Monitoring Gases Content in Modern Agriculture: A Density Functional Theory Study of the Adsorption Behavior and Sensing Properties of CO2 on MoS2 Doped GeSe Monolayer
Abstract
:1. Introduction
2. Methods
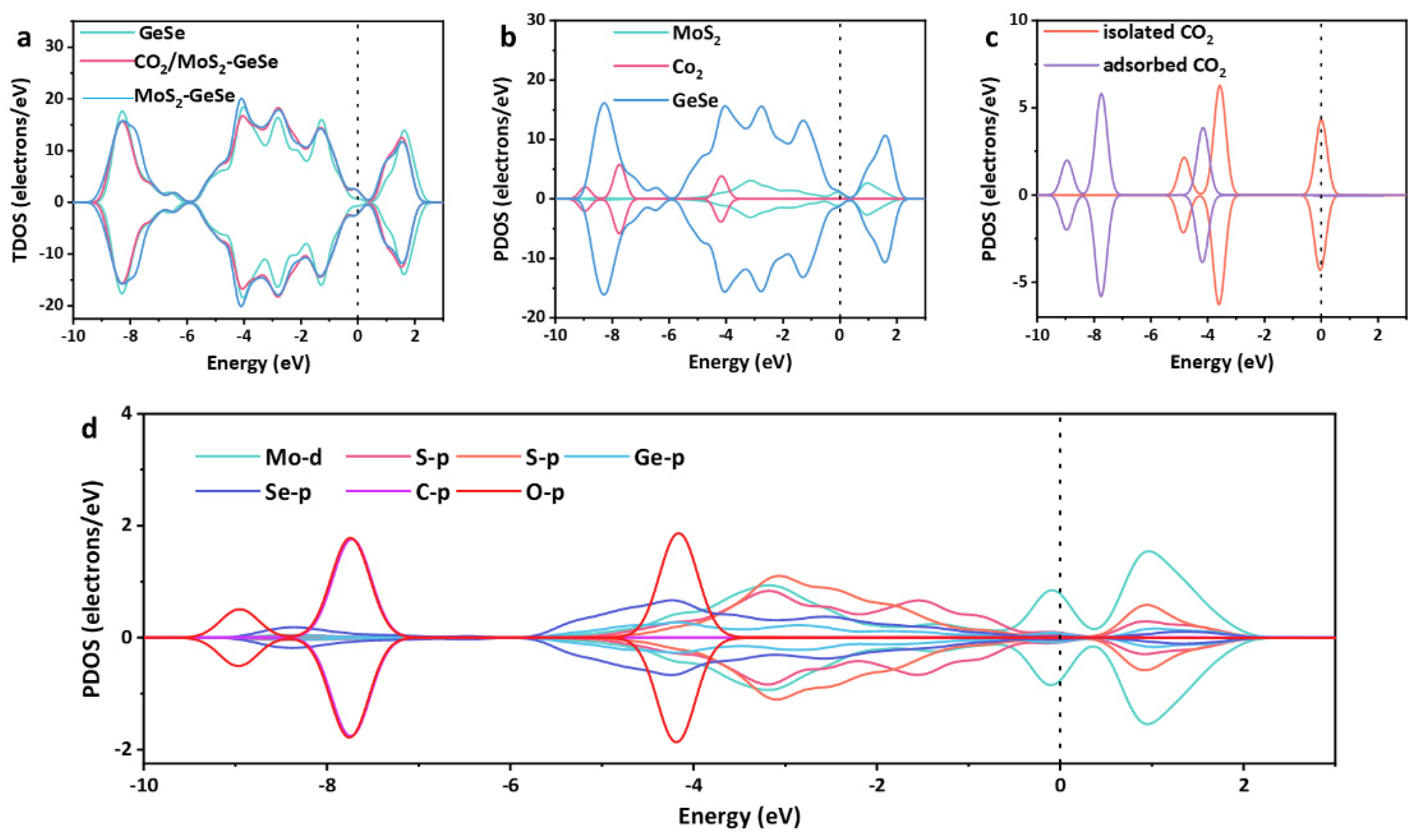
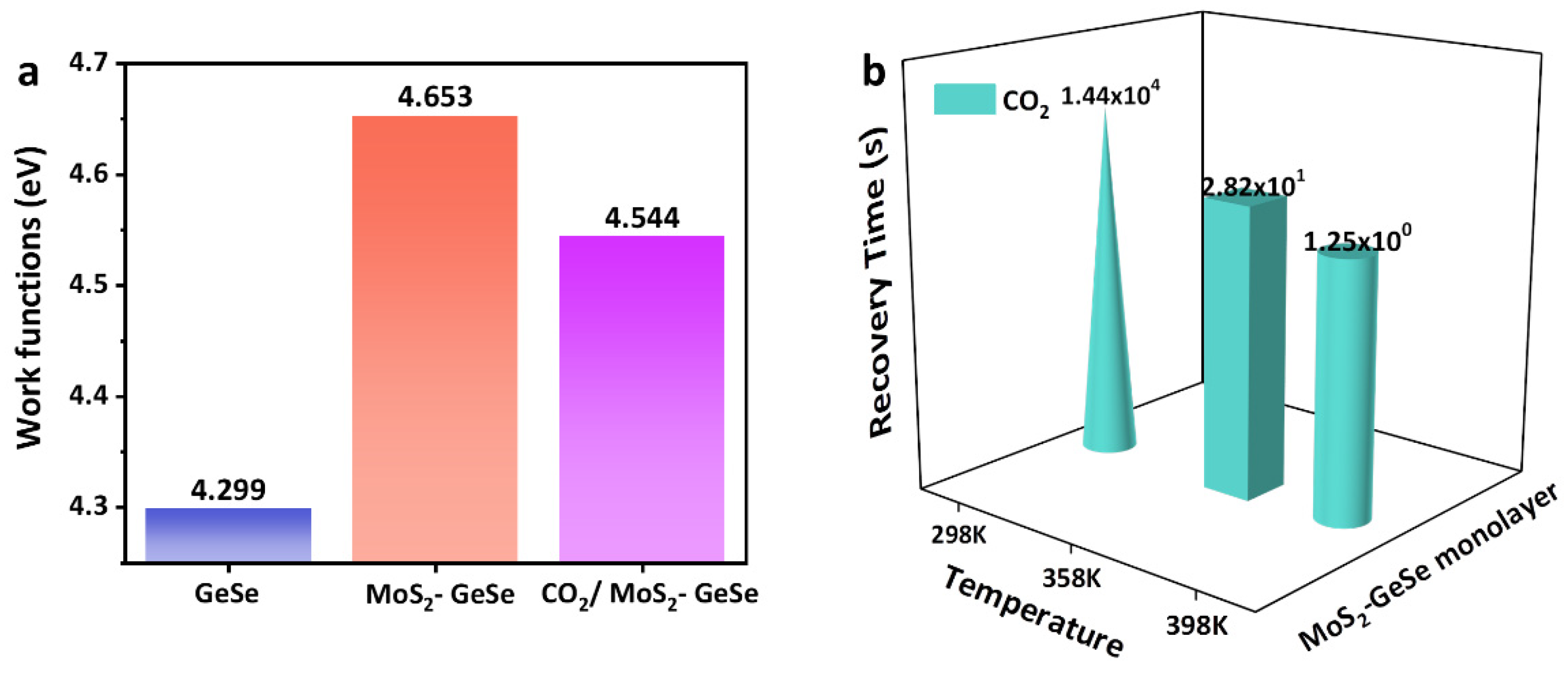
3. Results
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Alvarado, K.A.; Mill, A.; Pearce, J.M.; Vocaet, A.; Denkenberger, D. Scaling of greenhouse crop production in low sunlight scenarios. Sci. Total Environ. 2020, 707, 136012. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.; Berenguel, M.; Guzmán, J.L.; Ramírez-Arias, A. Modeling and Control of Greenhouse Crop Growth; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Timmermans, G.H.; Hemming, S.; Baeza, E.; Van Thoor, E.A.; Schenning, A.P.; Debije, M.G. Advanced optical materials for sunlight control in greenhouses. Adv. Opt. Mater. 2020, 8, 2000738. [Google Scholar] [CrossRef]
- Körner, C. Plant CO2 responses: An issue of definition, time and resource supply. New Phytol. 2006, 172, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, S.G.; Rogers, H.H.; Prior, S.A.; Peterson, C.M. Elevated CO2 and plant structure: A review. Glob. Change Biol. 1999, 5, 807–837. [Google Scholar] [CrossRef] [Green Version]
- Gerhart, L.M.; Ward, J.K. Plant responses to low [CO2] of the past. New Phytol. 2010, 188, 674–695. [Google Scholar] [CrossRef]
- Leakey, A.D.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Morison, J.; Lawlor, D. Interactions between increasing CO2 concentration and temperature on plant growth. Plant Cell Environ. 1999, 22, 659–682. [Google Scholar] [CrossRef] [Green Version]
- Bazzaz, F.; McConnaughay, K. Plant plant interactions in elevated CO2 environments. Aust. J. Bot. 1992, 40, 547–563. [Google Scholar] [CrossRef]
- Makino, A.; Mae, T. Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol. 1999, 40, 999–1006. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Li, Y.; Chen, D.; Zhang, Y. First-principles insight into Ni-doped InN monolayer as a noxious gases scavenger. Appl. Surf. Sci. 2019, 494, 1267. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhang, G.; Tang, J. Pd-doped MoS_2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Cui, H.; Zheng, K.; Xie, Z.; Yu, J.; Chen, X. Tellurene Nanoflake-Based NO2 Sensors with Superior Sensitivity and a Sub-Parts-per-Billion Detection Limit. ACS Appl. Mater. Interfaces 2020, 12, 47704–47713. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Guozhi, Z.; Xiaoxing, Z.; Ju, T. Rh-doped MoSe2 as toxic gas scavenger: A first-principles study. Nanoscale Adv. 2018, 1, 772–780. [Google Scholar]
