Printed Textile-Based Ag2O–Zn Battery for Body Conformal Wearable Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used
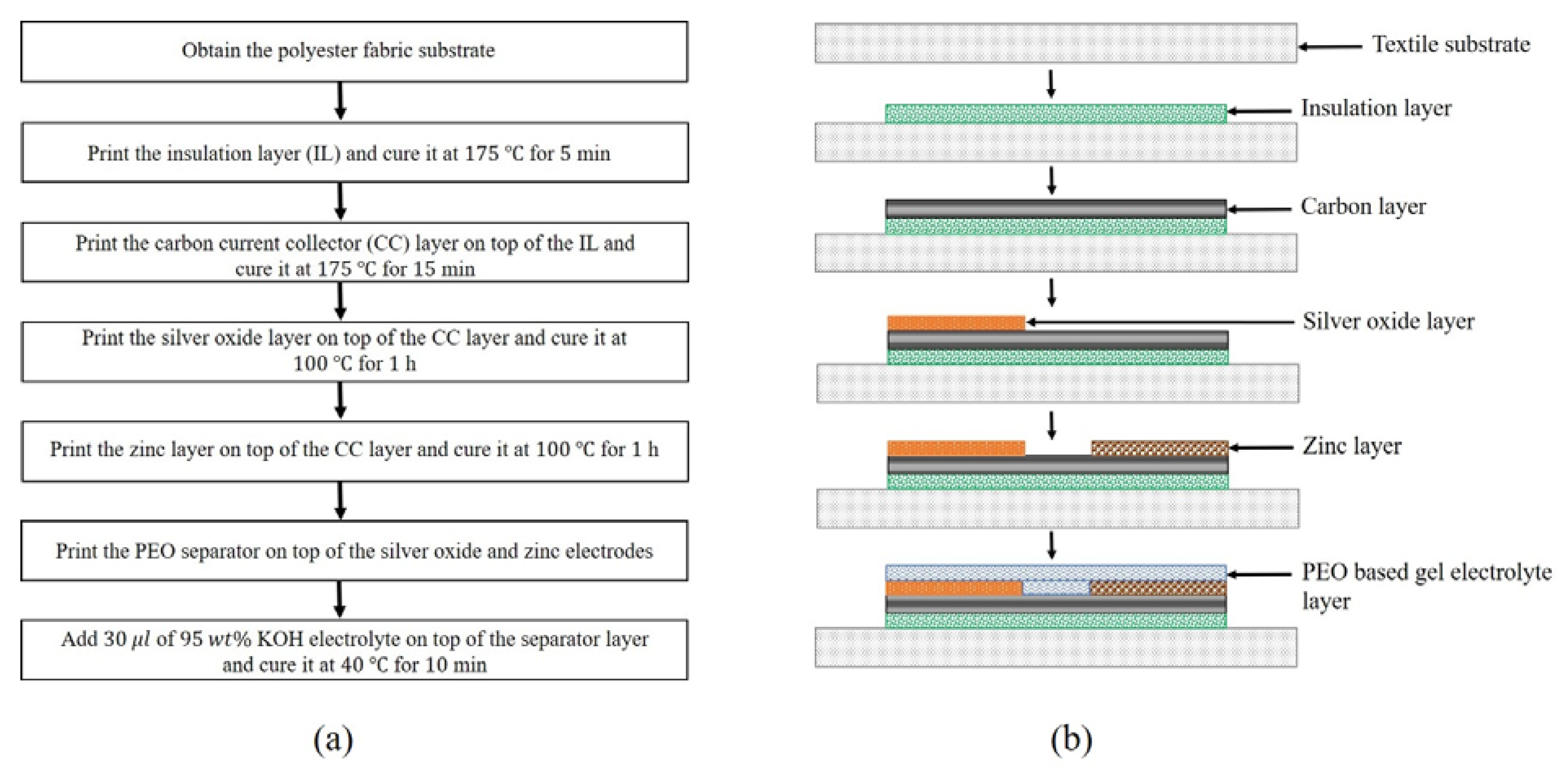
2.2. Battery Fabrication
2.3. Theoretical Calculations
2.4. Characterization Methods
3. Results
3.1. HRSEM Characterization
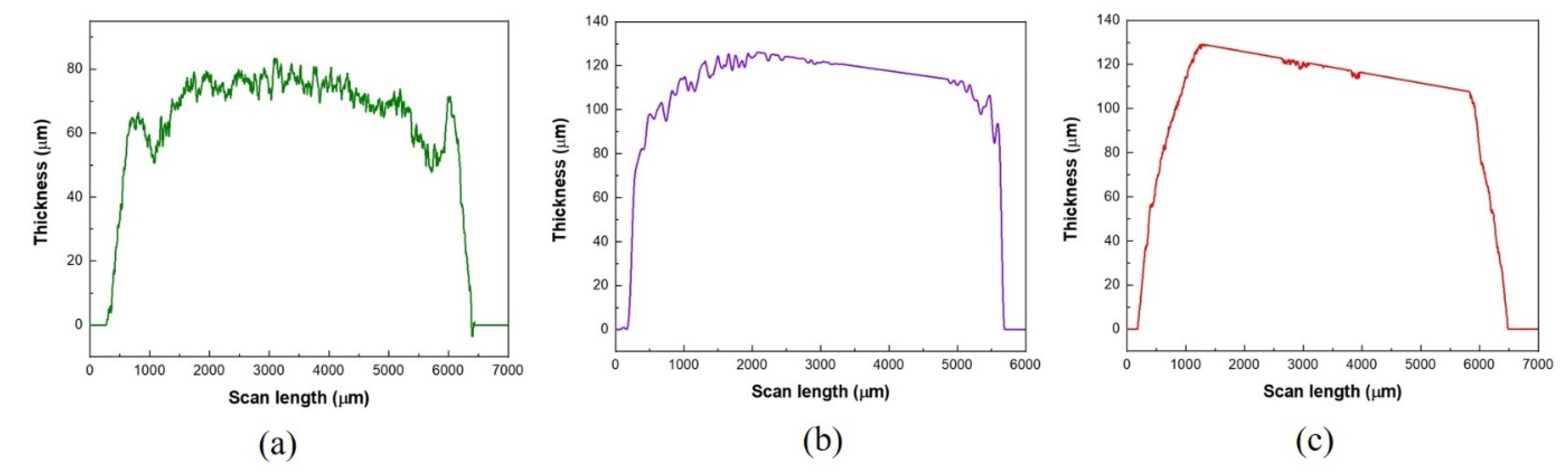
3.2. Surface Profiler Characterization
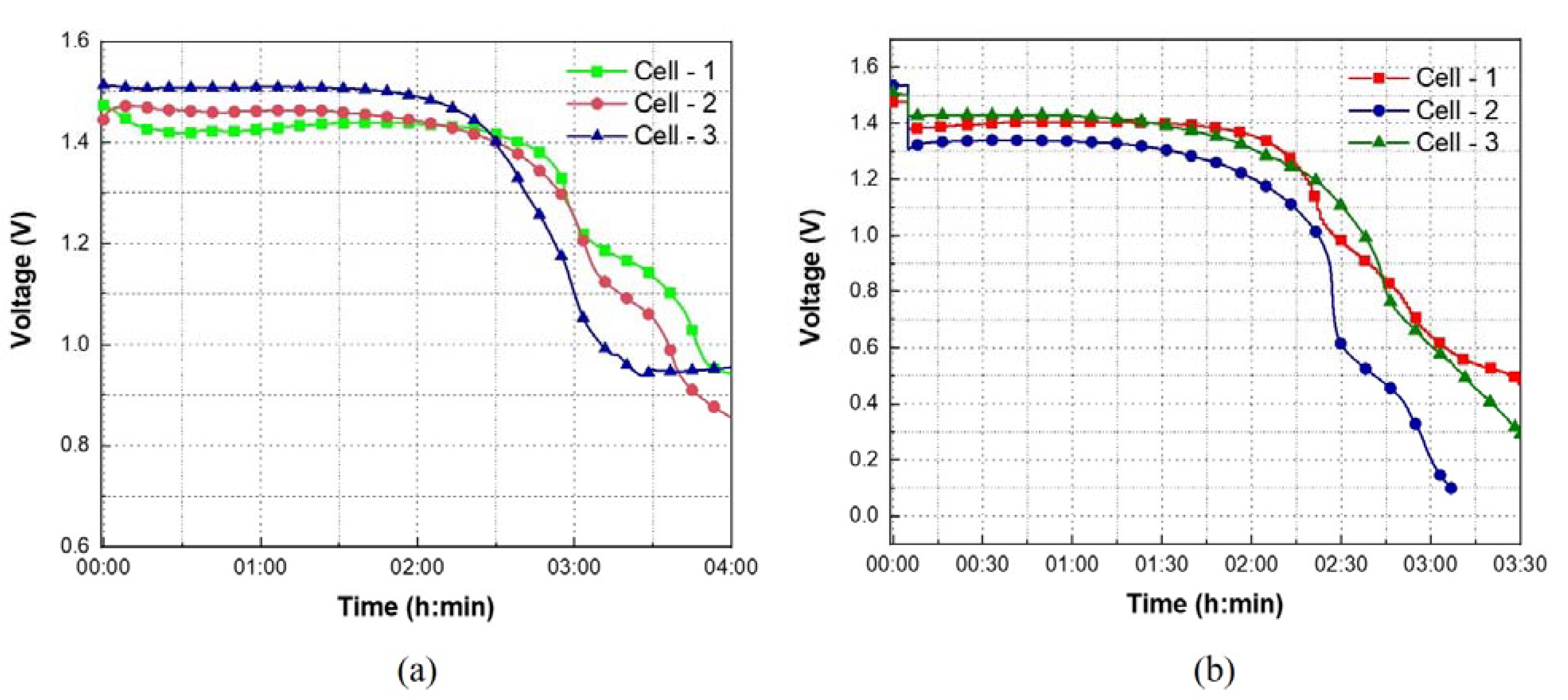
3.3. OCV and Discharge Profiles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, S.; Hong, S.; Kim, J.; Lee, S.; Hyeon, T.; Lee, M.; Kim, D. Wearable Fall Detector using Integrated Sensors and Energy Devices. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lanata, A.; Valenza, G.; Nardelli, M.; Gentili, C.; Scilingo, E.P. Complexity Index from a Personalized Wearable Monitoring System for Assessing Remission in Mental Health. IEEE J. Biomed. Health Inform. 2014, 19, 132–139. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart Bandages: The Future of Wound Care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.; Takahata, K. A Hydrogel-Based Passive Wireless Sensor using a Flex-Circuit Inductive Transducer. Sens. Actuators Phys. 2009, 155, 58–65. [Google Scholar] [CrossRef]
- Punjiya, M.; Rezaei, H.; Zeeshan, M.A.; Sonkusale, S. A Flexible pH Sensing Smart Bandage with Wireless CMOS Readout for Chronic Wound Monitoring. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017; pp. 1700–1702. [Google Scholar] [CrossRef]
