Rapid Determination of the ‘Legal Highs’ 4-MMC and 4-MEC by Spectroelectrochemistry: Simultaneous Cyclic Voltammetry and In Situ Surface-Enhanced Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation for Electrochemistry and Raman Spectroscopy
2.3. Substrate Characterization
2.4. Spectroelectrochemical Determinations
2.5. Raman Measurements
2.6. Interference Study
2.7. Reproducibility of the EC-SERS Substrate
2.8. Urine Analysis
3. Results and Discussion
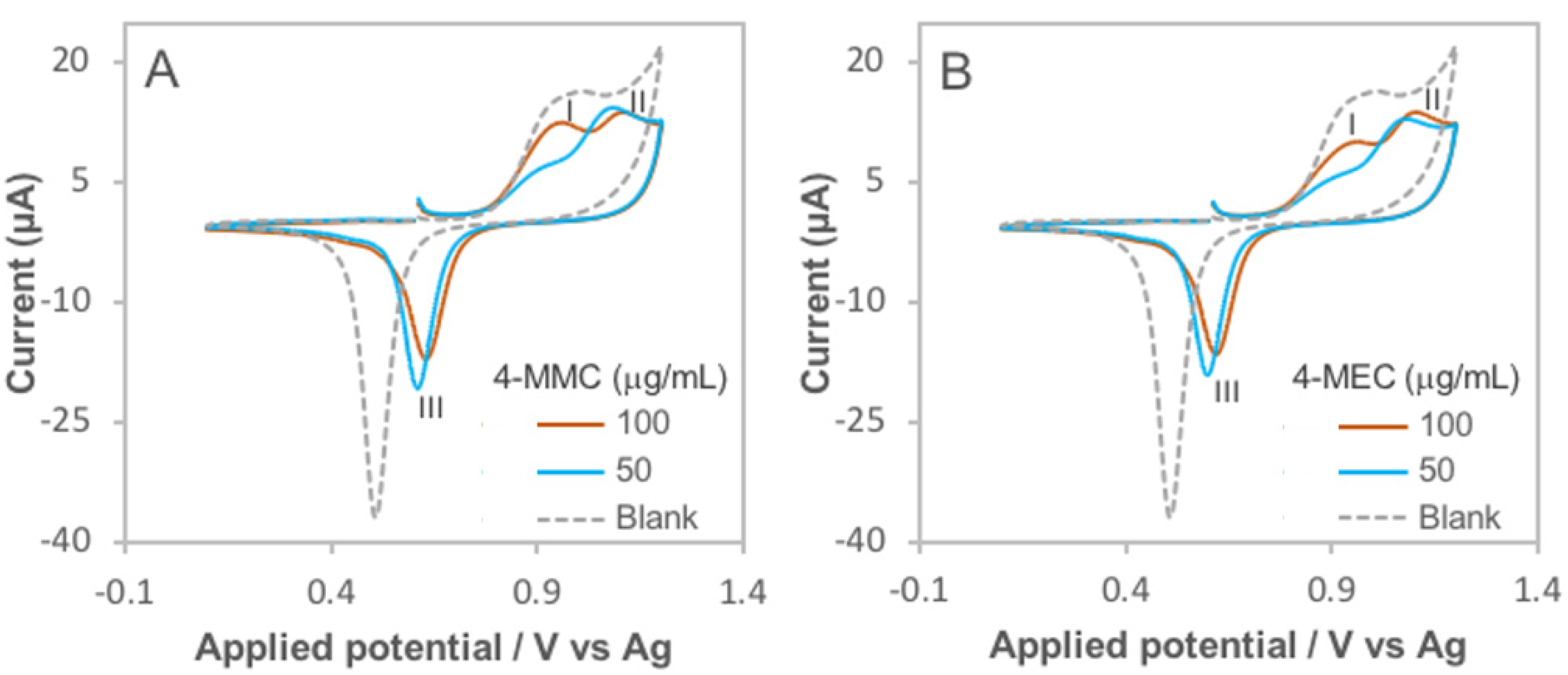
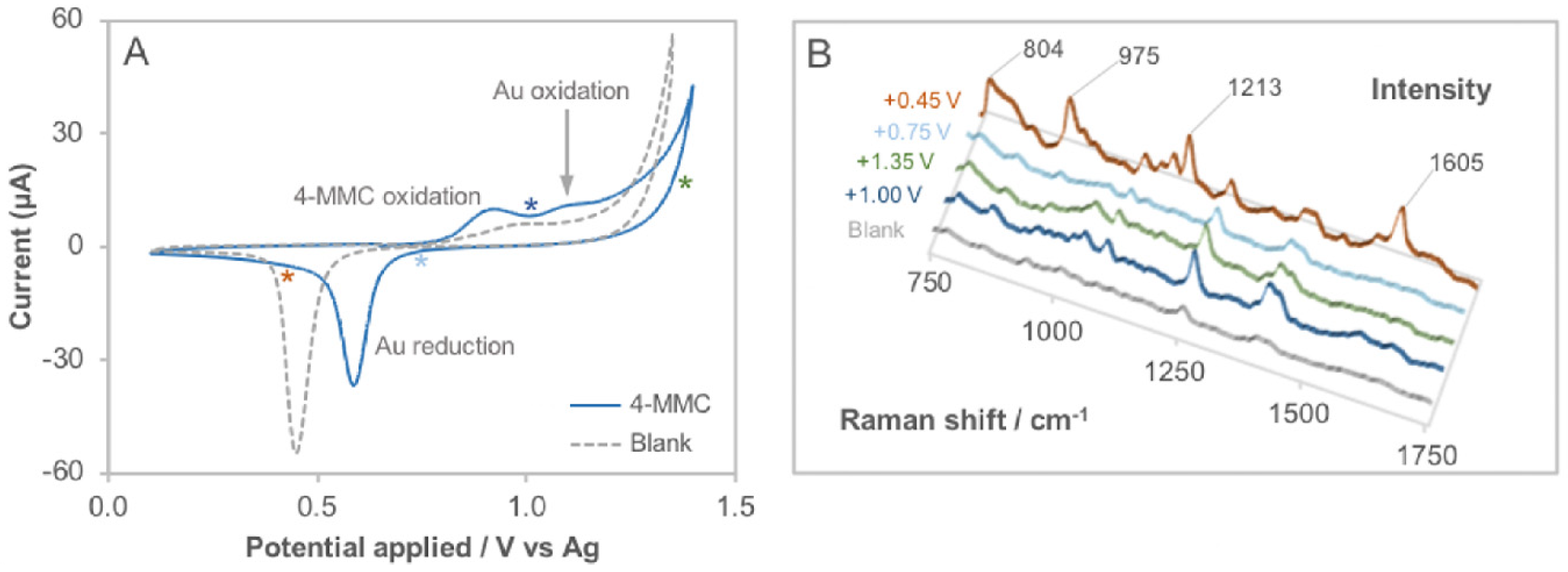
