A Wearable Biofeedback Device to Increase Gait Swing Time Could Have Positive Effects on Gait among Older Adults
Abstract
:1. Introduction
2. Materials and Methods
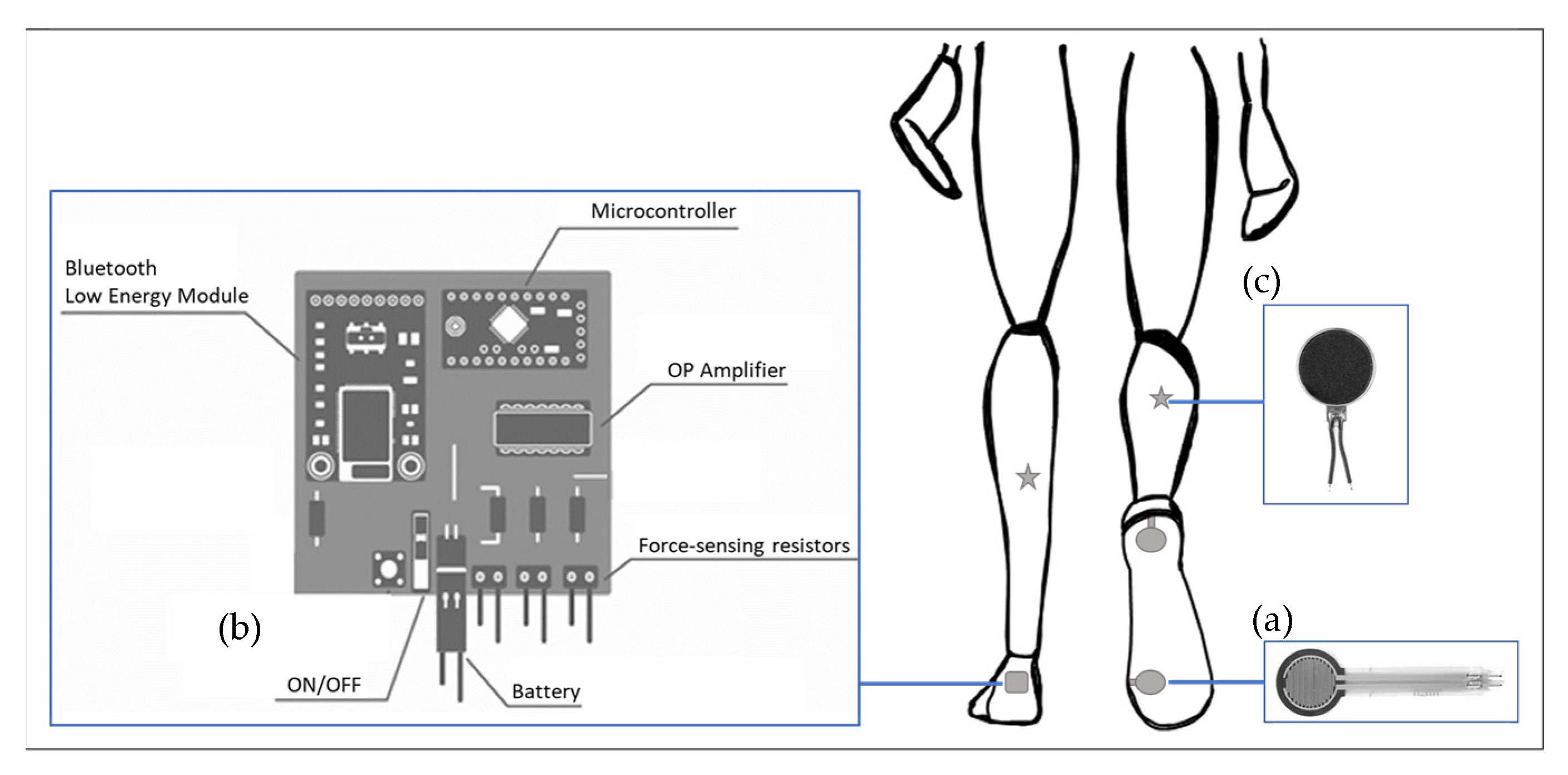
2.1. Smart Insole System
2.2. Data Acquisition from the Smart Insole System
2.3. Participants
2.4. Experimental Design
2.5. Variables and Data Sources from Reference Systems
2.5.1. Gait Data Collection
2.5.2. Walking Mobility Test
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voukelatos, A.; Merom, D.; Rissel, C.; Sherrington, C.; Watson, W.; Waller, K. The effect of walking on falls in older people: The ’Easy Steps to Health’ randomized controlled trial study protocol. BMC Public Health 2011, 11, 888. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.S.; Kim, E.Y. Comparing self-selected speed walking of the elderly with self-selected slow, moderate, and fast speed walking of young adults. Ann. Rehabil. Med. 2014, 38, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silder, A.; Heiderscheit, B.; Thelen, D.G. Active and passive contributions to joint kinetics during walking in older adults. J. Biomech. 2008, 41, 1520–1527. [Google Scholar] [CrossRef] [Green Version]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Age-related differences in walking stability. Age Ageing 2003, 32, 137–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubenstein, L.Z. Falls in older people: Epidemiology, risk factors and strategies for prevention. Age Ageing 2006, 35, ii37–ii41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callisaya, M.L.; Blizzard, L.; Schmidt, M.D.; McGinley, J.L.; Srikanth, V.K. Ageing and gait variability-a population-based study of older people. Age Ageing 2010, 39, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W.; Verghese, J.; Holtzer, R.; Mahoney, J.R.; Oh-Park, M. Trunk sway during walking among older adults: Norms and correlation with gait velocity. Gait Posture 2014, 40, 676–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerrigan, D.C.; Todd, M.K.; Della Croce, U.; Lipsitz, L.A.; Collins, J.J. Biomechanical gait alterations independent of speed in the healthy elderly: Evidence for specific limiting impairments. Arch. Phys Med. Rehabil. 1998, 79, 317–322. [Google Scholar] [CrossRef]
- Kirkwood, R.N.; de Souza Moreira, B.; Vallone, M.L.; Mingoti, S.A.; Dias, R.C.; Sampaio, R.F. Step length appears to be a strong discriminant gait parameter for elderly females highly concerned about falls: A cross-sectional observational study. Physiotherapy 2011, 97, 126–131. [Google Scholar] [CrossRef]
- Elhadi, M.M.O.; Ma, C.Z.; Wong, D.W.C.; Wan, A.H.P.; Lee, W.C.C. Comprehensive Gait Analysis of Healthy Older Adults Who Have Undergone Long-Distance Walking. J. Aging Phys. Act. 2017, 25, 367–377. [Google Scholar] [CrossRef]