- Heping, C.; Kai, Z.; Yingying, Z.; Huaiyu, Y.; Xianping, C. Superior Selectivity and Sensitivity of C3N Sensor in Probing Toxic Gases NO2 and SO2. IEEE Electron Device Lett. 2017, 39, 284–287. [Google Scholar]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 1500101. [Google Scholar] [CrossRef]
- Gui, Y.; Sun, H.; Wei, H.; Duan, S.; Tang, C.; Zhang, X. Effect of nickel doping on adsorption of SF6 decomposition products over MoS2 surface. JOM 2019, 71, 3971–3979. [Google Scholar] [CrossRef]
- Jiang, T.; He, Q.; Bi, M.; Chen, X.; Sun, H.; Tao, L. First-principles calculations of adsorption sensitivity of Au-doped MoS2 gas sensor to main characteristic gases in oil. J. Mater. Sci. 2021, 56, 13673–13683. [Google Scholar] [CrossRef]
- Peng, Z.; Tao, L.-Q.; Zheng, K.; Yu, J.; Wang, G.; Sun, H.; Zhu, C.; Zou, S.; Chen, X. Gas Sensor Based on Semihydrogenated and Semifluorinated h-BN for SF₆ Decomposition Components Detection. IEEE Trans. Electron Devices 2021, 68, 1878–1885. [Google Scholar] [CrossRef]
- Sun, H.; Gui, Y.; Wei, H.; Long, Y.; Wang, Q.; Tang, C. DFT study of SF6 decomposed products on Pd–TiO2: Gas sensing mechanism study. Adsorption 2019, 25, 1643–1653. [Google Scholar] [CrossRef]
- Xia, S.-Y.; Tao, L.-Q.; Jiang, T.; Sun, H.; Li, J. Rh-doped h-BN monolayer as a high sensitivity SF6 decomposed gases sensor: A DFT study. Appl. Surf. Sci. 2021, 536, 147965. [Google Scholar] [CrossRef]
- Liu, Z.; Gui, Y.; Xu, L.; Chen, X. Adsorption and sensing performances of transition metal (Ag, Pd, Pt, Rh, and Ru) modified WSe2 monolayer upon SF6 decomposition gases (SOF2 and SO2F2). Appl. Surf. Sci. 2022, 581, 152365. [Google Scholar] [CrossRef]
- Liu, Z.; Gui, Y.; Xu, L.; Chen, X. Adsorption and gas-sensing properties of Aun (n = 1–3) cluster doped MoTe2 for NH3, NO2, and SO2 gas molecules. Surf. Interfaces 2022, 30, 101883. [Google Scholar] [CrossRef]
- Wang, X.; Gui, Y.; Ding, Z.; Xu, H.; Zeng, H.; Chen, X. Density functional theory study of Pd, Pt, and Au modified GeSe for adsorption and sensing of dissolved gases in transformer oil. Surf. Interfaces 2022, 31, 101994. [Google Scholar] [CrossRef]