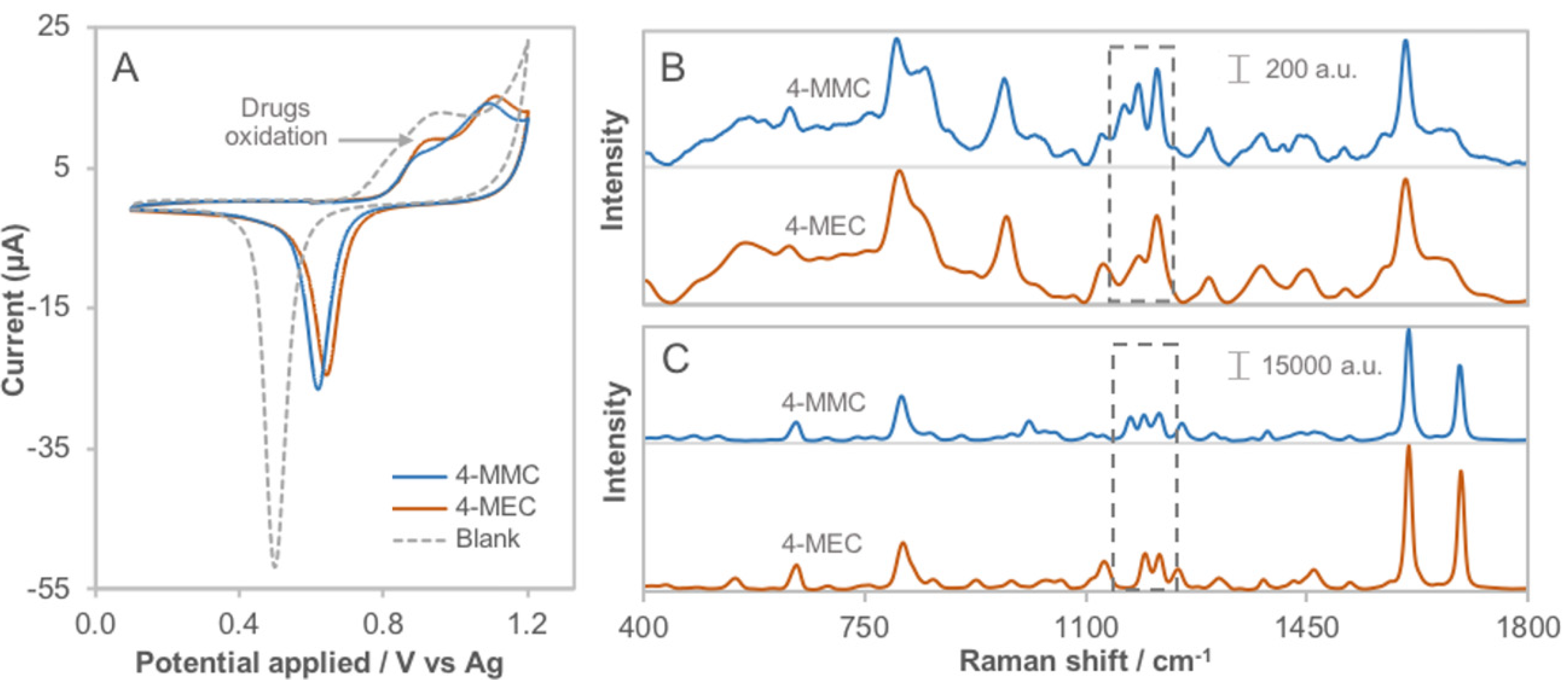
3.1. Determination of the Electro-Activity
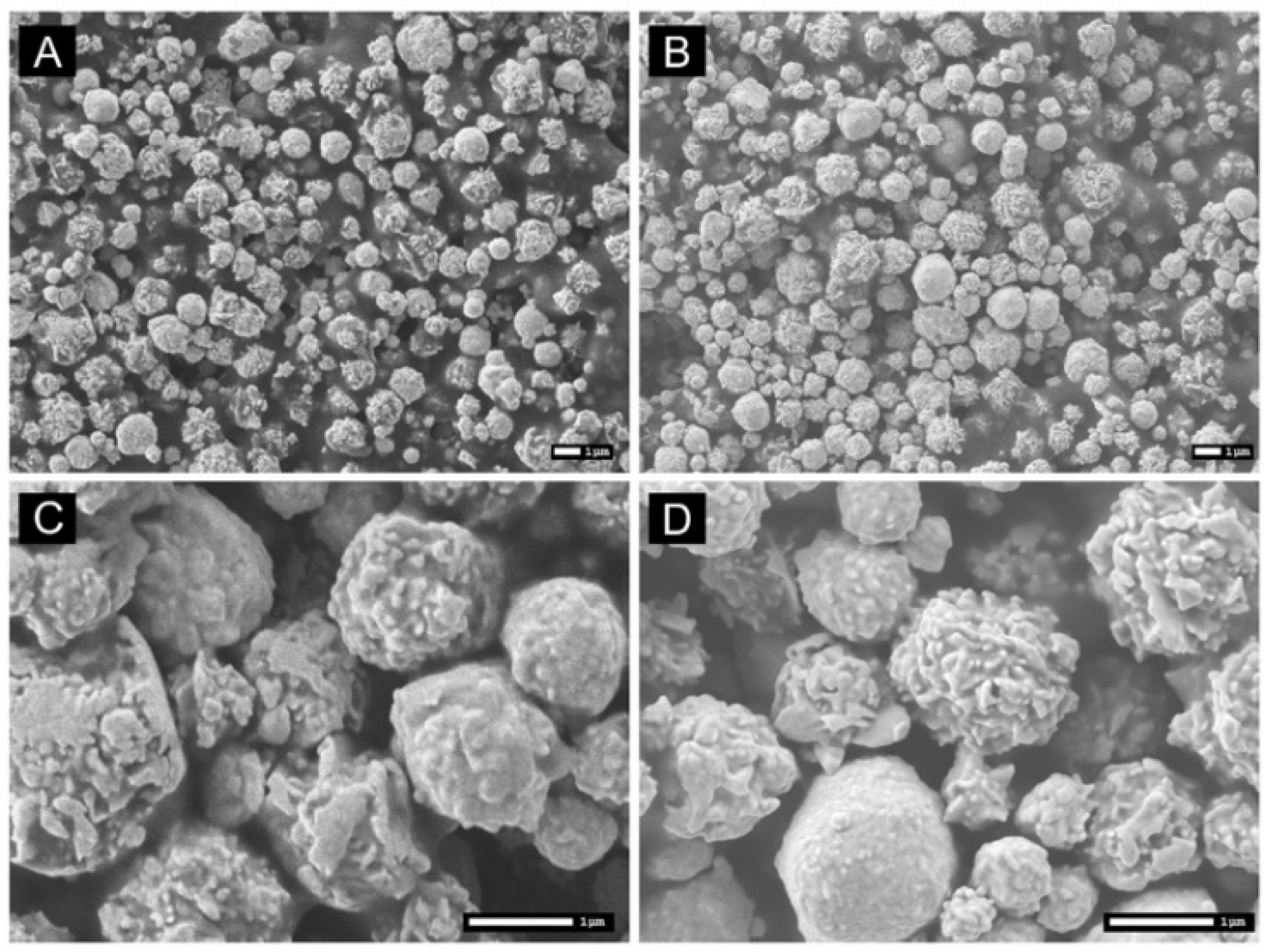
3.2. Substrate Characterization
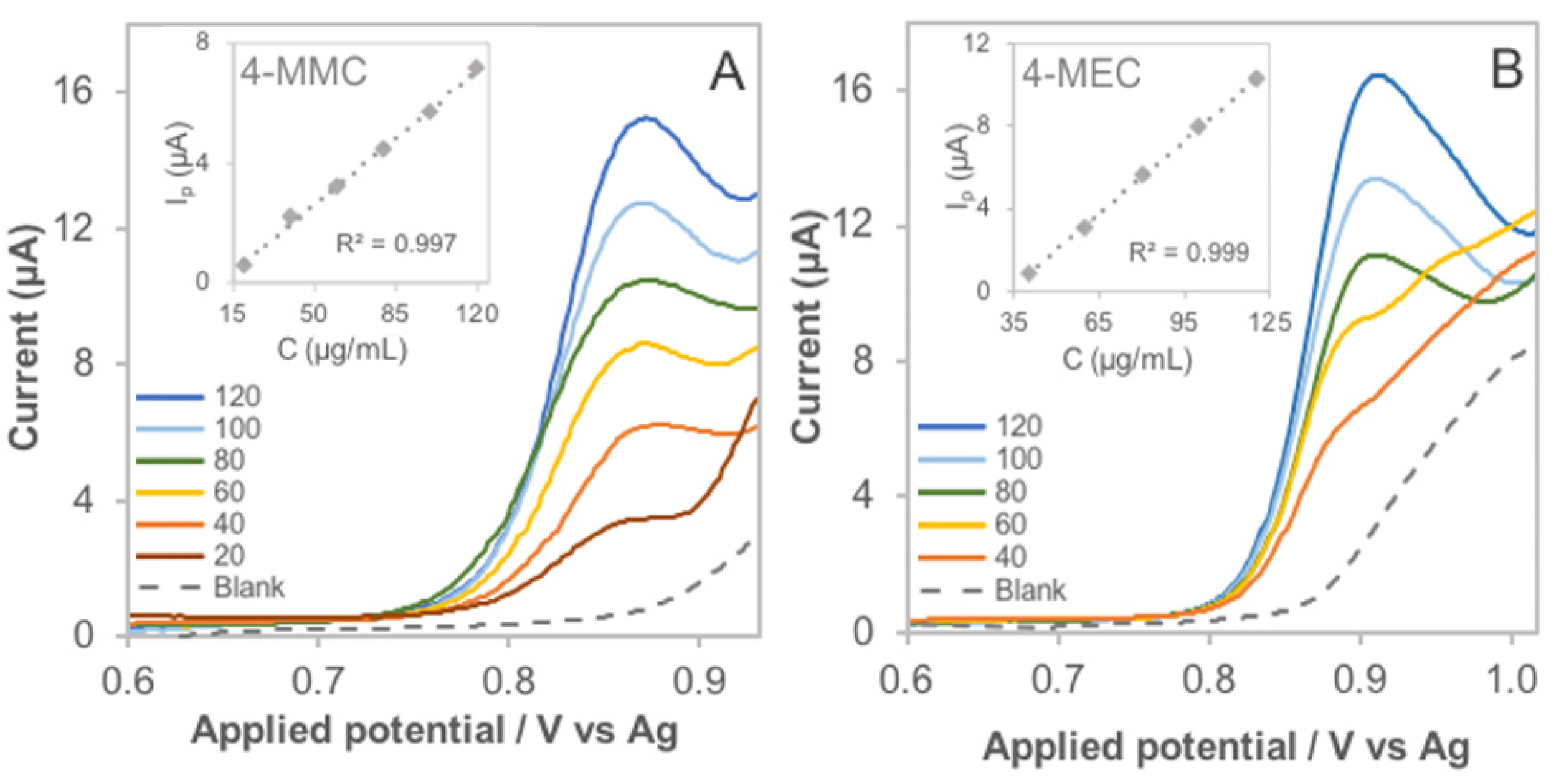
3.3. Spectroelectrochemical Sensing
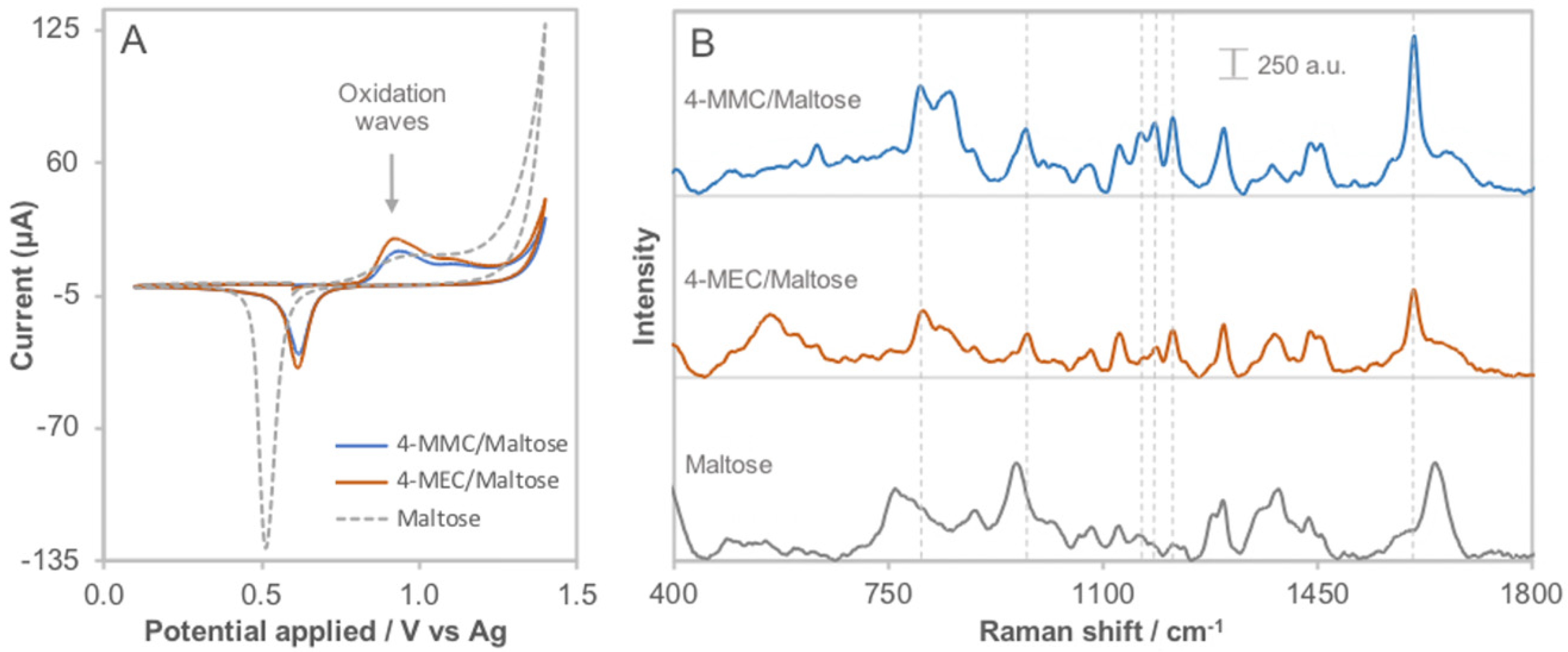
3.4. Interference Study
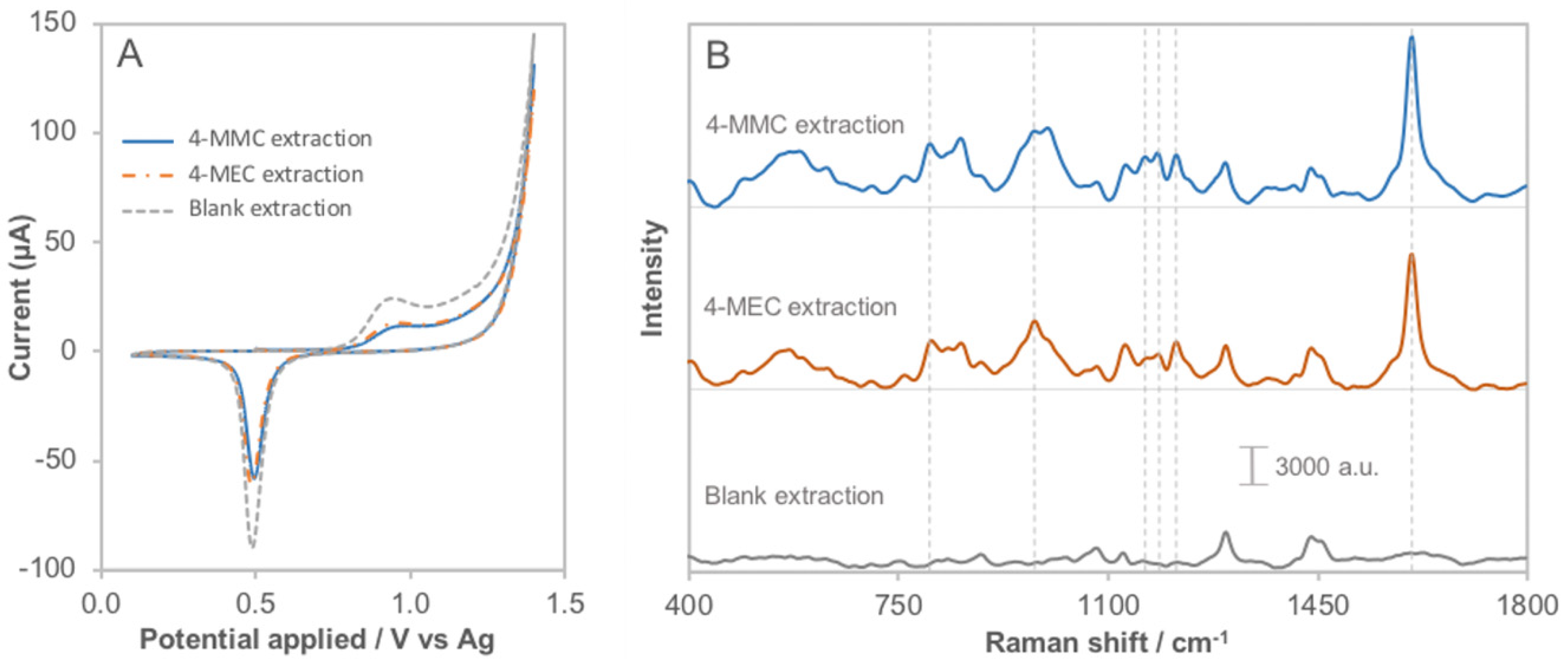
3.5. Applicability of the Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Office on Drugs and Crime (UNODC). World Drug Report 2020; United Nations Office on Drugs and Crime (UNODC): Vienna, Austria, 2020. [Google Scholar] [CrossRef]
