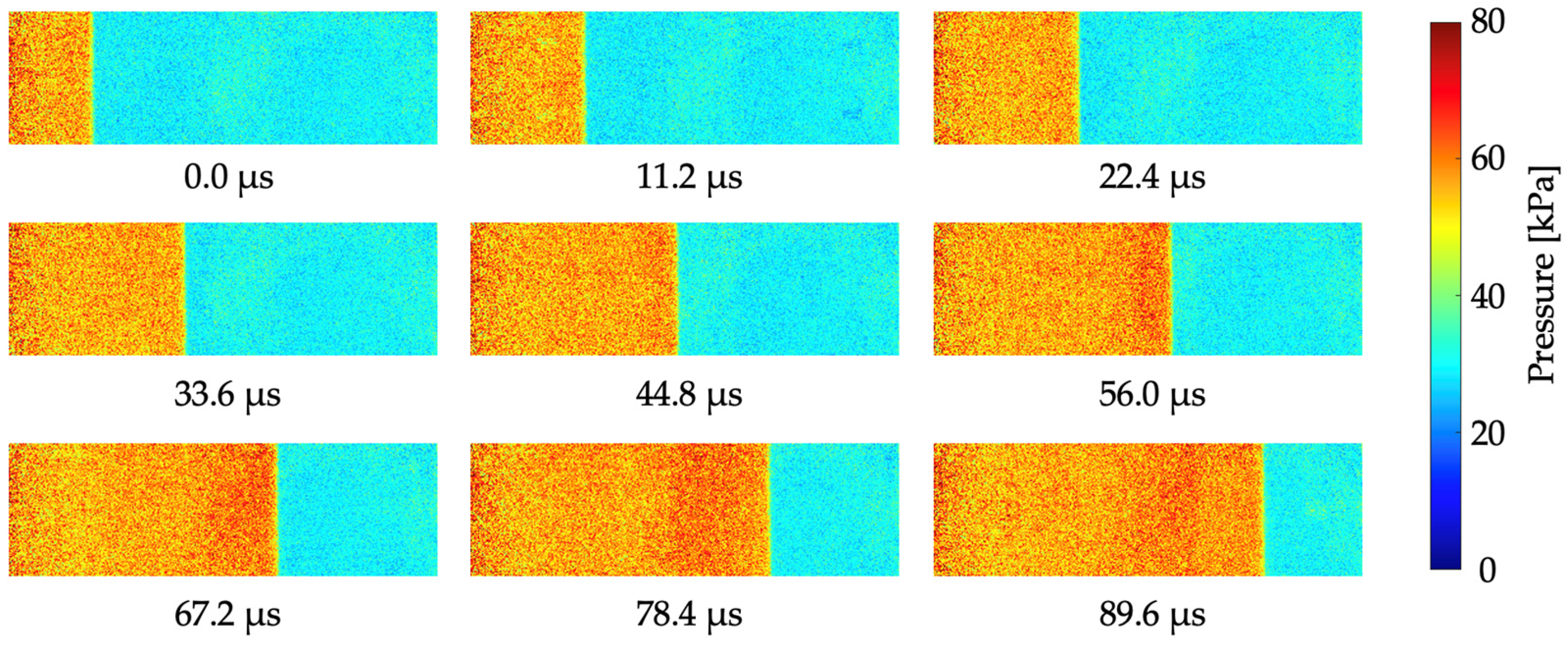
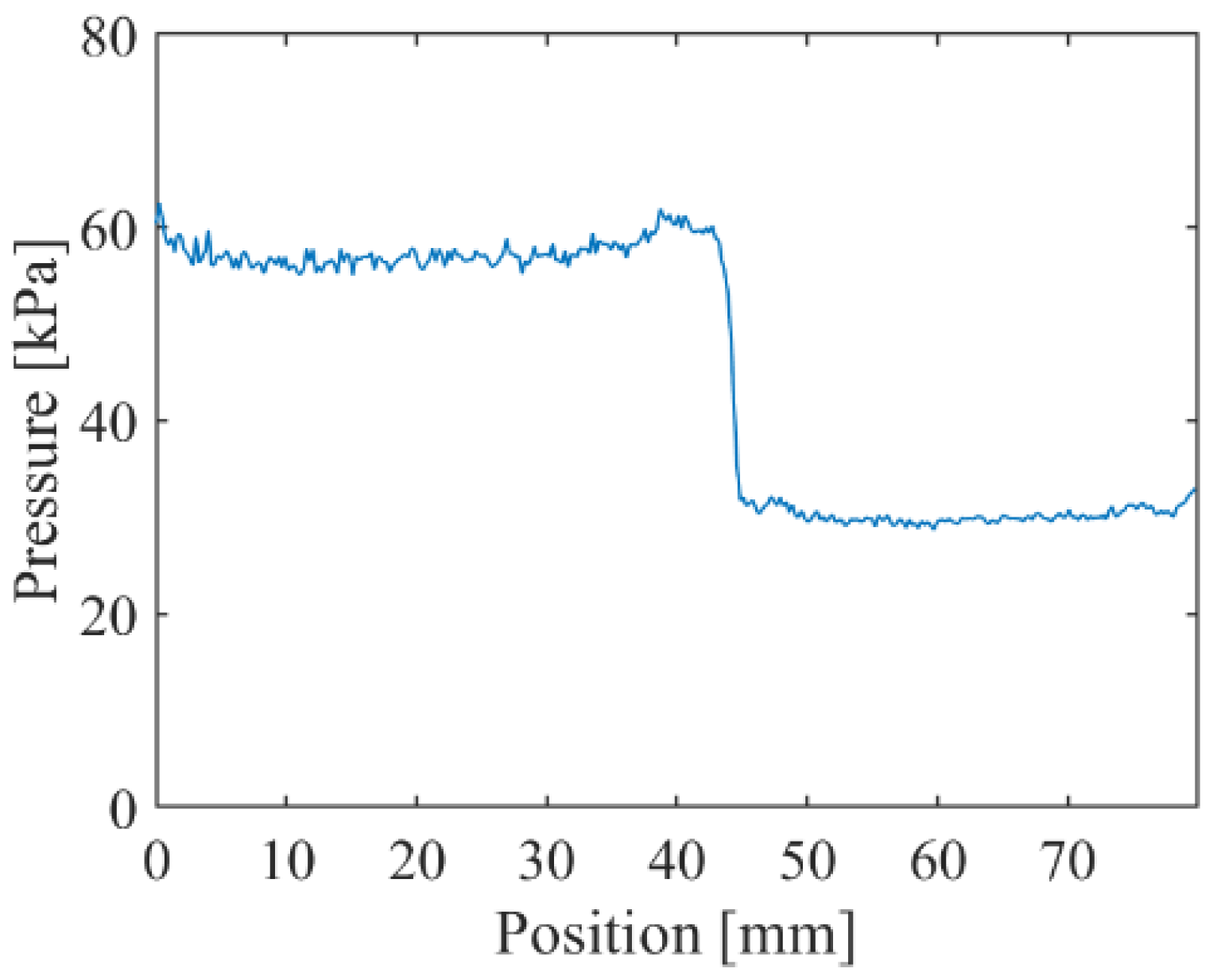
| Anodization Time | Dipping Solvent | Dipping Concentration |
|---|---|---|
| 30 min | Ethanol + Dichloromethane | 0.10–1.00 mM 1 |
| Ethanol | 0.10 mM | |
| 60 min | Dichloromethane | 0.10 mM |
| Ethanol + Dichloromethane | 0.01–1.00 mM | |
| 90 min | Ethanol + Dichloromethane | 0.10–1.0 mM |
| Pretreatment | Solution | NaOH aq (0.3 wt%) |
| Dipping time | 10 min | |
| Anodization | Electrolyte | Phosphoric acid (0.3 mol/L) |
| Current density | 12.5 mA/cm2 | |
| Temperature | 30 °C | |
| Post-treatment | Solution | Phosphoric acid (0.3 mol/L) |
| Temperature | 30 °C | |
| Dipping time | 10 min |
| Test Piece 1 | Test Piece 2 | Test Piece 3 | |
|---|---|---|---|
| AA30 min | AA60 min | AA90 min | |
| texp = 1.5 μs | 1.0 μs | 2.5 μs | 3.0 μs |
| texp = 3.0 μs | 0.9 μs | 2.8 μs | 3.2 μs |
| texp = 10.0 μs | 1.1 μs | 1.4 μs | 2.5 μs |
