Aerogel-Lined Capillaries for Raman Signal Gain of Aqueous Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Generation of the Gel–Particle Dispersion
2.3. Fabrication of Aerogel-Lined Capillaries
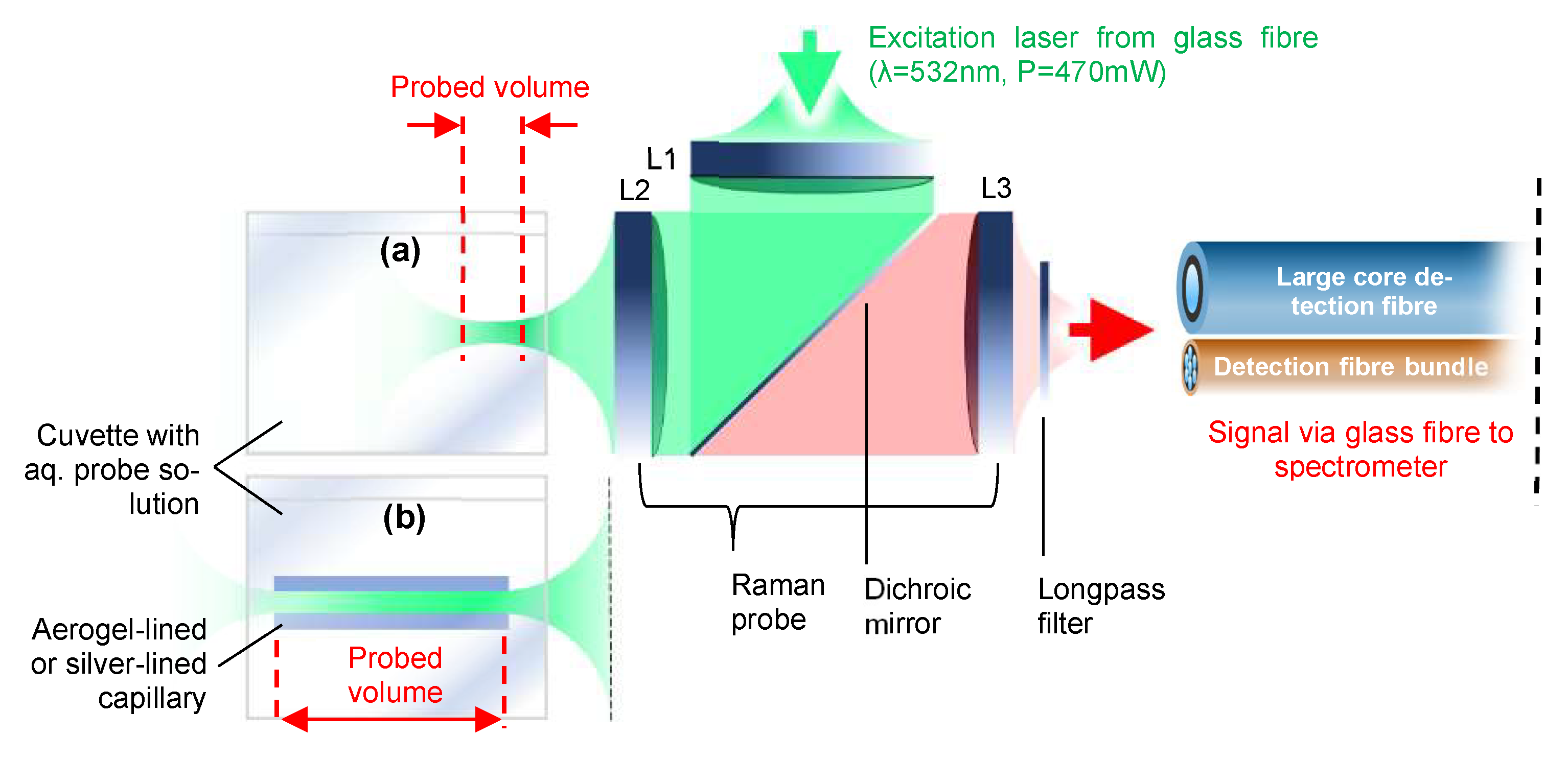
2.4. Experimental Setup for Raman Measurement of Aqueous Mixtures
3. Results
3.1. Fabrication of Aerogel-Lined Capillaries
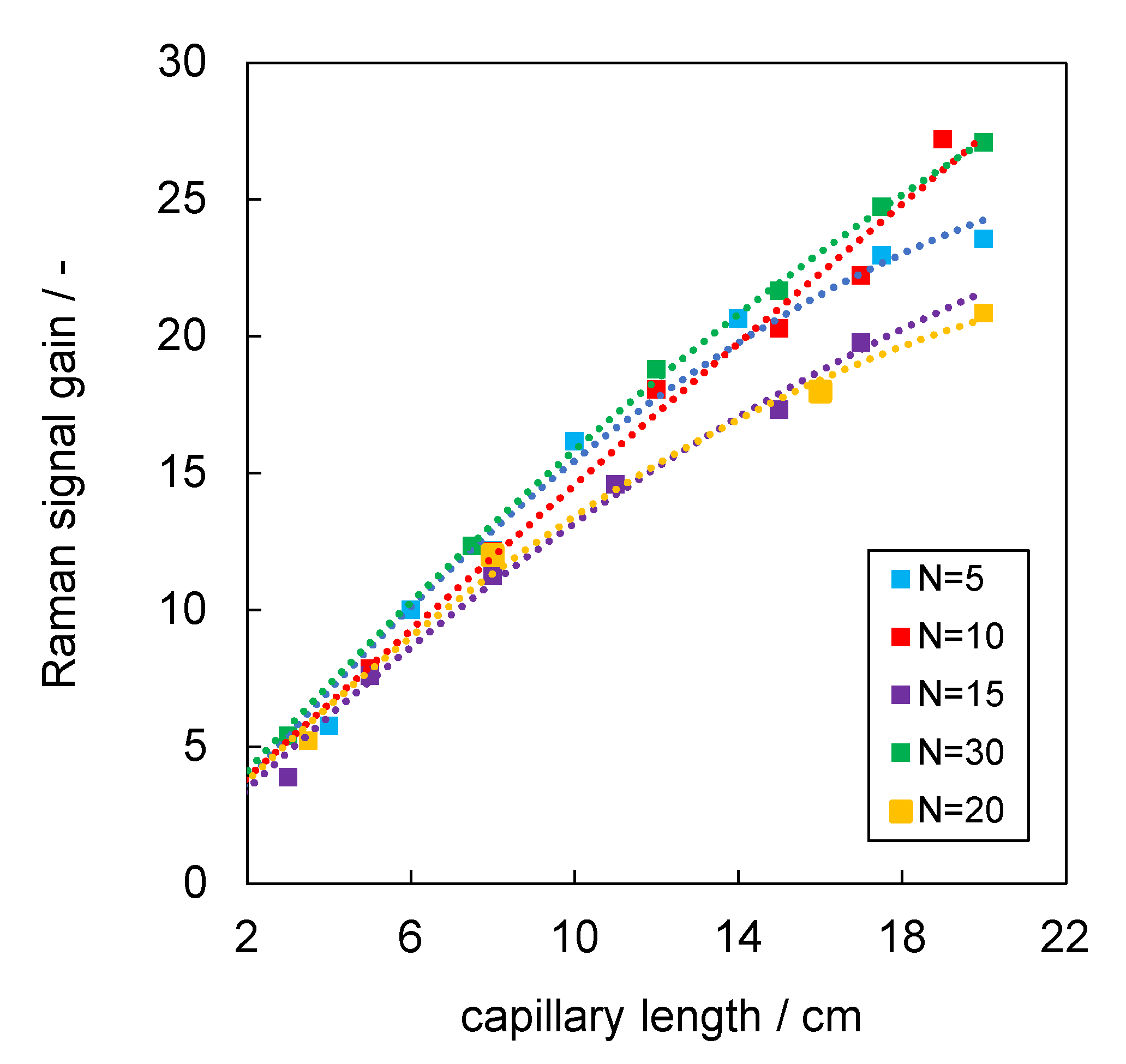
3.2. Analysis of Raman Signal Gain of Aerogel-Lined and Silver-Lined Capillaries in Liquid Water
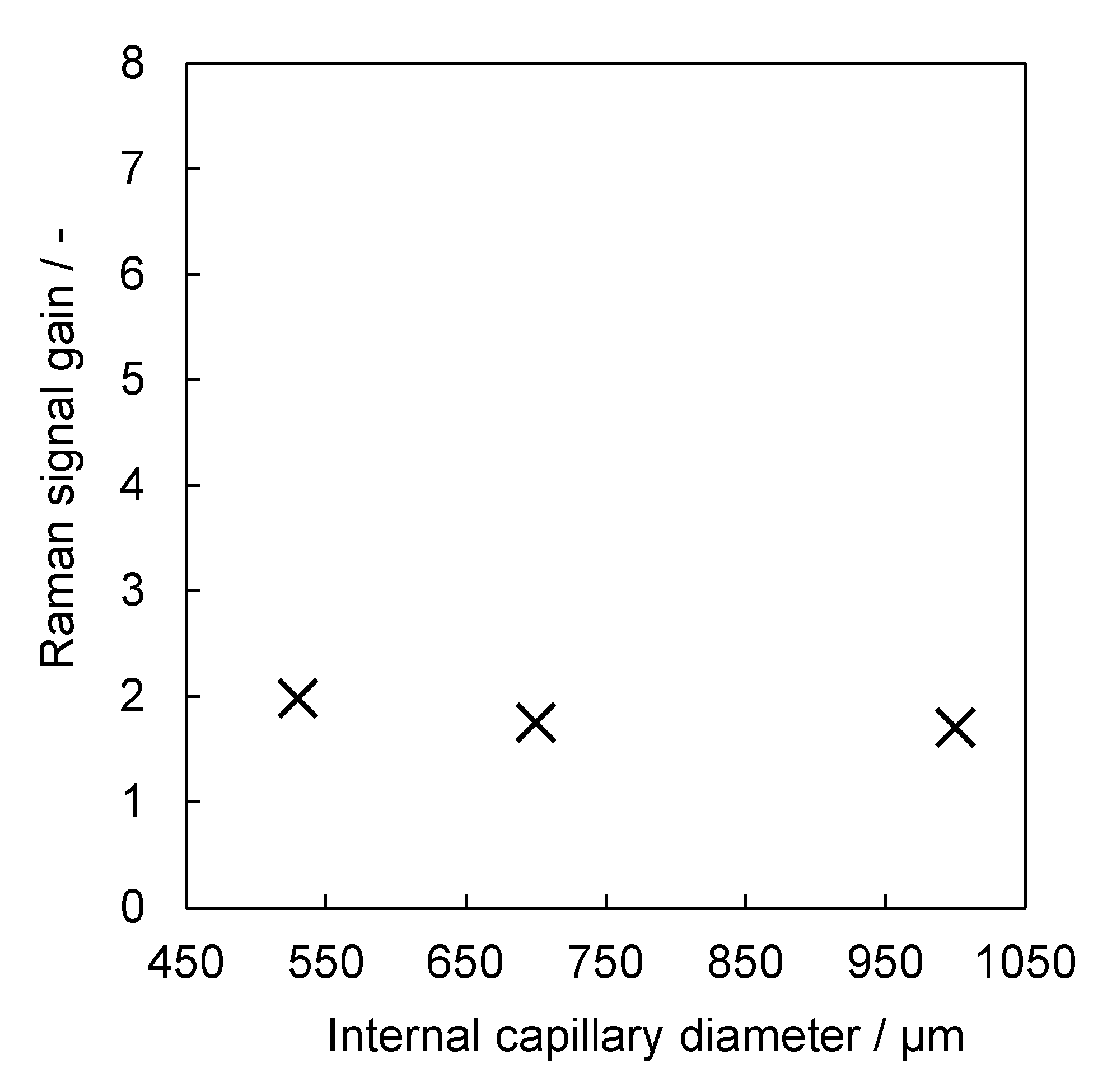
3.3. Correlation of Raman Signal Gain and Internal Diameter of Aerogel-Lined Capillaries
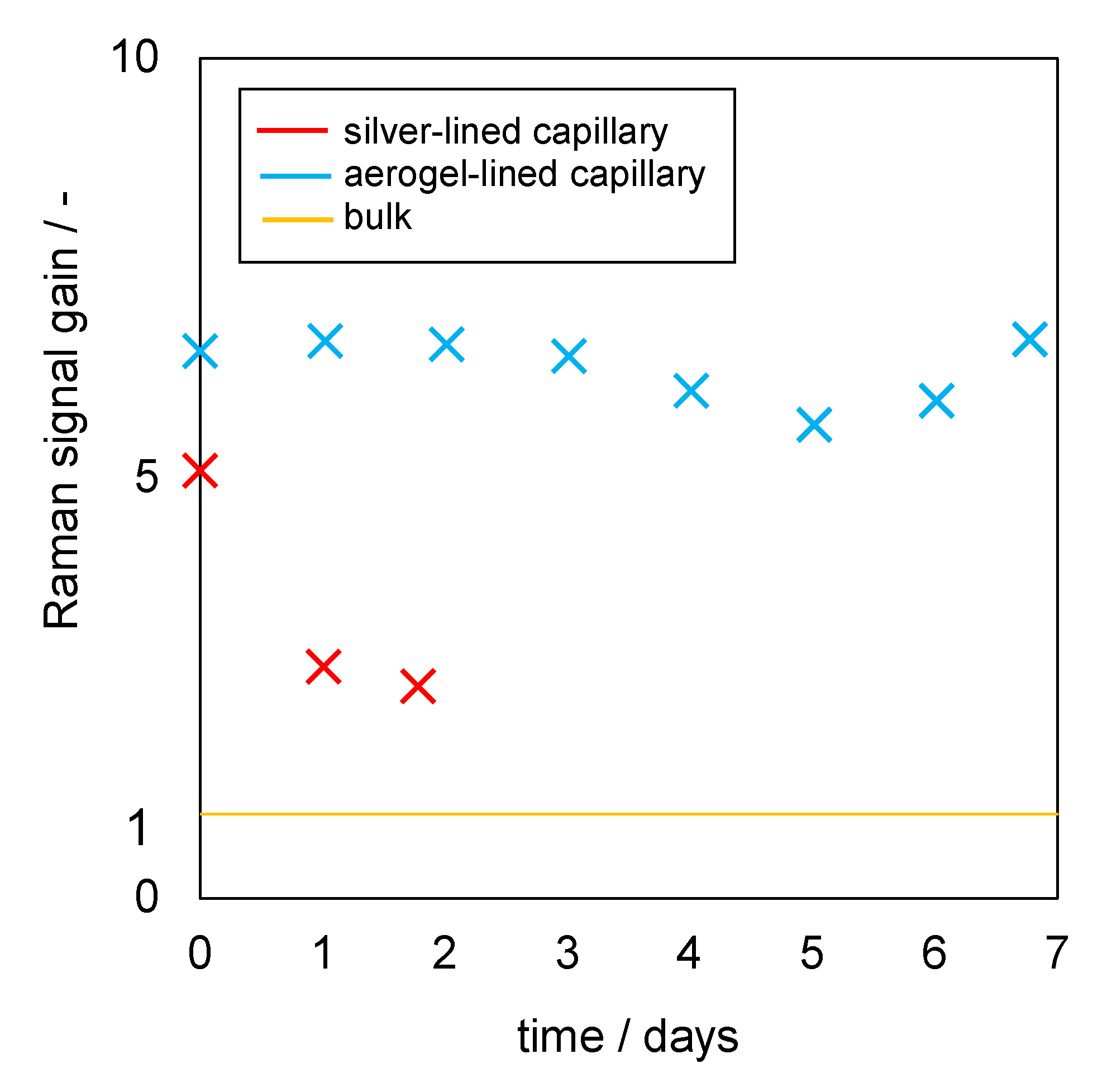
3.4. Raman Signal Gain Stability of Aerogel-Lined and Silver-Lined Capillaries in an Aqueous Mixture of Glucose, Ethanol and Acetic Acid
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Beer, T.; Burggraeve, A.; Fonteyne, M.; Saerens, L.; Remon, J.P.; Vervaet, C. Near Infrared and Raman Spectroscopy for the In-Process Monitoring of Pharmaceutical Production Processes. Int. J. Pharm. 2011, 417, 32–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buric, M.P.; Chen, K.P.; Falk, J.; Woodruff, S.D. Improved Sensitivity Gas Detection by Spontaneous Raman Scattering. Appl. Opt. 2009, 48, 