- Smith, J.P.; Metters, J.P.; Irving, C.; Sutcliffe, O.B.; Banks, C.E. Forensic electrochemistry: The electroanalytical sensing of synthetic cathinone-derivatives and their accompanying adulterants in “legal high” products. Analyst 2014, 139, 389–400. [Google Scholar] [CrossRef]
- Gibbons, S.; Zloh, M. An analysis of the ‘legal high’ mephedrone. Bioorg. Med. Chem. Lett. 2010, 20, 4135–4139. [Google Scholar] [CrossRef]
- The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report 2020: Trends and Developments; The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA): Lisbon, Portugal, 2020. [Google Scholar] [CrossRef]
- Simmler, L.; Buser, T.; Donzelli, M.; Schramm, Y.; Dieu, L.-H.; Huwyler, J.; Chaboz, S.; Hoener, M.; Liechti, M. Pharmacological characterization of designer cathinonesin vitro. J. Cereb. Blood Flow Metab. 2013, 168, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Papaseit, E.; Pérez-Mañá, C.; de Sousa Fernandes Perna, E.B.; Olesti, E.; Mateus, J.; Kuypers, K.P.; Theunissen, E.L.; Fonseca, F.; Torrens, M.; Ramaekers, J.G.; et al. Mephedrone and Alcohol Interactions in Humans. Front. Pharmacol. 2020, 10, 1588. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime (UNODC). Recommended Methods for the Identification and Analysis of Synthetic Cathinones in Seized Materials; United Nations Office on Drugs and Crime (UNODC): Vienna, Austria, 2020. Available online: https://www.unodc.org/documents/scientific/Recommended_methods_for_the_Identification_and_Analysis_of_Synthetic_Cathinones_in_Seized_Materials-Rev..pdf (accessed on 20 November 2021).