- McAndrew Young, P.M.; Dingwell, J.B. Voluntarily changing step length or step width affects dynamic stability of human walking. Gait Posture 2012, 35, 472–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirker, W.; Katzenschlager, R. Gait disorders in adults and the elderly: A clinical guide. Wien. Klin. Wochenschr. 2017, 129, 81–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.E.; Madigan, M.L. Healthy older adults have insufficient hip range of motion and plantar flexor strength to walk like healthy young adults. J. Biomech. 2014, 47, 1104–1109. [Google Scholar] [CrossRef] [Green Version]
- Boyer, K.A.; Johnson, R.T.; Banks, J.J.; Jewell, C.; Hafer, J.F. Systematic review and meta-analysis of gait mechanics in young and older adults. Exp. Gerontol. 2017, 95, 63–70. [Google Scholar] [CrossRef]
- Atkinson, H.H.; Rosano, C.; Simonsick, E.M.; Williamson, J.D.; Davis, C.; Ambrosius, W.T.; Rapp, S.R.; Cesari, M.; Newman, A.B.; Harris, T.B.; et al. Cognitive Function, Gait Speed Decline, and Comorbidities: The Health, Aging and Body Composition Study. J. Gerontol. Ser. A 2007, 62, 844–850. [Google Scholar] [CrossRef] [Green Version]
- Judge, J.O.; Davis, R.B., 3rd; Ounpuu, S. Step length reductions in advanced age: The role of ankle and hip kinetics. J. Gerontol. A Biol. Sci. Med. Sci. 1996, 51, M303–M312. [Google Scholar] [CrossRef]
- Eikema, D.J.; Forrester, L.W.; Whitall, J. Manipulating the stride length/stride velocity relationship of walking using a treadmill and rhythmic auditory cueing in non-disabled older individuals. A short-term feasibility study. Gait Posture 2014, 40, 712–714. [Google Scholar] [CrossRef] [Green Version]
- Jerome, G.J.; Ko, S.-u.; Kauffman, D.; Studenski, S.A.; Ferrucci, L.; Simonsick, E.M. Gait characteristics associated with walking speed decline in older adults: Results from the Baltimore Longitudinal Study of Aging. Arch. Gerontol. Geriatr. 2015, 60, 239–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persch, L.N.; Ugrinowitsch, C.; Pereira, G.; Rodacki, A.L.F. Strength training improves fall-related gait kinematics in the elderly: A randomized controlled trial. Clin. Biomech. 2009, 24, 819–825. [Google Scholar] [CrossRef]
- Buesing, C.; Fisch, G.; O’Donnell, M.; Shahidi, I.; Thomas, L.; Mummidisetty, C.K.; Williams, K.J.; Takahashi, H.; Rymer, W.Z.; Jayaraman, A. Effects of a wearable exoskeleton stride management assist system (SMA®) on spatiotemporal gait characteristics in individuals after stroke: A randomized controlled trial. J. NeuroEng. Rehabil. 2015, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Tate, J.J.; Milner, C.E. Real-Time Kinematic, Temporospatial, and Kinetic Biofeedback During Gait Retraining in Patients: A Systematic Review. Phys. Ther. 2007, 90, 1123–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluge, F.; Gaßner, H.; Hannink, J.; Pasluosta, C.; Klucken, J.; Eskofier, B.M. Towards Mobile Gait Analysis: Concurrent Validity and Test-Retest Reliability of an Inertial Measurement System for the Assessment of Spatio-Temporal Gait Parameters. Sensors 2017, 17, 1525. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait Analysis Using Wearable Sensors. Sensors 2012, 12, 2255. [Google Scholar] [CrossRef]