- Wang, X.; Gui, Y.; Sun, N.; Ding, Z.; Chen, X. A DFT calculation: Gas sensitivity of defect GeSe to air decomposition products (CO, NO and NO2). IEEE Sens. J. 2022, 19, 905. [Google Scholar]
- Hu, Y.; Zhang, S.; Sun, S.; Xie, M.; Cai, B.; Zeng, H. GeSe monolayer semiconductor with tunable direct band gap and small carrier effective mass. Appl. Phys. Lett. 2015, 107, 122107. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Wang, X.; Yang, Y.; Liu, S.-C.; Shi, W.; Li, Y.; Xing, X.; Xue, D.-J.; Hu, J.-S. Strain-engineering the in-plane electrical anisotropy of GeSe monolayers. Phys. Chem. Chem. Phys. 2020, 22, 914–918. [Google Scholar] [CrossRef]
- Zhao, H.; Mao, Y.; Mao, X.; Shi, X.; Xu, C.; Wang, C.; Zhang, S.; Zhou, D. Band structure and photoelectric characterization of GeSe monolayers. Adv. Funct. Mater. 2018, 28, 1704855. [Google Scholar] [CrossRef]
- Lv, X.; Wei, W.; Mu, C.; Huang, B.; Dai, Y. Two-dimensional GeSe for high performance thin-film solar cells. J. Mater. Chem. A 2018, 6, 5032–5039. [Google Scholar] [CrossRef]
- Mao, Y.; Long, L.; Yuan, J.; Zhong, J.; Zhao, H. Toxic gases molecules (NH3, SO2 and NO2) adsorption on GeSe monolayer with point defects engineering. Chem. Phys. Lett. 2018, 706, 501–508. [Google Scholar] [CrossRef]
- Sun, H.; Tao, L.-Q.; Li, T.; Gao, X.; Sang, T.; Li, Y.; Wei, Y.; Wang, G.; Peng, Z.; Gui, Y. TiO2–Doped GeSe Monolayer: A highly selective gas sensor for SF6 decomposed species detection based on DFT method. Appl. Surf. Sci. 2022, 572, 151212. [Google Scholar] [CrossRef]
- Sun, H.; Tao, L.-Q.; Zhang, F.; Li, T.; He, R.; Gao, X.; Sang, T.; Wang, G.; Peng, Z.; Zhu, C. Metal Oxide Nanoparticles (XO, X= Cu, Zn, Ni) Doped GeSe Monolayer: Theoretical Exploration of a Novel H2S Gas Sensor for Health and Industrial Monitoring. IEEE Sens. J. 2021, 21, 26542–26548. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Peng, Y.; Gui, Y.; Sun, H. Pd and Pt decorated GeSe monolayers as promising materials for SOF2 and SO2F2 sensing. Appl. Surf. Sci. 2021, 560, 150028. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, J.I.; Lee, S.G.; Jang, S.S. A density functional theory (DFT) study of CO2 adsorption on Mg-rich minerals by enhanced charge distribution. Comput. Mater. Sci. 2014, 95, 181–186. [Google Scholar] [CrossRef]
