Negative Effects of Annealed Seed Layer on the Performance of ZnO-Nanorods Based Nitric Oxide Gas Sensor
Abstract
:1. Introduction
2. Experimental Process
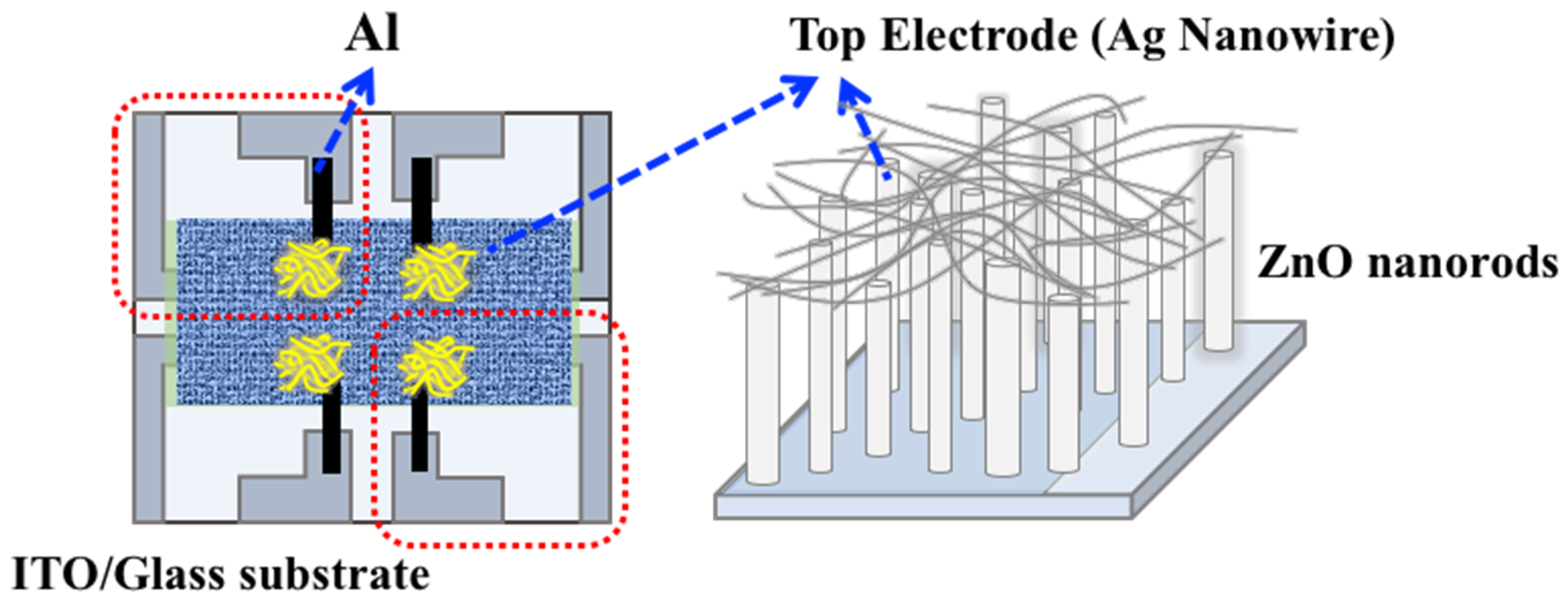
2.1. Sensor Device Fabrication
2.2. Response Measurement Set-Up
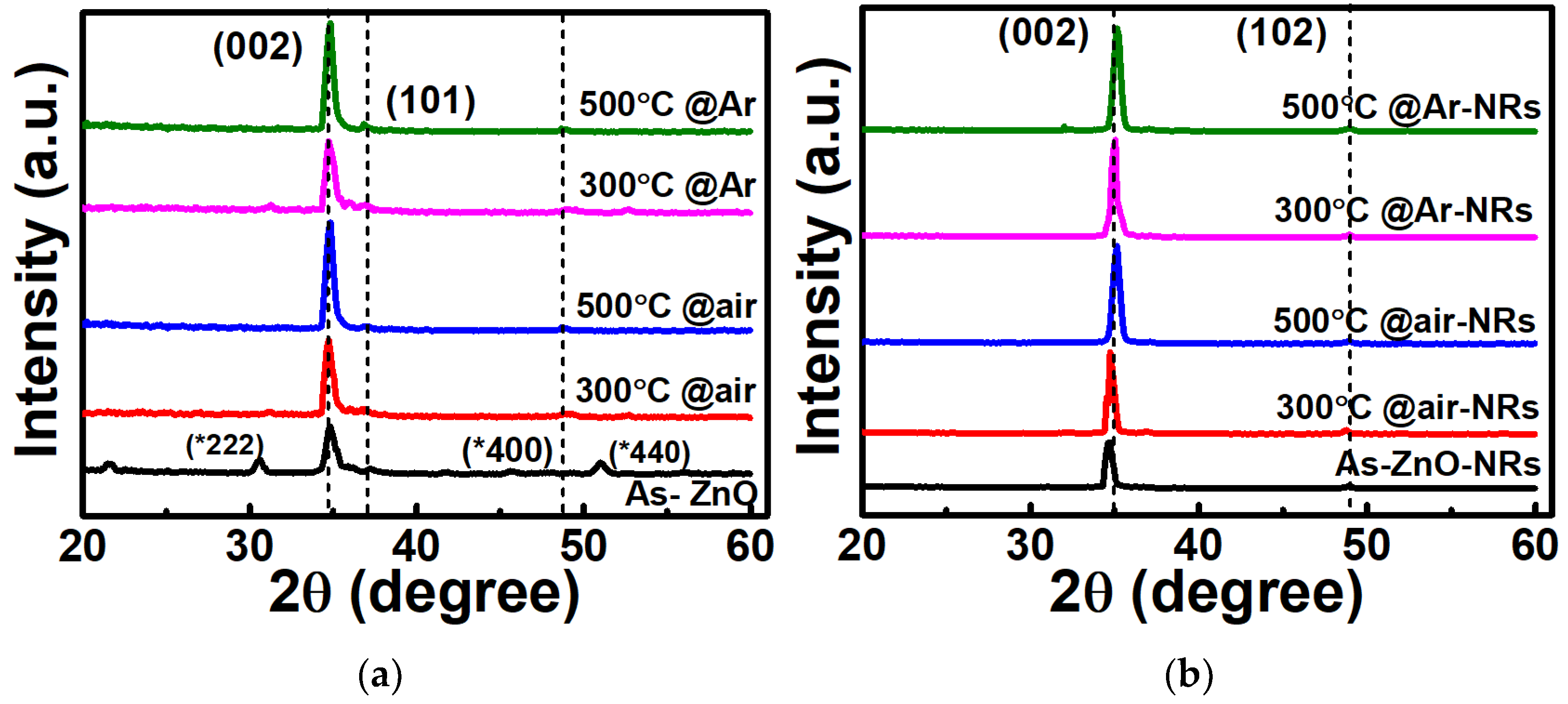
2.3. Material Characterization
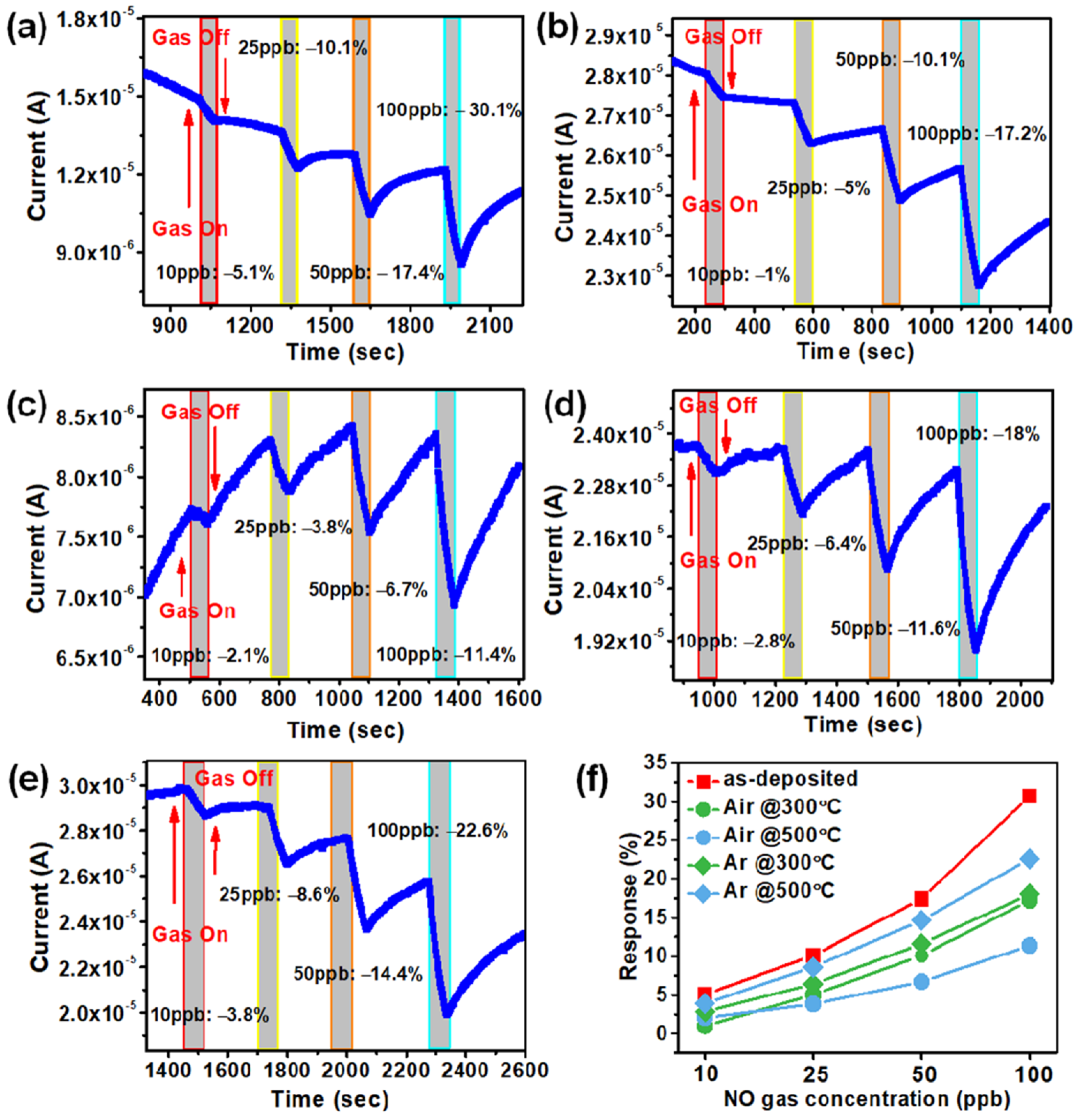
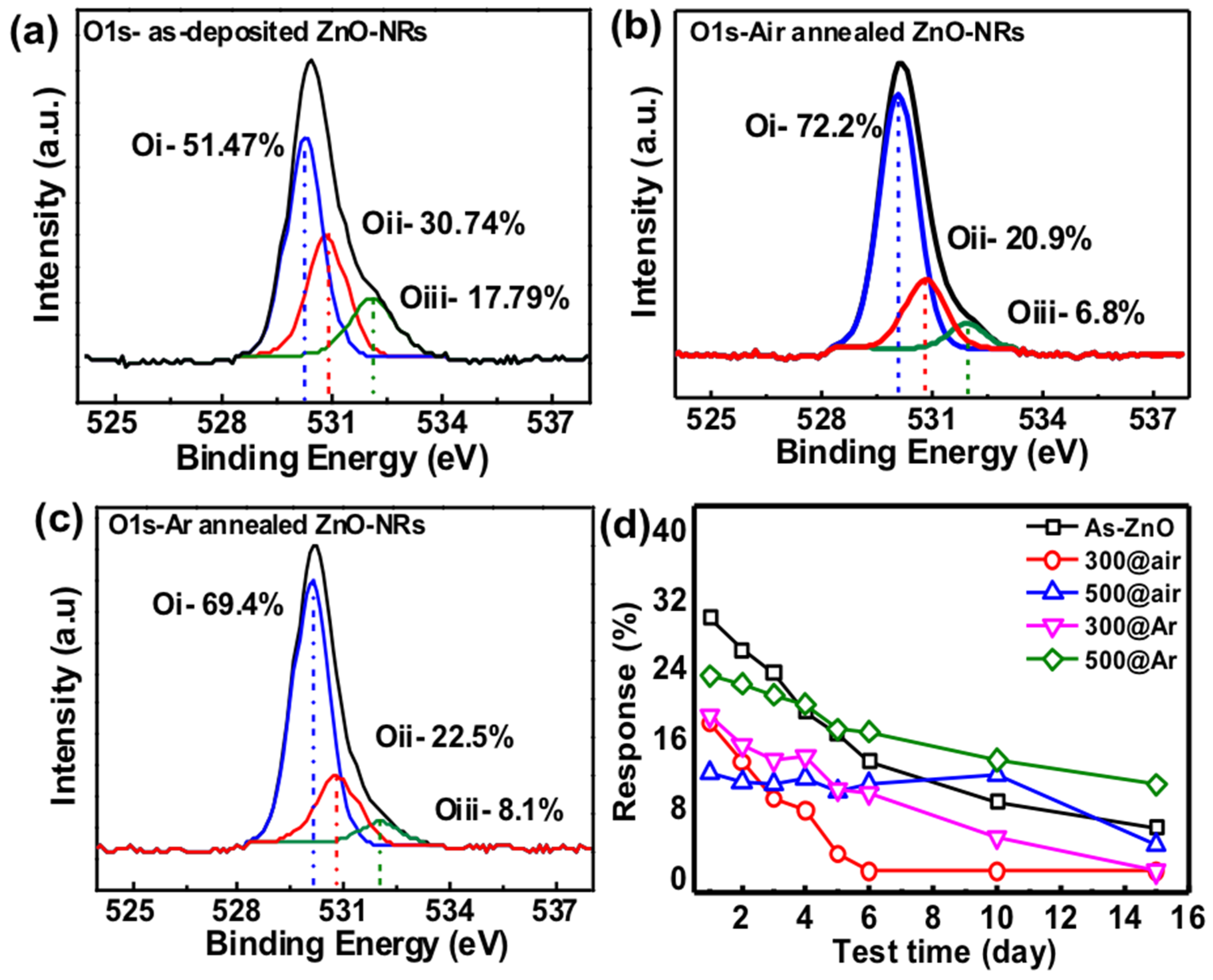
3. Results and Discussion
Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bowe, B.; Xie, Y.; Yan, Y.Y.; Al-Aly, Z. Burden of Cause-Specific Mortality Associated with PM2.5 Air Pollution in the United States. JAMA Netw. Open 2019, 2, e1915834. [Google Scholar] [CrossRef] [Green Version]
- Holgate, S.T. ‘Every breath we take: The lifelong impact of air pollution’—A call for action. Prof. Issues Clin. Med. 2017, 17, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Münzel, T.; Daiber, A. The air pollution constituent particulate matter (PM2.5) destabilizes coronary artery plaques. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1365–1367. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [Green Version]
- Fauzi, F.; Rianjanu, A.; Santoso, I.; Triyana, K. Gas and humidity sensing with quartz crystal microbalance (QCM) coated with graphene-based materials—A mini review. Sens. Actuators A Phys. 2021, 330, 112837. [Google Scholar] [CrossRef]
- Rianjanu, A.; Fauzi, F.; Triyana, K.; Wasisto, H.S. Electrospun Nanofibers for Quartz Crystal Microbalance Gas Sensors: A Review. ACS Appl. Nano Mater. 2021, 4, 9957–9975. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, S.J.; Hsueh, T.J. A WO3 nanoparticles NO gas sensor prepared by hot-wire CVD. IEEE Electron. Device Lett. 2017, 38, 266–269. [Google Scholar] [CrossRef]