- Bartlett, C.-A.; Taylor, S.; Fernandez, C.; Wanklyn, C.; Burton, D.; Enston, E.; Raniczkowska, A.; Black, M.; Murphy, L. Disposable screen printed sensor for the electrochemical detection of methamphetamine in undiluted saliva. Chem. Cent. J. 2016, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, J.; Alvarado-Gámez, A.L.; Arroyo-Mora, L.E.; Barquero-Quirós, M. Electrochemical determination of novel psychoactive substances by differential pulse voltammetry using a microcell for boron-doped diamond electrode and screen-printed electrodes based on carbon and platinum. J. Electroanal. Chem. 2021, 882, 114994. [Google Scholar] [CrossRef]
- Dronova, M.; Smolianitski, E.; Lev, O. Electrooxidation of New Synthetic Cannabinoids: Voltammetric Determination of Drugs in Seized Street Samples and Artificial Saliva. Anal. Chem. 2016, 88, 4487–4494. [Google Scholar] [CrossRef]
- Nissim, R.; Compton, R.G. Absorptive stripping voltammetry for cannabis detection. Chem. Cent. J. 2015, 9, 41. [Google Scholar] [CrossRef]
- Goodchild, S.A.; Hubble, L.J.; Mishra, R.K.; Li, Z.H.; Goud, K.Y.; Barfidokht, A.; Shah, R.; Bagot, K.S.; McIntosh, A.J.S.; Wang, J. Ionic Liquid-Modified Disposable Electrochemical Sensor Strip for Analysis of Fentanyl. Anal. Chem. 2019, 91, 3747–3753. [Google Scholar] [CrossRef]
- Ott, C.; Cunha-Silva, H.; Kuberski, S.L.; Cox, J.A.; Arcos-Martínez, M.J.; Arroyo-Mora, L.E. Electrochemical detection of fentanyl with screen-printed carbon electrodes using square-wave adsorptive stripping voltammetry for forensic applications. J. Electroanal. Chem. 2020, 873, 114425. [Google Scholar] [CrossRef]
- Elbardisy, H.M.; Foster, C.W.; Cumba, L.; Antonides, L.H.; Gilbert, N.; Schofield, C.J.; Belal, T.S.; Talaat, W.; Sutcliffe, O.B.; Daabees, H.G.; et al. Analytical determination of heroin, fentanyl and fentalogues using high-performance liquid chromatography with diode array and amperometric detection. Anal. Methods 2019, 11, 1053–1063. [Google Scholar] [CrossRef]
- Alvarado-Gámez, A.L.; Alonso-Lomillo, M.A.; Dominguez-Renedo, O.; Arcos-Martínez, M.J. A Chronoamperometric Screen Printed Carbon Biosensor Based on Alkaline Phosphatase Inhibition for W(VI) Determination in Water, Using 2-Phospho-l-Ascorbic Acid Trisodium Salt as a Substrate. Sensors 2015, 15, 2232–2243. [Google Scholar] [CrossRef]
- Ferrari, A.G.-M.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Waddell, S.; Fernandez, C.; Inverarity, C.; Prabhu, R. Extending the capability of forensic electrochemistry to the novel psychoactive substance benzylpiperazine. Sens. Bio-Sens. Res. 2017, 13, 28–39. [Google Scholar] [CrossRef]
- Pînzaru, S.C.; Pavel, I.; Leopold, N.; Kiefer, W. Identification and characterization of pharmaceuticals using Raman and surface-enhanced Raman scattering. J. Raman Spectrosc. 2004, 35, 338–346. [Google Scholar] [CrossRef]
- Omar, J.; Slowikowski, B.; Guillou, C.; Reniero, F.; Holland, M.; Boix, A. Identification of new psychoactive substances (NPS) by Raman spectroscopy. J. Raman Spectrosc. 2019, 50, 41–51. [Google Scholar] [CrossRef]
- Fortuni, B.; Fujita, Y.; Ricci, M.; Inose, T.; Aubert, R.; Lu, G.; Hutchison, J.A.; Hofkens, J.; Latterini, L.; Uji-I, H. A novel method for in situ synthesis of SERS-active gold nanostars on polydimethylsiloxane film. Chem. Commun. 2017, 53, 5121–5124. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.Y.F.; Rusin, C.J.; McDermott, M.T. Gold nanostars as a colloidal substrate for in-solution SERS measurements using a handheld Raman spectrometer. Analyst 2020, 145, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Bonnier, F.; Casey, A.; Shanahan, A.E.; Byrne, H.J. Surface enhanced Raman scattering with gold nanoparticles: Effect of particle shape. Anal. Methods 2014, 6, 9116–9123. [Google Scholar] [CrossRef]
- Chen, K.-H.; Pan, M.-J.; Jargalsaikhan, Z.; Ishdorj, T.-O.; Tseng, F.-G. Development of Surface-Enhanced Raman Scattering (SERS)-Based Surface-Corrugated Nanopillars for Biomolecular Detection of Colorectal Cancer. Biosensors 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Herrera, G.M.; Padilla, A.C.; Hernandez-Rivera, S.P. Surface Enhanced Raman Scattering (SERS) Studies of Gold and Silver Nanoparticles Prepared by Laser Ablation. Nanomaterials 2013, 3, 158–172. [Google Scholar] [CrossRef]
- Stevenson, H.; Bacon, A.; Joseph, K.M.; Gwandaru, W.R.W.; Bhide, A.; Sankhala, D.; Dhamu, V.N.; Prasad, S. A Rapid Response Electrochemical Biosensor for Detecting Thc In Saliva. Sci. Rep. 2019, 9, 12701. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hong, S.; Li, X. Effects of cations and anions as aggregating agents on SERS detection of cotinine (COT) and trans-3′-hydroxycotinine (3HC). J. Colloid Interface Sci. 2013, 410, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugan, K.; Squire, K.; Tan, A.; Zhao, Y.; Kraai, J.A.; Rorrer, G.L.; Wang, A.X. Trace Detection of Tetrahydrocannabinol in Body Fluid via Surface-Enhanced Raman Scattering and Principal Component Analysis. ACS Sens. 2019, 4, 1109–1117. [Google Scholar] [CrossRef]