- Bamberg, S.J.M.; Benbasat, A.Y.Y.; Scarborough, D.M.M.; Krebs, D.E.E.; Paradiso, J.A.A. Gait Analysis Using a Shoe-Integrated Wireless Sensor System. IEEE Trans. Inf. Technol. Biomed. 2008, 12, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Afiah, I.N.; Nakashima, H.; Loh, P.Y.; Muraki, S. An exploratory investigation of changes in gait parameters with age in elderly Japanese women. Springerplus 2016, 5, 1069. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Youm, C.; Park, H.; Lee, M.; Noh, B. Characteristics of Gait Variability in the Elderly While Walking on a Treadmill with Gait Speed Variation. Int. J. Environ. Res. Public Health 2021, 18, 4704. [Google Scholar] [CrossRef]
- Osoba, M.Y.; Rao, A.K.; Agrawal, S.K.; Lalwani, A.K. Balance and gait in the elderly: A contemporary review. Laryngoscope Investig. Otolaryngol. 2019, 4, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, P.M.; Barrett, R.S. Swing phase mechanics of healthy young and elderly men. Hum. Mov. Sci. 2001, 20, 427–446. [Google Scholar] [CrossRef]
- Cudejko, T.; Button, K.; Willott, J.; Al-Amri, M. Applications of Wearable Technology in a Real-Life Setting in People with Knee Osteoarthritis: A Systematic Scoping Review. J. Clin. Med. 2021, 10, 5645. [Google Scholar] [CrossRef]
- Small, S.R.; Bullock, G.S.; Khalid, S.; Barker, K.; Trivella, M.; Price, A.J. Current clinical utilisation of wearable motion sensors for the assessment of outcome following knee arthroplasty: A scoping review. BMJ. Open 2019, 9, e033832. [Google Scholar] [CrossRef] [Green Version]
- Mariani, B.; Hoskovec, C.; Rochat, S.; Büla, C.; Penders, J.; Aminian, K. 3D gait assessment in young and elderly subjects using foot-worn inertial sensors. J. Biomech. 2010, 43, 2999–3006. [Google Scholar] [CrossRef]
- Layne, C.S.; Malaya, C.A.; Levine, J.T. The effects of muscle vibration on gait control: A review. Somatosens. Mot. Res. 2019, 36, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Grasso, R.; Lacquaniti, F. Influence of Leg Muscle Vibration on Human Walking. J. Neurophysiol. 2000, 84, 1737–1747. [Google Scholar] [CrossRef]
- Ma, C.Z.-H.; Lee, W.C.-C. A wearable vibrotactile biofeedback system improves balance control of healthy young adults following perturbations from quiet stance. Hum. Mov. Sci. 2017, 55, 54–60. [Google Scholar] [CrossRef]
- Creaby, M.W.; Franettovich Smith, M.M. Retraining running gait to reduce tibial loads with clinician or accelerometry guided feedback. J. Sci. Med. Sport. 2016, 19, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Lisini Baldi, T.; Aggravi, M.; Ulivelli, M.; Cioncoloni, D.; Niccolini, V.; Donati, L.; Prattichizzo, D. Wearable haptic anklets for gait and freezing improvement in Parkinson’s disease: A proof-of-concept study. Neurol. Sci. 2020, 41, 3643–3651. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, P.; Lorenzoni, V.; Derie, R.; Six, J.; Gerlo, J.; Leman, M.; De Clercq, D. Music-based biofeedback to reduce tibial shock in over-ground running: A proof-of-concept study. Sci. Rep. 2021, 11, 4091. [Google Scholar] [CrossRef]