- Kaur, M.; Kailasaganapathi, S.; Ramgir, N.; Datta, N.; Kumar, S.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Gas dependent sensing mechanism in ZnO nanobelt sensor. Appl. Surf. Sci. 2017, 394, 258–266. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc oxide nanostructures for NO2 gas–sensor applications: A review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Hu, L.-L.; Zan, H.-W.; Tseng, T.-Y. Highly sensitive nitric oxide gas sensor based on ZnO-nanorods vertical resistor operated at room temperature. Nanotechnology 2019, 30, 095501. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Simanjuntak, F.M.; Wu, Y.-C.; Kumar, A.; Zan, H.-W.; Tseng, T.-Y. Sensing performance of gas sensors fabricated from controllably grown ZnO-based nanorods on seed layers. J. Mater. Sci. 2020, 55, 8850–8860. [Google Scholar] [CrossRef]
- Shouli, B.; Liangyuan, C.; Dianqing, L.; Wensheng, Y.; Pengcheng, Y.; Zhiyong, L.; Aifan, C.; Liu, C.C. Different morphologies of ZnO nanorods and their sensing property. Sens. Actuators B Chem. 2010, 146, 129–137. [Google Scholar] [CrossRef]
- Alfaro Cruz, M.R.; Ceballos-Sanchez, O.; Luévano-Hipólito, E.; Torres-Martínez, L.M. ZnO thin films deposited by RF magnetron sputtering: Effects of the annealing and atmosphere conditions on the photocatalytic hydrogen production. Int. J. Hydrog. Energy 2018, 43, 10301–10310. [Google Scholar] [CrossRef]
- Li, J.; Yang, D.; Zhu, X. Effects of aging time and annealing temperature on structural and optical properties of sol-gel ZnO thin films. AIP Adv. 2017, 7, 065213. [Google Scholar] [CrossRef] [Green Version]
- Malik, G.; Mourya, S.; Jaiswal, J.; Chandra, R. Effect of annealing parameters on optoelectronic properties of highly ordered ZnO thin films. Mater. Sci. Semicond. Process. 2019, 100, 200–213. [Google Scholar] [CrossRef]