4424–4429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saerens, L.; Dierickx, L.; Lenain, B.; Vervaet, C.; Remon, J.P.; Beer, T. De Raman Spectroscopy for the In-Line Polymer-Drug Quantification and Solid State Characterization during a Pharmaceutical Hot-Melt Extrusion Process. Eur. J. Pharm. Biopharm. 2011, 77, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Braeuer, A. In Situ Spectroscopic Techniques at High Pressure; Elsevier: Amsterdam, The Netherlands, 2015; Volume 7, ISBN 9780444634221. [Google Scholar]
- Schwab, S.D.; McCreery, R.L. Remote, Long-Pathlength Cell for High-Sensitivity Raman Spectroscopy. Appl. Spectrosc. 1987, 41, 126–130. [Google Scholar] [CrossRef]
- Trägårdh, J.; Pikálek, T.; Šerý, M.; Akimov, D.; Meyer, T.; Popp, J.; Cižmár, T. Coherent Anti-Stokes Raman Scattering Microscopy through a Multimode Fiber Endoscope. In Biomedical Spectroscopy, Microscopy, and Imaging; International Society for Optics and Photonics: Bellingham, WA, USA, 2020. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shende, C.; Farquharson, S.; Farquharson, D.; Brouillette, C. Measurements of Bicarbonate in Water Containing Ocean-Level Sulfate Using a Simple Multi-Pass Optical Raman System. Oceans 2021, 2, 330–336. [Google Scholar] [CrossRef]