- Wu, C.; Yang, W.; Wang, J.; Kannaiyan, R.; Gates, I.D. CO2 adsorption and dissociation on single and double iron atomic molybdenum disulfide catalysts: A DFT study. Fuel 2021, 305, 121547. [Google Scholar] [CrossRef]
- Yan, X.; Li, Y.; Zhao, J.; Wang, Z. Density Functional Theory Study on CO2 Adsorption by Ce-Promoted CaO in the Presence of Steam. Energy Fuels 2020, 34, 6197–6208. [Google Scholar] [CrossRef]
- Ye, J.; Liu, C.; Ge, Q. DFT Study of CO2 Adsorption and Hydrogenation on the In2O3 Surface. J. Phys. Chem. C 2011, 116, 7817–7825. [Google Scholar] [CrossRef]
- Wang, C.; Fang, Y.; Duan, H.; Liang, G.; Long, M. DFT study of CO2 adsorption properties on pristine, vacancy and doped graphenes. Solid State Commun. 2021, 5948, 114436. [Google Scholar] [CrossRef]
- Wang, G.; Zheng, K.; Huang, Y.; Yu, J.; Tao, L.Q. Investigation of the positive effect of doping Al atom to the adsorption of CO2 on BN nanosheets: A DFT study. Phys. Chem. Chem. Phys. 2020, 22, 17. [Google Scholar] [CrossRef]
- He, X.; Gui, Y.; Liu, K.; Xu, L. Comparison of sensing and electronic properties of C2H2 on different transition metal oxide nanoparticles (Fe2O3, NiO, TiO2) modified BNNT (10, 0). Appl. Surf. Sci. 2020, 521, 146463. [Google Scholar] [CrossRef]
- He, X.; Gui, Y.; Xie, J.; Liu, X.; Wang, Q.; Tang, C. A DFT study of dissolved gas (C2H2, H2, CH4) detection in oil on CuO-modified BNNT. Appl. Surf. Sci. 2020, 500, 144030. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Für Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Delley, B. Time dependent density functional theory with DMol3. J. Phys. Condens. Matter 2010, 22, 384208. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA—Type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.; Grimme, S. Density functional theory including dispersion corrections for intermolecular interactions in a large benchmark set of biologically relevant molecules. Phys. Chem. Chem. Phys. 2006, 8, 5287–5293. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Density functional theory with London dispersion corrections. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 211–228. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Moellmann, J.; Ehrlich, S.; Tonner, R.; Grimme, S. A DFT-D study of structural and energetic properties of TiO2 modifications. J. Phys. Condens. Matter 2012, 24, 424206. [Google Scholar] [CrossRef]