- Matsuda, M.; Mataki, Y.; Mutsuzaki, H.; Yoshikawa, K.; Takahashi, K.; Enomoto, K.; Sano, K.; Mizukami, M.; Tomita, K.; Ohguro, H.; et al. Immediate effects of a single session of robot-assisted gait training using Hybrid Assistive Limb (HAL) for cerebral palsy. J. Phys. Ther. Sci. 2018, 30, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Al-Amri, M.; Nicholas, K.; Button, K.; Sparkes, V.; Sheeran, L.; Davies, J.L. Inertial Measurement Units for Clinical Movement Analysis: Reliability and Concurrent Validity. Sensors 2018, 18, 719. [Google Scholar] [CrossRef] [Green Version]
- Ritt, M.; Schülein, S.; Lubrich, H.; Bollheimer, L.C.; Sieber, C.C.; Gaßmann, K.G. High-Technology Based Gait Assessment in Frail People: Associations between Spatio-Temporal and Three-Dimensional Gait Characteristics with Frailty Status across Four Different Frailty Measures. J. Nutr. Health Aging 2017, 21, 346–353. [Google Scholar] [CrossRef]
- Barry, E.; Galvin, R.; Keogh, C.; Horgan, F.; Fahey, T. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta-analysis. BMC Geriatr. 2014, 14, 14. [Google Scholar] [CrossRef]
- Pondal, M.; del Ser, T. Normative Data and Determinants for the Timed “Up and Go” Test in a Population-Based Sample of Elderly Individuals Without Gait Disturbances. J. Geriatr. Phys. Ther. 2008, 31, 57–63. [Google Scholar] [CrossRef]
- Kurosawa, C.; Shimazu, N.; Yamamoto, S. Where do healthy older adults take more time during the Timed Up and Go test? J. Phys. Sci. 2020, 32, 663–668. [Google Scholar] [CrossRef]
- Ibrahim, A.; Singh, D.K.A.; Shahar, S. ‘Timed Up and Go’ test: Age, gender and cognitive impairment stratified normative values of older adults. PLoS ONE 2017, 12, e0185641. [Google Scholar] [CrossRef]
- Pereiro, A.X.; Campos-Magdaleno, M.; Navarro-Pardo, E.; Juncos-Rabadán, O.; Facal, D. Normative scores for the Timed Up & Go in a Spanish sample of community-dweller adults with preserved functionality. Atención Primaria 2021, 53, 102065. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Brouwer, B.J.; Culham, E.G. Use of Visual Feedback in Retraining Balance Following Acute Stroke. Phys. Ther. 2000, 80, 886–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, P.P.; Si Tou, J.I.; Tse, M.M.; Ng, S.S. Reliability and Validity of the Timed Up and Go Test With a Motor Task in People With Chronic Stroke. Arch. Phys. Med. Rehabil. 2017, 98, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Vítečková, S.; Horáková, H.; Poláková, K.; Krupička, R.; Růžička, E.; Brožová, H. Agreement between the GAITRite(®) System and the Wearable Sensor BTS G-Walk(®) for measurement of gait parameters in healthy adults and Parkinson’s disease patients. PeerJ 2020, 8, e8835. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.; Newell, K.M. Intraindividual Variability of Neuromotor Function Predicts Falls Risk in Older Adults and those with Type 2 Diabetes. J. Mot. Behav. 2019, 51, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Kluft, N.; van Dieën, J.H.; Pijnappels, M. The degree of misjudgment between perceived and actual gait ability in older adults. Gait Posture 2017, 51, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Morio, Y.; Izawa, K.P.; Omori, Y.; Katata, H.; Ishiyama, D.; Koyama, S.; Yamano, Y. The Relationship between Walking Speed and Step Length in Older Aged Patients. Diseases 2019, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, E.J.; Merbitz, C.; Mroczek, K.; Dugan, S.A.; Suh, W.W. HEMIPLEGIC GAIT: Relationships Between Walking Speed and Other Temporal Parameters: 1. Am. J. Phys. Med. Rehabil. 1997, 76, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.P.; Kory, R.C.; Clarkson, B.H. Walking patterns in healthy old men. J. Gerontol. 1969, 24, 169–178. [Google Scholar] [CrossRef]
- Miyake, T.; Tsukune, M.; Kobayashi, Y.; Sugano, S.; Fujie, M.G. Relationship between magnitude of applied torque in pre-swing phase and gait change for prevention of trip in elderly people. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 6154–6157. [Google Scholar]
- Tudor-Locke, C.; Rowe, D.A. Using cadence to study free-living ambulatory behaviour. Sports Med. 2012, 42, 381–398. [Google Scholar] [CrossRef]
- Alcock, L.; Vanicek, N.; O’Brien, T.D. Alterations in gait speed and age do not fully explain the changes in gait mechanics associated with healthy older women. Gait Posture 2013, 37, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, T.; Woodward, J.; Wu, M.M.; Jackson, B.; Souza, P.; Siegel, J.; Dhar, S.; Gordon, K.E. Walking With Ears: Altered Auditory Feedback Impacts Gait Step Length in Older Adults. Front. Sports Act. Living 2020, 2, 38. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, K.; Hayashi, Y.; Tawara, A.; Iwata, H. Development of a vibratory cueing system using an implicit method to increase walking speed in patients with stroke: A proof-of-concept study. ROBOMECH J. 2020, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Han, D.; Kim, J.; Lee, S. Whole-Body Vibration Combined with Treadmill Training Improves Walking Performance in Post-Stroke Patients: A Randomized Controlled Trial. Med. Sci. Monit. 2017, 23, 4918–4925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Iersel, M.B.; Munneke, M.; Esselink, R.A.J.; Benraad, C.E.M.; Olde Rikkert, M.G.M. Gait velocity and the Timed-Up-and-Go test were sensitive to changes in mobility in frail elderly patients. J. Clin. Epidemiol. 2008, 61, 186–191. [Google Scholar] [CrossRef]