- Ding, H.; Hu, D.J.J.; Yu, X.; Liu, X.; Zhu, Y.; Wang, G. Review on All-Fiber Online Raman Sensor with Hollow Core Microstructured Optical Fiber. Photonics 2022, 9, 134. [Google Scholar] [CrossRef]
- Trutna, W.R.; Byer, R.L. Multiple-Pass Raman Gain Cell. Appl. Opt. 1980, 19, 301. [Google Scholar] [CrossRef]
- Adler-Golden, S.M.; Goldstein, N.; Bien, F.; Matthew, M.W.; Gersh, M.E.; Cheng, W.K.; Adams, F.W. Laser Raman Sensor for Measurement of Trace-Hydrogen Gas. Appl. Opt. 1992, 31, 831. [Google Scholar] [CrossRef]
- White, J.U. Long Optical Paths of Large Aperture. J. Opt. Soc. Am. 1942, 32, 285–288. [Google Scholar] [CrossRef]
- Owyoung, A. Sensitivity Limitations for CW Stimulated Raman Spectroscopy. Opt. Commun. 1977, 22, 323–328. [Google Scholar] [CrossRef]
- Herriott, D.; Kogelnik, H.; Kompfner, R. Off-Axis Paths in Spherical Mirror Interferometers. Appl. Opt. 1964, 3, 523–526. [Google Scholar] [CrossRef] [Green Version]
- Pearman, W.F.; Carter, J.C.; Angel, S.M.; Chan, J.W.-J. Quantitative Measurements of CO2 and CH4 Using a Multipass Raman Capillary Cell. Appl. Opt. 2008, 47, 4627–4632. [Google Scholar] [CrossRef]
- Guo, J.; Luo, Z.; Liu, Q.; Yang, D.; Dong, H.; Huang, S.; Kong, A.; Wu, L. High-Sensitivity Raman Gas Probe for in Situ Multi-Component Gas Detection. Sensors 2021, 21, 3539. [Google Scholar] [CrossRef]