- Sener, E.; Bayram, O.; Hasar, U.C.; Simsek, O. Structural and optical properties of RF sputtered ZnO thin films: Annealing effect. Phys. B Condens. Matter 2021, 605, 412421. [Google Scholar] [CrossRef]
- Harale, N.S.; Kamble, A.S.; Tarwal, N.L.; Mulla, I.S.; Rao, V.K.; Kim, J.H.; Patil, P.S. Hydrothermally grown ZnO nanorods arrays for selective NO2 gas sensing: Effect of anion generating agents. Ceram. Int. 2016, 42, 12807–12814. [Google Scholar] [CrossRef]
- Wang, X.; Sun, F.; Huang, Y.; Duan, Y.; Yin, Z. A patterned ZnO nanorod array/gas sensor fabricated by mechanoelectrospinning-assisted selective growth. Chem. Commun. 2015, 51, 3117–3120. [Google Scholar] [CrossRef] [PubMed]
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Panda, D.; Simanjuntak, F.M.; Chandrasekaran, S.; Pattanayak, B.; Singh, P.; Tseng, T.-Y. Barrier Layer Induced Switching Stability in Ga:ZnO Nanorods Based Electrochemical Metallization Memory. IEEE Trans. Nanotechnol. 2020, 19, 764–768. [Google Scholar] [CrossRef]
- Suresh Kumar, P.; Sundaramurthy, J.; Mangalaraj, D.; Nataraj, D.; Rajarathnam, D.; Srinivasan, M.P. Enhanced super-hydrophobic and switching behavior of ZnO nanostructured surfaces prepared by simple solution—Immersion successive ionic layer adsorption and reaction process. J. Colloid Interface Sci. 2011, 363, 51–58. [Google Scholar] [CrossRef]
- Pimentel, A.; Ferreira, S.H.; Nunes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. Microwave synthesized ZnO nanorod arrays for UV sensors: A seed layer annealing temperature study. Materials 2016, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Simanjuntak, F.M.; Kumar, A.; Tseng, T. Resistive switching behavior of Ga doped ZnO-nanorods film conductive bridge random access memory. Thin Solid Film. 2018, 660, 828–833. [Google Scholar] [CrossRef]