- Xia, Y.; Pan, A.; Su, Y.-Q.; Zhao, S.; Li, Z.; Davey, A.K.; Zhao, L.; Maboudian, R.; Carraro, C. In-situ synthesized N-doped ZnO for enhanced CO2 sensing: Experiments and DFT calculations. Sens. Actuators B Chem. 2022, 357, 131359. [Google Scholar] [CrossRef]
- Baltanás, M.A.; Reimers, W.G.; Branda, M.M. CO, CO2 and H2 adsorption on ZnO, CeO2, and ZnO/CeO2 surfaces: DFT simulations. J. Mol. Modeling 2014, 20, 2270-1–2270-10. [Google Scholar]
- Wang, D.; Chen, Y.; Liu, Z.; Ling, L.; Shi, C.; Qin, H.; Hu, J. CO2-sensing properties and mechanism of nano-SnO2 thick-film sensor. Sens. Actuators B Chem. 2016, 227, 73–84. [Google Scholar] [CrossRef]
- Chen, H.-Y.T.; Tosoni, S.; Pacchioni, G. A DFT study of the acid–base properties of anatase TiO2 and tetragonal ZrO2 by adsorption of CO and CO2 probe molecules. Surf. Sci. 2016, 652, 163–171. [Google Scholar] [CrossRef]
- Li, J.-H.; Wu, J.; Yu, Y.-X. DFT exploration of sensor performances of two-dimensional WO3 to ten small gases in terms of work function and band gap changes and I-V responses. Appl. Surf. Sci. 2021, 546, 149104. [Google Scholar] [CrossRef]
- Bundgaard, E.; Krebs, F.C. Low band gap polymers for organic photovoltaics. Sol. Energy Mater. Sol. Cells 2007, 91, 954–985. [Google Scholar] [CrossRef] [Green Version]
- Soukoulis, C.M. Photonic Band Gap Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 315. [Google Scholar]
- Yablonovitch, E. Photonic band-gap structures. JOSA B 1993, 10, 283–295. [Google Scholar] [CrossRef]
- Lang, N.; Kohn, W. Theory of metal surfaces: Work function. Phys. Rev. B 1971, 3, 1215. [Google Scholar] [CrossRef]
- Michaelson, H.B. The work function of the elements and its periodicity. J. Appl. Phys. 1977, 48, 4729–4733. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Tao, L.Q.; Li, T.; Gao, X.; Wang, G.; Peng, Z.; Zhu, C.; Zou, S.; Gui, Y.; Xia, S.Y.; et al. Sensing Characteristics of Toxic C₄F₇N Decomposition Products on Metallic- Nanoparticle Co-Doped BN Monolayer: A First Principles Study. IEEE Sens. J. 2021, 21, 13082–13089. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, G.; Tian, S.; Zhang, X. First-Principles Insight into Pd-Doped ZnO Monolayers as a Promising Scavenger for Dissolved Gas Analysis in Transformer Oil. ACS Omega 2020, 5, 17801–17807. [Google Scholar] [CrossRef]