- Drużbicki, M.; Przysada, G.; Guzik, A.; Brzozowska-Magoń, A.; Kołodziej, K.; Wolan-Nieroda, A.; Majewska, J.; Kwolek, A. The Efficacy of Gait Training Using a Body Weight Support Treadmill and Visual Biofeedback in Patients with Subacute Stroke: A Randomized Controlled Trial. BioMed. Res. Int. 2018, 2018, 3812602. [Google Scholar] [CrossRef] [Green Version]
- Soangra, R.; Lockhart, T.E. Dual-Task Does Not Increase Slip and Fall Risk in Healthy Young and Older Adults during Walking. Appl. Bionics Biomech. 2017, 2017, 1014784. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Duysens, J. Significance of load receptor input during locomotion: A review. Gait Posture 2000, 11, 102–110. [Google Scholar] [CrossRef]


| Parameter | Without B ± SD | 95%CI | Biofeedback ± SD | 95%CI | p-Value |
|---|---|---|---|---|---|
| Cadence | 119.400 ± 7.20 | 112.74–126.06 | 112.826 ± 10.29 | 103.31–122.34 | 0.000 * |
| Swing Time (s) | 0.38 ± 0.03 | [0.37, 0.39] | 0.40 ± 0.05 | [0.39, 0.42] | 0.000 * |
| Stride Length(cm) | 135.75 ± 11.30 | 125.30–146.20 | 141.88 ± 12.24 | 130.57–153.20 | 0.022 * |
| Hip Flexion | 27.1831 ± 4.08 | 23.40–30.96 | 31.189 ± 5.81 | 25.75–36.62 | 0.030 * |
| Parameter | Without B ± SD | 95%CI | Biofeedback ± SD | 95%CI |
|---|---|---|---|---|
| Velocity (cm/s) | 134.557 ± 9.44 | [125.82, 143.29] | 131.979 ± 12.21 | [120.68, 143.28] |
| Norm Velocity | 1.570 ± 0.184 | [1.40,1.74] | 1.539 ± 0.181 | [1.371, 1.71] |
| Peak Joint angle | ||||
| Hip Extension | −11.88 ± 3.88 | [−15.47, −8.30] | −14.61 ± 5.19 | [−19.42, −9.81] |
| Knee Flexion | 63.14 ± 5.17 | [58.35, 67.92] | 65.75 ± 8.27 | [58.09, 73.40] |
| Knee Extension | −4.58 ± 2.52 | [−6.91, −2.25] | −3.72 ± 2.52 | [−7.16, −0.29] |
| Dorsiflexion | 12.61 ± 2.82 | [10.00, 15.22] | 13.08 ± 2.95 | [10.35, 15.80] |
| Plantarflexion | −20.12 ± 3.53 | [−23.39, −16.85] | −20.77 ± 4.56 | [−24.99, −16.55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraldo-Pedroza, A.; Lee, W.C.-C.; Lam, W.-K.; Coman, R.; Alici, G. A Wearable Biofeedback Device to Increase Gait Swing Time Could Have Positive Effects on Gait among Older Adults. Sensors 2022, 22, 102. https://doi.org/10.3390/s22010102
Giraldo-Pedroza A, Lee WC-C, Lam W-K, Coman R, Alici G. A Wearable Biofeedback Device to Increase Gait Swing Time Could Have Positive Effects on Gait among Older Adults. Sensors. 2022; 22(1):102. https://doi.org/10.3390/s22010102
Chicago/Turabian StyleGiraldo-Pedroza, Alexandra, Winson Chiu-Chun Lee, Wing-Kai Lam, Robyn Coman, and Gursel Alici. 2022. "A Wearable Biofeedback Device to Increase Gait Swing Time Could Have Positive Effects on Gait among Older Adults" Sensors 22, no. 1: 102. https://doi.org/10.3390/s22010102
APA StyleGiraldo-Pedroza, A., Lee, W. C.-C., Lam, W.-K., Coman, R., & Alici, G. (2022). A Wearable Biofeedback Device to Increase Gait Swing Time Could Have Positive Effects on Gait among Older Adults. Sensors, 22(1), 102. https://doi.org/10.3390/s22010102








