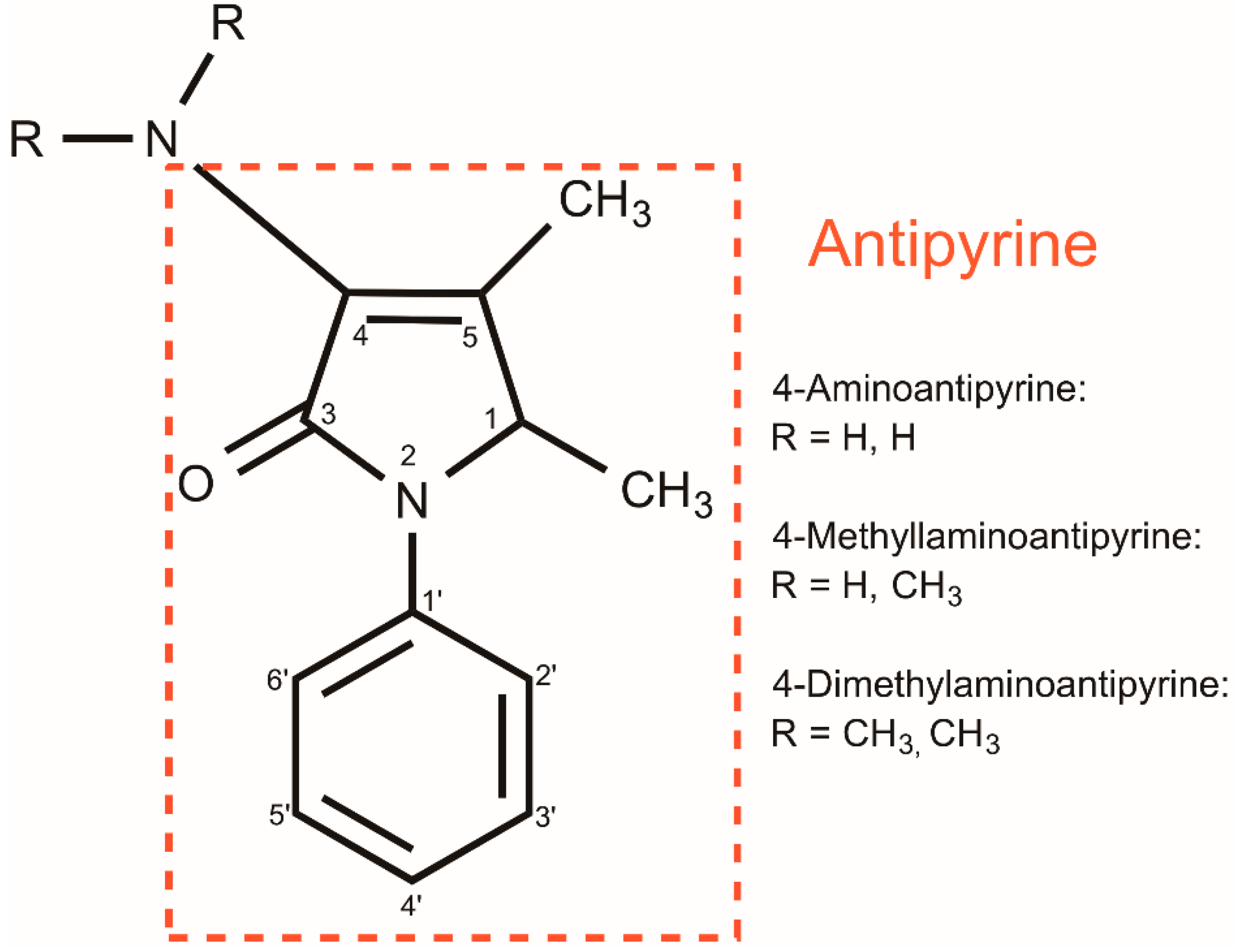
4-Dimethylaminoantipyrine as a Broad Electrochemical Indicator for Immunosensors Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Biological Reagents
2.2. Electrochemical Apparatus
2.3. Conjugation of the Probe and Electrochemical Measurements
2.4. Immunosensing Tests
2.5. Atomic Force Microscopy (AFM) Topographical Analysis
3. Results and Discussion
3.1. Surface Interactions
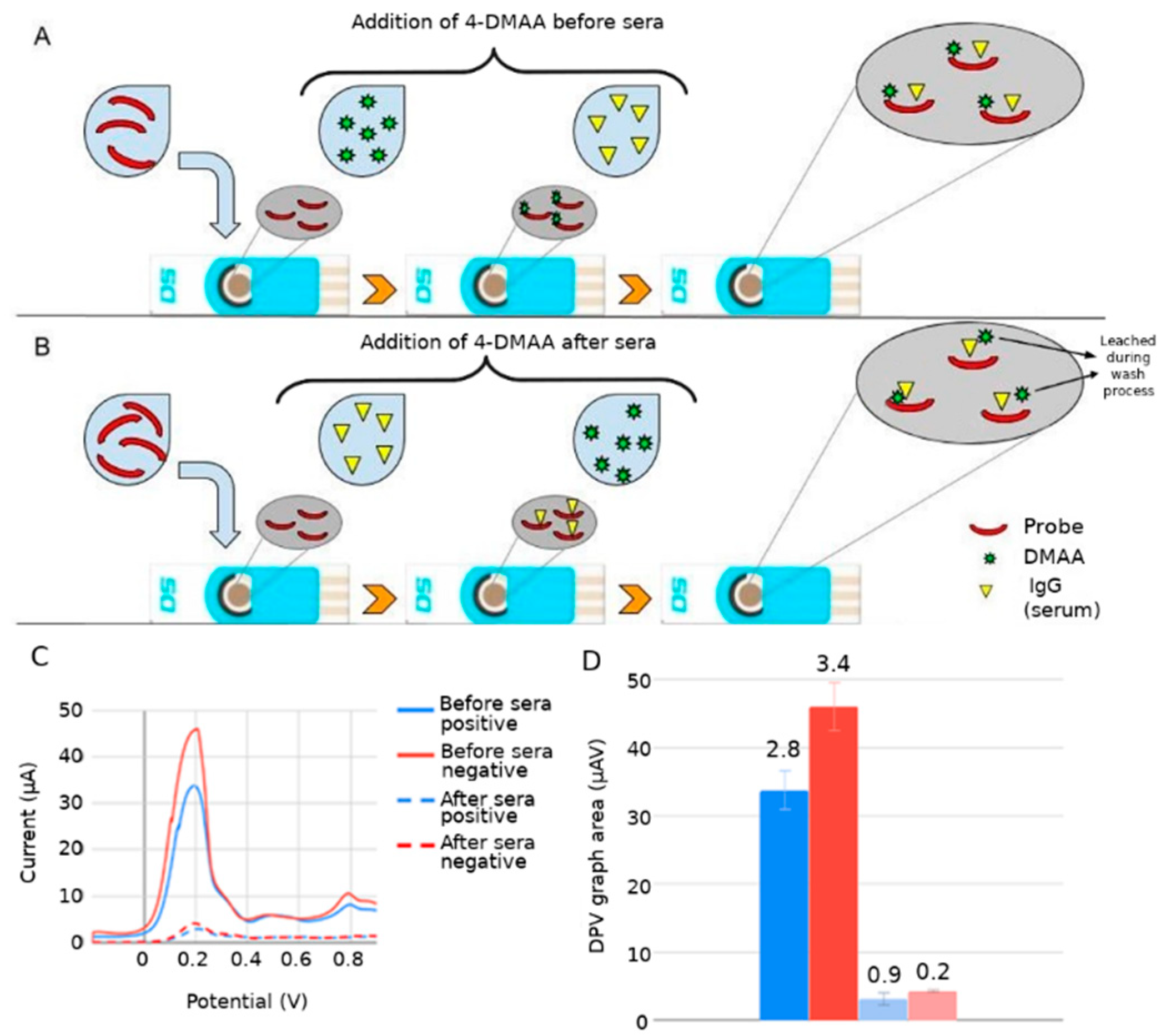
3.2. Assembly of Bioelectrodes and Importance of the Immobilization Sequence of 4-DMAA on the Surface for Detection
3.3. Affinity of 4-DMAA by Different Peptide Probes
3.4. Topographic Analysis AFM
3.5. Additional Considerations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferapontova, E.E. Electrochemical Indicators for DNA Electroanalysis. Curr. Anal. Chem. 2011, 7, 51–62. [Google Scholar] [CrossRef]
- Balaguez, R.A.; Ricordi, V.G.; Duarte, R.C.; Toldo, J.M.; Santos, C.M.; Schneider, P.H.; Gonçalves, P.F.B.; Rodembusch, F.S.; Alves, D. Bis-Arylsulfenyl- and Bis-Arylselanyl-Benzo-2,1,3-Thiadiazoles: Synthesis and Photophysical Characterization. RSC Adv. 2016, 6, 49613–49624. [Google Scholar] [CrossRef]
- Rodrigues, L.P.; Ferreira, D.C.; Ferreira, L.F.; Cuadros-Orellana, S.; de Oliveira, G.C.; Brito-Madurro, A.G.; de Oliveira, R.J.; Abrahão, O.; Madurro, J.M. Electropolymerization of Hydroxyphenylacetic Acid Isomers and the Development of a Bioelectrode for the Diagnosis of Bacterial Meningitis. J. Appl. Electrochem. 2015, 45, 1277–1287. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z.; Lee, N.Y.; Kim, J.S.; Lee, E.-C. Electrochemical DNA Detection Using Hoechst Dyes in Microfluidic Chips. Curr. Appl. Phys. 2012, 12, 1493–1496. [Google Scholar] [CrossRef]
- Tran, L.D.; Nguyen, B.H.; Van Hieu, N.; Tran, H.V.; Nguyen, H.L.; Nguyen, P.X. Electrochemical Detection of Short HIV Sequences on Chitosan/Fe3O4 Nanoparticle Based Screen Printed Electrodes. Mater. Sci. Eng. C 2011, 31, 477–485. [Google Scholar] [CrossRef]