- Simanjuntak, F.; Singh, P.; Chandrasekaran, S.; Lumbantoruan, F.J.; Yang, C.-C.; Huang, C.-J.; Lin, C.-C.; Tseng, T.-Y. Role of Nanorods Insertion Layer in ZnO-based Electrochemical Metallization Memory Cell. Semicond. Sci. Technol. 2017, 32, 124003. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, C.M.; Park, C.H.; Lim, J.H.; Park, G.C.; Joo, J. Effect of Annealing Temperature on Morphology and Electrical Property of Hydrothermally-Grown ZnO Nanorods/p-Si Heterojunction Diodes. J. Nanosci. Nanotechnol. 2018, 19, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Chen, T.; Wan, J.; Ru, G.P.; Li, B.Z.; Qu, X.P. The effect of pre-annealing of sputtered ZnO seed layers on growth of ZnO nanorods through a hydrothermal method. Appl. Phys. A Mater. Sci. Process. 2009, 94, 775–780. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, C.K.; Park, J.S. Physical properties of RF-sputtered ZnO thin films: Effects of two-step deposition. J. Korean Phys. Soc. 2007, 50, 1073–1078. [Google Scholar] [CrossRef]
- Simanjuntak, F.M.; Ohno, T.; Samukawa, S. Influence of rf sputter power on ZnO film characteristics for transparent memristor devices. AIP Adv. 2019, 9, 105216. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.P.; He, G.; Zhu, L.Q.; Liu, M.; Pan, S.S.; Zhang, L.D. Effect of oxygen partial pressure on the structural and optical properties of ZnO film deposited by reactive sputtering. Appl. Surf. Sci. 2007, 253, 9414–9421. [Google Scholar] [CrossRef]
- Sett, D.; Basak, D. Highly enhanced H2gas sensing characteristics of Co:ZnO nanorods and its mechanism. Sens. Actuators B Chem. 2017, 243, 475–483. [Google Scholar] [CrossRef]