- Cui, H.; Yan, C.; Jia, P.; Cao, W. Adsorption and sensing behaviors of SF6 decomposed species on Ni-doped C3N monolayer: A first-principles study. Appl. Surf. Sci. 2020, 512, 145759. [Google Scholar] [CrossRef]
- Cui, H.; Jia, P.; Peng, X.; Li, P. Adsorption and sensing of CO and C2H2 by S-defected SnS2 monolayer for DGA in transformer oil: A DFT study. Mater. Chem. Phys. 2020, 249, 123006. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, Z.; Cui, H. Pristine and Cu decorated hexagonal InN monolayer, a promising candidate to detect and scavenge SF6 decompositions based on first-principle study. J. Hazard. Mater. 2019, 363, 346–357. [Google Scholar] [CrossRef] [PubMed]



| 2D Materials | Gas | Eads (eV) | Ref. |
|---|---|---|---|
| ZnO | CO2 | +0.01 | [49] |
| ZnO | CO2 | +0.44 | [50] |
| SnO2 | CO2 | −0.663 | [51] |
| TiO2 | CO2 | −0.17 | [52] |
| ZrO2 | CO2 | −0.05 | [52] |
| WO₃ | CO2 | 0.22 | [53] |
| Eb | Eads | ΔQ | WFs | Recovery Time | |
|---|---|---|---|---|---|
| MoS2–GeSe Monolayer | −5.994 eV | / | / | 4.653 eV | / |
| CO2/MoS2–GeSe Monolayer | / | −0.955 eV | 0.266 | 4.544 eV | 14,400 s (298 K) 28.2 s (358 K) 1.25 s (398 K) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Li, Y. Monitoring Gases Content in Modern Agriculture: A Density Functional Theory Study of the Adsorption Behavior and Sensing Properties of CO2 on MoS2 Doped GeSe Monolayer. Sensors 2022, 22, 3860. https://doi.org/10.3390/s22103860
Gao X, Li Y. Monitoring Gases Content in Modern Agriculture: A Density Functional Theory Study of the Adsorption Behavior and Sensing Properties of CO2 on MoS2 Doped GeSe Monolayer. Sensors. 2022; 22(10):3860. https://doi.org/10.3390/s22103860
Chicago/Turabian StyleGao, Xin, and Yunwu Li. 2022. "Monitoring Gases Content in Modern Agriculture: A Density Functional Theory Study of the Adsorption Behavior and Sensing Properties of CO2 on MoS2 Doped GeSe Monolayer" Sensors 22, no. 10: 3860. https://doi.org/10.3390/s22103860
APA StyleGao, X., & Li, Y. (2022). Monitoring Gases Content in Modern Agriculture: A Density Functional Theory Study of the Adsorption Behavior and Sensing Properties of CO2 on MoS2 Doped GeSe Monolayer. Sensors, 22(10), 3860. https://doi.org/10.3390/s22103860