- Urcuyo, R.; Cortes, E.; Rubert, A.A.; Benitez, G.; Montero, M.L.; Tognalli, N.G.; Fainstein, A.; Vela, M.E.; Salvarezza, R.C. Aromatic and Aliphatic Thiol Self-Assembled Monolayers on Au: Anchoring and Delivering Copper Species. J. Phys. Chem. C 2011, 115, 24707–24717. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Pérez-Junquera, A.; González-García, M.B.; Perales-Rondon, J.V.; Heras, A.; Colina, A.; Hernández-Santos, D.; Fanjul-Bolado, P. Quantitative Raman spectroelectrochemistry using silver screen-printed electrodes. Electrochim. Acta 2018, 264, 183–190. [Google Scholar] [CrossRef]
- Zhao, L.; Blackburn, J.; Brosseau, C.L. Quantitative Detection of Uric Acid by Electrochemical-Surface Enhanced Raman Spectroscopy Using a Multilayered Au/Ag Substrate. Anal. Chem. 2014, 87, 441–447. [Google Scholar] [CrossRef]
- Ibáñez, D.; Pérez-Junquera, A.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Spectroelectrochemical elucidation of B vitamins present in multivitamin complexes by EC-SERS. Talanta 2020, 206, 120190. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Pérez-Junquera, A.; Hernández-Santos, D.; Fanjul-Bolado, P. In situ Activation of Thick-film Disposable Copper Electrodes for Sensitive Detection of Malachite Green Using Electrochemical Surface-enhanced Raman Scattering (EC-SERS). Electroanalysis 2018, 30, 1095–1099. [Google Scholar] [CrossRef]
- Moldovan, R.; Vereshchagina, E.; Milenko, K.; Iacob, B.-C.; Bodoki, A.E.; Falamas, A.; Tosa, N.; Muntean, C.M.; Farcău, C.; Bodoki, E. Review on combining surface-enhanced Raman spectroscopy and electrochemistry for analytical applications. Anal. Chim. Acta 2021, 339250. [Google Scholar] [CrossRef]
- Hernández, S.; Perales-Rondon, J.V.; Heras, A.; Colina, A. Electrochemical SERS and SOERS in a single experiment: A new methodology for quantitative analysis. Electrochim. Acta 2020, 334, 135561. [Google Scholar] [CrossRef]
- Wu, D.-Y.; Li, J.-F.; Ren, B.; Tian, Z.-Q. Electrochemical surface-enhanced Raman spectroscopy of nanostructures. Chem. Soc. Rev. 2008, 37, 1025–1041. [Google Scholar] [CrossRef]
- Wu, W.; Liu, L.; Dai, Z.; Liu, J.; Yang, S.; Zhou, L.; Xiao, X.; Jiang, C.; Roy, V.A. Low-Cost, Disposable, Flexible and Highly Reproducible Screen Printed SERS Substrates for the Detection of Various Chemicals. Sci. Rep. 2015, 5, srep10208. [Google Scholar] [CrossRef] [PubMed]
- Dendisová, M.; Němečková, Z.; Člupek, M.; Prokopec, V. EC-SERS study of phenolic acids sorption behavior on Au, Ag and Cu substrates—Effect of applied potential and metal used. Appl. Surf. Sci. 2019, 470, 716–723. [Google Scholar] [CrossRef]
- Li, D.; Li, D.-W.; Fossey, J.S.; Long, Y.-T. Portable Surface-Enhanced Raman Scattering Sensor for Rapid Detection of Aniline and Phenol Derivatives by On-Site Electrostatic Preconcentration. Anal. Chem. 2010, 82, 9299–9305. [Google Scholar] [CrossRef]
- Robinson, A.M.; Harroun, S.G.; Bergman, J.; Brosseau, C.L. Portable Electrochemical Surface-Enhanced Raman Spectroscopy System for Routine Spectroelectrochemical Analysis. Anal. Chem. 2012, 84, 1760–1764. [Google Scholar] [CrossRef]
- Bindesri, S.D.; Jebailey, R.; Albarghouthi, N.; Pye, C.C.; Brosseau, C.L. Spectroelectrochemical and computational studies of tetrahydrocannabinol (THC) and carboxy-tetrahydrocannabinol (THC-COOH). Analyst 2020, 145, 1849–1857. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- ChemAxon. Online Platform for Chemical Calculations. 2021. Available online: https://chemicalize.com/app/calculation (accessed on 1 March 2021).
- Zhang, W.; Bas, A.D.; Ghali, E.; Choi, Y. Passive behavior of gold in sulfuric acid medium. Trans. Nonferrous Met. Soc. China 2015, 25, 2037–2046. [Google Scholar] [CrossRef]
- Burke, L.D.; Nugent, P.F. The electrochemistry of gold: I the redox behaviour of the metal in aqueous media. Gold Bull. 1997, 30, 43–53. [Google Scholar] [CrossRef]
- Vilas-Boas, Â.; Valderrama, P.; Fontes, N.; Geraldo, D.; Bento, F. Evaluation of total polyphenol content of wines by means of voltammetric techniques: Cyclic voltammetry vs. differential pulse voltammetry. Food Chem. 2019, 276, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, S.; Perales-Rondon, J.V.; Arnaiz, A.; Perez-Estebanez, M.; Gomez, E.; Colina, A.; Heras, A. Determination of nicotinamide in a multivitamin complex by electrochemical-surface enhanced Raman spectroscopy. J. Electroanal. Chem. 2020, 879, 114743. [Google Scholar] [CrossRef]
- Cortés, E.; Tognalli, N.G.; Fainstein, A.; Vela, M.E.; Salvarezza, R.C. Ag-modified Au nanocavity SERS substrates. Phys. Chem. Chem. Phys. 2009, 11, 7469–7475. [Google Scholar] [CrossRef] [PubMed]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Moores, A.; Goettmann, F. The plasmon band in noble metal nanoparticles: An introduction to theory and applications. New J. Chem. 2006, 30, 1121–1132. [Google Scholar] [CrossRef]