- Jin, X.B.; Li, Y.X.; Su, Y.; Guo, Z.; Gu, C.P.; Huang, J.R.; Meng, F.L.; Huang, X.J.; Li, M.Q.; Liu, J.H. Porous and single-crystalline ZnO nanobelts: Fabrication with annealing precursor nanobelts, and gas-sensing and optoelectronic performance. Nanotechnology 2016, 27, 355702. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Quasi-one dimensional metal oxide semiconductors: Preparation, characterization and application as chemical sensors. Prog. Mater. Sci. 2009, 54, 1–67. [Google Scholar] [CrossRef]
- Li, C.C.; Du, Z.F.; Li, L.M.; Yu, H.C.; Wan, Q.; Wang, T.H. Surface-depletion controlled gas sensing of ZnO nanorods grown at room temperature. Appl. Phys. Lett. 2007, 91, 2005–2008. [Google Scholar] [CrossRef]
- Wang, S.H.; Shen, C.Y.; Lien, Z.J.; Wang, J.H. Nitric oxide sensing properties of a surface acoustic wave sensor with copper-ion-doped polyaniline/tungsten oxide nanocomposite film. Sens. Actuators B Chem. 2017, 243, 1075–1082. [Google Scholar] [CrossRef]
- Yuliarto, B.; Ramadhani, M.F.; Wieno, H. Nugraha Fabrications of NO Gas Sensors Based on ZnO Nanorod Thin Films. Int. J. Mater. Sci. Eng. 2014, 2, 15–18. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, L.; Yang, J.; Du, P.; Xu, L.; Wang, J. Highly Sensitive and Fast Optoelectronic Room-Temperature NO2 Gas Sensor Based on ZnO Nanorod-Assembled Macro-/Mesoporous Film. ACS Appl. Electron. Mater. 2020, 2, 580–589. [Google Scholar] [CrossRef]
- Hongsith, N.; Wongrat, E.; Kerdcharoen, T.; Choopun, S. Sensor response formula for sensor based on ZnO nanostructures. Sens. Actuators B Chem. 2010, 144, 67–72. [Google Scholar] [CrossRef]