- Yang, D.; Guo, J.; Liu, Q.; Luo, Z.; Yan, J.; Zheng, R. Highly Sensitive Raman System for Dissolved Gas Analysis in Water. Appl. Opt. 2016, 55, 7744–7747. [Google Scholar] [CrossRef]
- Cubillas, A.M.; Unterkofler, S.; Euser, T.G.; Etzold, B.J.M.; Jones, A.C.; Sadler, P.J.; Wasserscheid, P.; Russell, P.S.J. Photonic Crystal Fibres for Chemical Sensing and Photochemistry. Chem. Soc. Rev. 2013, 42, 8629–8648. [Google Scholar] [CrossRef] [Green Version]
- Konorov, S.O.; Addison, C.J.; Schulze, H.G.; Turner, R.F.B.; Blades, M.W. Hollow-Core Photonic Crystal Fiber-Optic Probes for Raman Spectroscopy. Opt. Lett. 2006, 31, 1911. [Google Scholar] [CrossRef]
- James, T.M.; Rupp, S.; Telle, H.H. Trace Gas and Dynamic Process Monitoring by Raman Spectroscopy in Metal-Coated Hollow Glass Fibres. Anal. Methods 2015, 7, 2568–2576. [Google Scholar] [CrossRef]
- Persichetti, G.; Testa, G.; Bernini, R. Optofluidic Jet Waveguide Enhanced Raman Spectroscopy. Sens. Actuators B Chem. 2015, 207, 732–739. [Google Scholar] [CrossRef]
- Fraval, H.R.; Brinker, C.J. Fluid Light Guide Having Hydrophobic Aerogel Cladding Layer. U.S. Patent 6,983,093, 3 January 2006. [Google Scholar]
- Özbakır, Y.; Jonáš, A.; Kiraz, A.; Erkey, C. Total Internal Reflection-Based Optofluidic Waveguides Fabricated in Aerogels. J. Sol-Gel Sci. Technol. 2017, 84, 522–534. [Google Scholar] [CrossRef]