| Average | 1.0 μs | 2.2 μs | 2.9 μs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yomo, K.; Ikami, T.; Fujita, K.; Nagai, H. Investigation of Formulations on Pyrene-Based Anodized-Aluminum Pressure-Sensitive Paints for Supersonic Phenomena. Sensors 2022, 22, 4430. https://doi.org/10.3390/s22124430
Yomo K, Ikami T, Fujita K, Nagai H. Investigation of Formulations on Pyrene-Based Anodized-Aluminum Pressure-Sensitive Paints for Supersonic Phenomena. Sensors. 2022; 22(12):4430. https://doi.org/10.3390/s22124430
Chicago/Turabian StyleYomo, Kazuma, Tsubasa Ikami, Koji Fujita, and Hiroki Nagai. 2022. "Investigation of Formulations on Pyrene-Based Anodized-Aluminum Pressure-Sensitive Paints for Supersonic Phenomena" Sensors 22, no. 12: 4430. https://doi.org/10.3390/s22124430
APA StyleYomo, K., Ikami, T., Fujita, K., & Nagai, H. (2022). Investigation of Formulations on Pyrene-Based Anodized-Aluminum Pressure-Sensitive Paints for Supersonic Phenomena. Sensors, 22(12), 4430. https://doi.org/10.3390/s22124430