- Mabbott, S.; Correa, E.; Cowcher, D.P.; Allwood, J.W.; Goodacre, R. Optimization of Parameters for the Quantitative Surface-Enhanced Raman Scattering Detection of Mephedrone Using a Fractional Factorial Design and a Portable Raman Spectrometer. Anal. Chem. 2012, 85, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Christie, R.; Horan, E.; Fox, J.; O’Donnell, C.; Byrne, H.J.; McDermott, S.; Power, J.; Kavanagh, P. Discrimination of cathinone regioisomers, sold as ‘legal highs’, by Raman spectroscopy. Drug Test. Anal. 2014, 6, 651–657. [Google Scholar] [CrossRef]
- Halouzka, V.; Halouzkova, B.; Jirovsky, D.; Hemzal, D.; Ondra, P.; Siranidi, E.; Kontos, A.G.; Falaras, P.; Hrbac, J. Copper nanowire coated carbon fibers as efficient substrates for detecting designer drugs using SERS. Talanta 2017, 165, 384–390. [Google Scholar] [CrossRef]
- Lin, Y.K.; Leong, H.Y.; Ling, T.C.; Lin, D.-Q.; Yao, S.-J. Raman spectroscopy as process analytical tool in downstream processing of biotechnology. Chin. J. Chem. Eng. 2021, 30, 204–211. [Google Scholar] [CrossRef]
- Wang, F.; Cao, S.; Yan, R.; Wang, Z.; Wang, D.; Yang, H. Selectivity/Specificity Improvement Strategies in Surface-Enhanced Raman Spectroscopy Analysis. Sensors 2017, 17, 2689. [Google Scholar] [CrossRef] [PubMed]
- Elie, L.; Elie, M.; Cave, G.; Vetter, M.; Croxton, R.; Baron, M. Microcrystalline testing used in combination with Raman micro-spectroscopy for absolute identification of novel psychoactive substances. J. Raman Spectrosc. 2016, 47, 1343–1350. [Google Scholar] [CrossRef]
- Farquharson, S.; Brouillette, C.; Smith, W.; Shende, C. A Surface-Enhanced Raman Spectral Library of Important Drugs Associated With Point-of-Care and Field Applications. Front. Chem. 2019, 7, 706. [Google Scholar] [CrossRef]
- Jones, L.E.; Stewart, A.; Peters, K.L.; McNaul, M.; Speers, S.J.; Fletcher, N.C.; Bell, S.E.J. Infrared and Raman screening of seized novel psychoactive substances: A large scale study of >200 samples. Analyst 2016, 141, 902–909. [Google Scholar] [CrossRef][Green Version]
- Stewart, S.P.; Bell, S.E.; Fletcher, N.; Bouazzaoui, S.; Ho, Y.C.; Speers, S.; Peters, K.L. Raman spectroscopy for forensic examination of β-ketophenethylamine “legal highs”: Reference and seized samples of cathinone derivatives. Anal. Chim. Acta 2012, 711, 1–6. [Google Scholar] [CrossRef]
- Szaniawska, A.; Kudelski, A. Applications of Surface-Enhanced Raman Scattering in Biochemical and Medical Analysis. Front. Chem. 2021, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Huang, B.-H.; Lin, T.-H. Surface-Enhanced Raman Scattering and Fluorescence on Gold Nanogratings. Nanomaterials 2020, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; Sleegers, N.; Kim, J.; Van Durme, F.; Samyn, N.; Wang, J.; De Wael, K. Electrochemical fingerprint of street samples for fast on-site screening of cocaine in seized drug powders. Chem. Sci. 2016, 7, 2364–2370. [Google Scholar] [CrossRef]
- Olesti, E.; Pujadas, M.; Papaseit, E.; Pérez-Mañá, C.; Pozo, Ó.J.; Farré, M.; de la Torre, R. GC–MS Quantification Method for Mephedrone in Plasma and Urine: Application to Human Pharmacokinetics. J. Anal. Toxicol. 2016, 41, 100–106. [Google Scholar] [CrossRef]
- Ayres, T.C.; Bond, J.W. A chemical analysis examining the pharmacology of novel psychoactive substances freely available over the internet and their impact on public (ill)health. Legal highs or illegal highs? BMJ Open 2012, 2, e000977. [Google Scholar] [CrossRef]



| Matrix. | EC-SERS Band | Raman Intensity RSD (n = 3) |
|---|---|---|
| 4-MMC/Maltose ratio 1:4 | 1605 cm−1 | 1.7% |
| 1213 cm−1 | 14% | |
| 4-MEC/Maltose ratio 1:4 | 1605 cm−1 | 1.4% |
| 1213 cm−1 | 12% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Hernández, J.; Ott, C.E.; Arcos-Martínez, M.J.; Colina, Á.; Heras, A.; Alvarado-Gámez, A.L.; Urcuyo, R.; Arroyo-Mora, L.E. Rapid Determination of the ‘Legal Highs’ 4-MMC and 4-MEC by Spectroelectrochemistry: Simultaneous Cyclic Voltammetry and In Situ Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 295. https://doi.org/10.3390/s22010295
González-Hernández J, Ott CE, Arcos-Martínez MJ, Colina Á, Heras A, Alvarado-Gámez AL, Urcuyo R, Arroyo-Mora LE. Rapid Determination of the ‘Legal Highs’ 4-MMC and 4-MEC by Spectroelectrochemistry: Simultaneous Cyclic Voltammetry and In Situ Surface-Enhanced Raman Spectroscopy. Sensors. 2022; 22(1):295. https://doi.org/10.3390/s22010295
Chicago/Turabian StyleGonzález-Hernández, Jerson, Colby Edward Ott, María Julia Arcos-Martínez, Álvaro Colina, Aránzazu Heras, Ana Lorena Alvarado-Gámez, Roberto Urcuyo, and Luis E. Arroyo-Mora. 2022. "Rapid Determination of the ‘Legal Highs’ 4-MMC and 4-MEC by Spectroelectrochemistry: Simultaneous Cyclic Voltammetry and In Situ Surface-Enhanced Raman Spectroscopy" Sensors 22, no. 1: 295. https://doi.org/10.3390/s22010295
APA StyleGonzález-Hernández, J., Ott, C. E., Arcos-Martínez, M. J., Colina, Á., Heras, A., Alvarado-Gámez, A. L., Urcuyo, R., & Arroyo-Mora, L. E. (2022). Rapid Determination of the ‘Legal Highs’ 4-MMC and 4-MEC by Spectroelectrochemistry: Simultaneous Cyclic Voltammetry and In Situ Surface-Enhanced Raman Spectroscopy. Sensors, 22(1), 295. https://doi.org/10.3390/s22010295