- Özbakir, Y.; Jonas, A.; Kiraz, A.; Erkey, C. Aerogels for Optofluidic Waveguides. Micromachines 2017, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Özbakır, Y.; Jonáš, A.; Kiraz, A.; Erkey, C. A New Type of Microphotoreactor with Integrated Optofluidic Waveguide Based on Solid-Air Nanoporous Aerogels. R. Soc. Open Sci. 2018, 5, 180802. [Google Scholar] [CrossRef] [Green Version]
- Yalizay, B.; Morova, Y.; Dincer, K.; Özbakir, Y.; Jonas, A.; Erkey, C.; Kiraz, A.; Akturk, S. Versatile Liquid-Core Optofluidic Waveguides Fabricated in Hydrophobic Silica Aerogels by Femtosecond-Laser Ablation. Opt. Mater. 2015, 47, 478–483. [Google Scholar] [CrossRef]
- Sprehn, G.A.; Hrubesh, L.W.; Poco, J.F.; Sandler, P.H. Aerogel-Clad Optical Fiber. U.S. Patent 5,684,907, 4 November 1997. [Google Scholar]
- Xiao, L.; Birks, T.A. Optofluidic Microchannels in Aerogel. Opt. Lett. 2011, 36, 3275–3277. [Google Scholar] [CrossRef] [Green Version]
- Hale, G.M.; Querry, M.R. Optical Constants of Water in the 200-Nm to 200-Μm Wavelength Region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef]
- Riffat, S.B.; Qiu, G. A Review of State-of-the-Art Aerogel Applications in Buildings. Int. J. Low-Carbon Technol. 2013, 8, 1–6. [Google Scholar] [CrossRef]