- Nasirizadeh, N.; Zare, H.R.; Pournaghi-Azar, M.H.; Hejazi, M.S. Introduction of Hematoxylin as an Electroactive Label for DNA Biosensors and Its Employment in Detection of Target DNA Sequence and Single-Base Mismatch in Human Papilloma Virus Corresponding to Oligonucleotide. Biosens. Bioelectron. 2011, 26, 2638–2644. [Google Scholar] [CrossRef]
- Lepecq, J.-B.; Paoletti, C. A Fluorescent Complex between Ethidium Bromide and Nucleic Acids: Physical—Chemical Characterization. J. Mol. Biol. 1967, 27, 87–106. [Google Scholar] [CrossRef]
- Waring, M.J. Complex Formation between Ethidium Bromide and Nucleic Acids. J. Mol. Biol. 1965, 13, 269–282. [Google Scholar] [CrossRef]
- Santos, P.M.P.; Antunes, A.M.M.; Noronha, J.; Fernandes, E.; Vieira, A.J.S.C. Scavenging Activity of Aminoantipyrines against Hydroxyl Radical. Eur. J. Med. Chem. 2010, 45, 2258–2264. [Google Scholar] [CrossRef]
- Malvar, D.D.C.; Aguiar, F.A.; Vaz, A.D.L.; Assis, D.C.; de Melo, M.C.; Jabor, V.A.; Kalapothakis, E.; Ferreira, S.H.; Clososki, G.C.; de Souza, G.E. Dipyrone Metabolite 4-MAA Induces Hypothermia and Inhibits PGE2-Dependent and -Independent Fever While 4-AA Only Blocks PGE2-Dependent Fever. Br. J. Pharmacol. 2014, 171, 3666–3679. [Google Scholar] [CrossRef] [Green Version]
- Zovico, P.V.C.; Cavatti, R.M.; Curty, V.M.; Barauna, V.G. Suplementos contendo DMAA: Mitos e verdades. RBNE-Rev. Bras. Nutr. Esportiva 2018, 12, 443–462. [Google Scholar]
- Levy, M.; Zylber-Katz, E.; Rosenkranz, B. Clinical Pharmacokinetics of Dipyrone and Its Metabolites. Clin. Pharmacokinet. 1995, 28, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Bacil, R.P.; Buoro, R.M.; Campos, O.S.; Ramos, M.A.; Sanz, C.G.; Serrano, S.H.P. Electrochemical Behaviour of Dipyrone (Metamizole) and Others Pyrazolones. Electrochim. Acta 2018, 273, 358–366. [Google Scholar] [CrossRef]