- Samanta, C.; Ghatak, A.; Raychaudhuri, A.K.; Ghosh, B. ZnO/Si nanowires heterojunction array-based nitric oxide (NO) gas sensor with noise-limited detectivity approaching 10 ppb. Nanotechnology 2019, 30, 305501. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Xie, M.; Luo, J.; Kan, H.; Wei, Q. Nitric oxide sensors using nanospiral ZnO thin film deposited by GLAD for application to exhaled human breath. RSC Adv. 2020, 10, 14877–14884. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.T.; Chang, S.J.; Ji, L.W.; Hsiao, Y.J.; Tang, I.T.; Lu, H.Y.; Chu, Y.L. High Sensitivity of NO Gas Sensors Based on Novel Ag-Doped ZnO Nanoflowers Enhanced with a UV Light-Emitting Diode. ACS Omega 2018, 3, 13798–13807. [Google Scholar] [CrossRef]
- Qiu, J.; Hu, X.; Min, X.; Quan, W.; Tian, R.; Ji, P.; Zheng, H.; Qin, W.; Wang, H.; Pan, T.; et al. Observation of Switchable Dual-Conductive Channels and Related Nitric Oxide Gas-Sensing Properties in the N-rGO/ZnO Heterogeneous Structure. ACS Appl. Mater. Interfaces 2020, 12, 19755–19767. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Cheng, Y.; Li, H.; Zhao, L.; He, Z.; Zhao, C.; Liu, F.; Ding, L. Selective-detection NO at room temperature on porous ZnO nanostructure by solid-state synthesis method. J. Colloid Interface Sci. 2019, 556, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Naderi, H.; Hajati, S.; Ghaedi, M.; Espinos, J.P. Highly selective few-ppm NO gas-sensing based on necklace-like nanofibers of ZnO/CdO n-n type I heterojunction. Sens. Actuators B Chem. 2019, 297, 126774. [Google Scholar] [CrossRef]
- Chou, C.Y.; Tseng, S.F.; Chang, T.L.; Tu, C.T.; Han, H.C. Controlled bridge growth of ZnO nanowires on laser-scribed graphene-based devices for NO gas detection. Appl. Surf. Sci. 2020, 508, 145204. [Google Scholar] [CrossRef]
- Li, C.; Song, B.Y.; Teng, Y.; Zhang, X.F.; Deng, Z.P.; Xu, Y.M.; Huo, L.H.; Gao, S. Biomass-derived hierarchical porous ZnO microtubules for highly selective detection of ppb-level nitric oxide at low temperature. Sens. Actuators B Chem. 2021, 333, 129627. [Google Scholar] [CrossRef]