- Qin, M.; Guo, H.; Dai, Z.; Yan, X.; Ning, X. Advances in Flexible and Wearable pH Sensors for Wound Healing Monitoring. J. Semicond. 2019, 40, 111607. [Google Scholar] [CrossRef]
- Scott, C.; Cameron, S.; Cundell, J.; Mathur, A.; Davis, J. Adapting Resistive Sensors for Monitoring Moisture in Smart Wound Dressings. Curr. Opin. Electrochem. 2020, 23, 31–35. [Google Scholar] [CrossRef]
- Milne, S.D.; Seoudi, I.; Al Hamad, H.; Talal, T.K.; Anoop, A.A.; Allahverdi, N.; Zakaria, Z.; Menzies, R.; Connolly, P. A Wearable Wound Moisture Sensor as an Indicator for Wound Dressing Change: An Observational Study of Wound Moisture and Status. Int. Wound J. 2016, 13, 1309–1314. [Google Scholar] [CrossRef]
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B. Smart Bandage for Monitoring and Treatment of Chronic Wounds. Small 2018, 14, 1703509. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, M.F.; Shamim, A. Low Cost Inkjet Printed Smart Bandage for Wireless Monitoring of Chronic Wounds. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Olgun, U.; Chen, C.; Volakis, J.L. Investigation of Rectenna Array Configurations for Enhanced RF Power Harvesting. IEEE Antennas Wirel. Propag. Lett. 2011, 10, 262–265. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, J.; Lin, H.; Huang, W. Flexible Fiber and Fabric Batteries. Adv. Mater. Technol. 2018, 3, 1700302. [Google Scholar] [CrossRef]