- Moro, G.; Cristofori, D.; Bottari, F.; Cattaruzza, E.; De Wael, K.; Moretto, L.M. Redesigning an Electrochemical MIP Sensor for PFOS: Practicalities and Pitfalls. Sensors 2019, 19, 4433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowda, J.I.; Nandibewoor, S.T. Electrochemical Behavior of 4-Aminophenazone Drug at a Graphite Pencil Electrode and Its Application in Real Samples. Ind. Eng. Chem. Res. 2012, 51, 15936–15941. [Google Scholar] [CrossRef]
- Gowda, J.I.; Buddanavar, A.T.; Nandibewoor, S.T. Fabrication of Multiwalled Carbon Nanotube-Surfactant Modified Sensor for the Direct Determination of Toxic Drug 4-Aminoantipyrine. J. Pharm. Anal. 2015, 5, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Gowda, J.I.; Nandibewoor, S.T. Binding and Conformational Changes of Human Serum Albumin upon Interaction with 4-Aminoantipyrine Studied by Spectroscopic Methods and Cyclic Voltammetry. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2014, 124, 397–403. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, R.; Li, C.; Xia, Q.; Zhang, P. The Interaction between 4-Aminoantipyrine and Bovine Serum Albumin: Multiple Spectroscopic and Molecular Docking Investigations. J. Hazard. Mater. 2011, 190, 574–581. [Google Scholar] [CrossRef]
- Jagadeesan, G.; Vijayakuma, V.; Palayam, M.; Suresh, G.; Krishnaswamy, G.; Aravindhan, S.; Peters, G.H.J. Pyrazole Based Inhibitors against Enzymes of Staphylococcus Aureus: A Computational Study. J. Proteomics Bioinform. 2015, 8, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Lemos, A.J.G.; Balvedi, R.P.A.; Rodovalho, V.R.; Resende, L.O.; Castro, A.C.H.; Cuadros-Orellana, S.; Madurro, J.M.; Brito-Madurro, A.G. Immunosensor Assembled on Polymeric Nanostructures for Clinical Diagnosis of C-Reactive Protein. Microchem. J. 2017, 133, 572–576. [Google Scholar] [CrossRef]
- Herzog, G.; Arrigan, D.W.M. Electrochemical Strategies for the Label-Free Detection of Amino Acids, Peptides and Proteins. Analyst 2007, 132, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Oliveira-Brett, A.M. Peptide Methionine Sulfoxide Reductase A (MsrA): Direct Electrochemical Oxidation on Carbon Electrodes. Bioelectrochemistry 2013, 89, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, J.; Yang, C.; Zhang, J. UV/H2O2 Degradation of 4-Aminoantipyrine: A Voltammetric Study. Chem. Eng. J. 2010, 161, 68–72. [Google Scholar] [CrossRef]