- Tabata, M.; Adachi, I.; Ishii, Y.; Kawai, H.; Sumiyoshi, T.; Yokogawa, H. Development of Transparent Silica Aerogel over a Wide Range of Densities. Nucl. Instrum. Methods Phys. Res. Sect. A 2010, 623, 339–341. [Google Scholar] [CrossRef] [Green Version]
- Adachi, I.; Sumiyoshi, T.; Hayashi, K.; Iida, N.; Enomoto, R.; Tsukada, K.; Suda, R.; Matsumoto, S.; Natori, K.; Yokoyama, M.; et al. Study of a Threshold Cherenkov Counter Based on Silica Aerogels with Low Refractive Indices. Nucl. Instrum. Methods Phys. Res. Sect. A 1995, 355, 390–398. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiske, F.; Dirauf, M.P.; Braeuer, A.S. Aerogel-Lined Capillaries for Raman Signal Gain of Aqueous Mixtures. Sensors 2022, 22, 4388. https://doi.org/10.3390/s22124388
Spiske F, Dirauf MP, Braeuer AS. Aerogel-Lined Capillaries for Raman Signal Gain of Aqueous Mixtures. Sensors. 2022; 22(12):4388. https://doi.org/10.3390/s22124388
Chicago/Turabian StyleSpiske, Felix, Martin Peter Dirauf, and Andreas Siegfried Braeuer. 2022. "Aerogel-Lined Capillaries for Raman Signal Gain of Aqueous Mixtures" Sensors 22, no. 12: 4388. https://doi.org/10.3390/s22124388
APA StyleSpiske, F., Dirauf, M. P., & Braeuer, A. S. (2022). Aerogel-Lined Capillaries for Raman Signal Gain of Aqueous Mixtures. Sensors, 22(12), 4388. https://doi.org/10.3390/s22124388