- Di, J.; Zhang, X.; Yong, Z.; Zhang, Y.; Li, D.; Li, R.; Li, Q. Carbon-nanotube Fibers for Wearable Devices and Smart Textiles. Adv. Mater. 2016, 28, 10529–10538. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.J.; Hong, W.G.; Kim, W.; Jun, Y.; Kim, B.H. A Novel Method for Applying Reduced Graphene Oxide Directly to Electronic Textiles from Yarns to Fabrics. Adv. Mater. 2013, 25, 5701–5705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, W.; Ren, J.; Weng, W.; Lin, H.; Zhang, Z.; Peng, H. Super-Stretchy Lithium-Ion Battery Based on Carbon Nanotube Fiber. J. Mater. Chem. 2014, 2, 11054–11059. [Google Scholar] [CrossRef]
- Xiong, P.; Peng, L.; Chen, D.; Zhao, Y.; Wang, X.; Yu, G. Two-Dimensional Nanosheets Based Li-Ion Full Batteries with high Rate Capability and Flexibility. Nano Energy 2015, 12, 816–823. [Google Scholar] [CrossRef]
- Yan, C.; Wang, X.; Cui, M.; Wang, J.; Kang, W.; Foo, C.Y.; Lee, P.S. Stretchable Silver-zinc Batteries Based on Embedded Nanowire Elastic Conductors. Adv. Energy Mater. 2014, 4, 1301396. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Han, J.; Zhang, X.; Sun, X.; Li, C.; Liu, W.; Li, Q.; Ma, Y. High-Performance Cable-Type Flexible Rechargeable Zn Battery Based on MnO2@ CNT Fiber Microelectrode. ACS Appl. Mater. Interfaces 2018, 10, 24573–24582. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, C.; Kim, J.H.; de Andrade, M.J.; Baughman, R.H.; Kim, S.J. Biscrolled Carbon Nanotube Yarn Structured Silver-Zinc Battery. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Kumar, R.; Shin, J.; Yin, L.; You, J.; Meng, Y.S.; Wang, J. All-printed, Stretchable Zn-Ag2O Rechargeable Battery Via Hyperelastic Binder for Self-powering Wearable Electronics. Adv. Energy Mater. 2017, 7, 1602096. [Google Scholar] [CrossRef]