| Probe ID | Probe Concentration | Target Disease | Serum Dilution | * IRB Protocol |
|---|---|---|---|---|
| LC1 | 38.0 μg.mL−1 | Visceral leishmaniasis | 1:100 | CEP 099/2003, 499/2008 |
| LT | 22.5 μg.mL−1 | Tegumentary leishmaniasis | 1:100 | CEP 099/2003, 499/2008 |
| D3 | 4.0 μg.mL−1 | Strongyloidiasis | 1:160 | CEP 553/2009 |
| M3R | 3.5 µg.mL−1 | Leprosy | 1:100 | CEP 449/2010, CAE N.23115003005/2009-36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, F.C.C.; Alves, R.P.; Valle, A.L.; Santos, F.d.A.A.; Dias, A.C.S.; Goulart, I.M.B.; Oliveira, E.G.A.; Oliveira, G.S.; Rodrigues, L.P.; Goulart, L.R. 4-Dimethylaminoantipyrine as a Broad Electrochemical Indicator for Immunosensors Platform. Sensors 2022, 22, 3681. https://doi.org/10.3390/s22103681
Melo FCC, Alves RP, Valle AL, Santos FdAA, Dias ACS, Goulart IMB, Oliveira EGA, Oliveira GS, Rodrigues LP, Goulart LR. 4-Dimethylaminoantipyrine as a Broad Electrochemical Indicator for Immunosensors Platform. Sensors. 2022; 22(10):3681. https://doi.org/10.3390/s22103681
Chicago/Turabian StyleMelo, Francielli C. C., Renata P. Alves, Anderson L. Valle, Fabiana de A. A. Santos, Ana Carolina S. Dias, Isabela M. B. Goulart, Eduardo G. A. Oliveira, Guedmiller S. Oliveira, Luciano P. Rodrigues, and Luiz R. Goulart. 2022. "4-Dimethylaminoantipyrine as a Broad Electrochemical Indicator for Immunosensors Platform" Sensors 22, no. 10: 3681. https://doi.org/10.3390/s22103681
APA StyleMelo, F. C. C., Alves, R. P., Valle, A. L., Santos, F. d. A. A., Dias, A. C. S., Goulart, I. M. B., Oliveira, E. G. A., Oliveira, G. S., Rodrigues, L. P., & Goulart, L. R. (2022). 4-Dimethylaminoantipyrine as a Broad Electrochemical Indicator for Immunosensors Platform. Sensors, 22(10), 3681. https://doi.org/10.3390/s22103681