| No. | Sensing Materials | Operating Temp. (°C) | Concentration | Response (%) | References |
|---|---|---|---|---|---|
| 1. | ZnO nanocrystals/N-rGO | 90 | 100 (ppb) | 2 | [41] |
| 2. | Ag-doped ZnO | 200 | 36 (ppb) | 90 | [40] |
| 3. | ZnO/p-Si nanowires | RT | 10 (ppm) | 35 | [38] |
| 4. | ZnO-NRs thin film | RT | 1 (ppm) | 57.1 | [11] |
| 5. | ZnO nanostructure | RT | 40 (ppm) | 23.6 | [42] |
| 6. | ZnO/CdO nanofibers | 215 | 3 (ppm) | 1.7 | [43] |
| 7. | ZnO nanowire | RT | 5 (ppm) | 15 | [44] |
| 8. | ZnO microtubules | 92 | 10 (ppm) | 78.5 | [45] |
| 9. | Nanospiral ZnO film | 150 | 100 (ppb) | 16.9 | [39] |
| 10. | ZnO-NRs (as-deposited) | RT | 100 (ppb) | 30 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, P.; Simanjuntak, F.M.; Hu, L.-L.; Tseng, T.-Y.; Zan, H.-W.; Chu, J.P. Negative Effects of Annealed Seed Layer on the Performance of ZnO-Nanorods Based Nitric Oxide Gas Sensor. Sensors 2022, 22, 390. https://doi.org/10.3390/s22010390
Singh P, Simanjuntak FM, Hu L-L, Tseng T-Y, Zan H-W, Chu JP. Negative Effects of Annealed Seed Layer on the Performance of ZnO-Nanorods Based Nitric Oxide Gas Sensor. Sensors. 2022; 22(1):390. https://doi.org/10.3390/s22010390
Chicago/Turabian StyleSingh, Pragya, Firman Mangasa Simanjuntak, Li-Lun Hu, Tseung-Yuen Tseng, Hsiao-Wen Zan, and Jinn P. Chu. 2022. "Negative Effects of Annealed Seed Layer on the Performance of ZnO-Nanorods Based Nitric Oxide Gas Sensor" Sensors 22, no. 1: 390. https://doi.org/10.3390/s22010390
APA StyleSingh, P., Simanjuntak, F. M., Hu, L.-L., Tseng, T.-Y., Zan, H.-W., & Chu, J. P. (2022). Negative Effects of Annealed Seed Layer on the Performance of ZnO-Nanorods Based Nitric Oxide Gas Sensor. Sensors, 22(1), 390. https://doi.org/10.3390/s22010390