- Lewandowski, A.; Skorupska, K.; Malinska, J. Novel Poly (Vinyl Alcohol)–KOH–H2O Alkaline Polymer Electrolyte. Solid State Ion. 2000, 133, 265–271. [Google Scholar] [CrossRef]
- Dirkse, T.P.; Vander Lugt, L.A.; Schnyders, H. The Reaction of Silver Oxides with Potassium Hydroxide. J. Inorg. Nucl. Chem. 1963, 25, 859–865. [Google Scholar] [CrossRef]
- Lorca, S.; Santos, F.; Fernández Romero, A.J. A Review of the use of GPEs in Zinc-Based Batteries. A Step Closer to Wearable Electronic Gadgets and Smart Textiles. Polymers 2020, 12, 2812. [Google Scholar] [CrossRef]
- Wang, Z.; Winslow, R.; Madan, D.; Wright, P.K.; Evans, J.W.; Keif, M.; Rong, X. Development of MnO2 Cathode Inks for Flexographically Printed Rechargeable Zinc-Based Battery. J. Power Sources 2014, 268, 246–254. [Google Scholar] [CrossRef]
- Braam, K.T.; Volkman, S.K.; Subramanian, V. Characterization and Optimization of a Printed, Primary Silver–zinc Battery. J. Power Sources 2012, 199, 367–372. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Steingart, D.A.; Nga, N.T.; Schwartz, D.E.; Whiting, G.L. A Flexible High Potential Printed Battery for Powering Printed Electronics. Appl. Phys. Lett. 2013, 102, 104. [Google Scholar] [CrossRef]
- Yokus, M.A.; Foote, R.J.J.S. Printed Stretchable Interconnects for Smart Garments: Design, Fabrication and Characterization. IEEE Sens. J. 2016, 16, 7967–7976. [Google Scholar] [CrossRef]


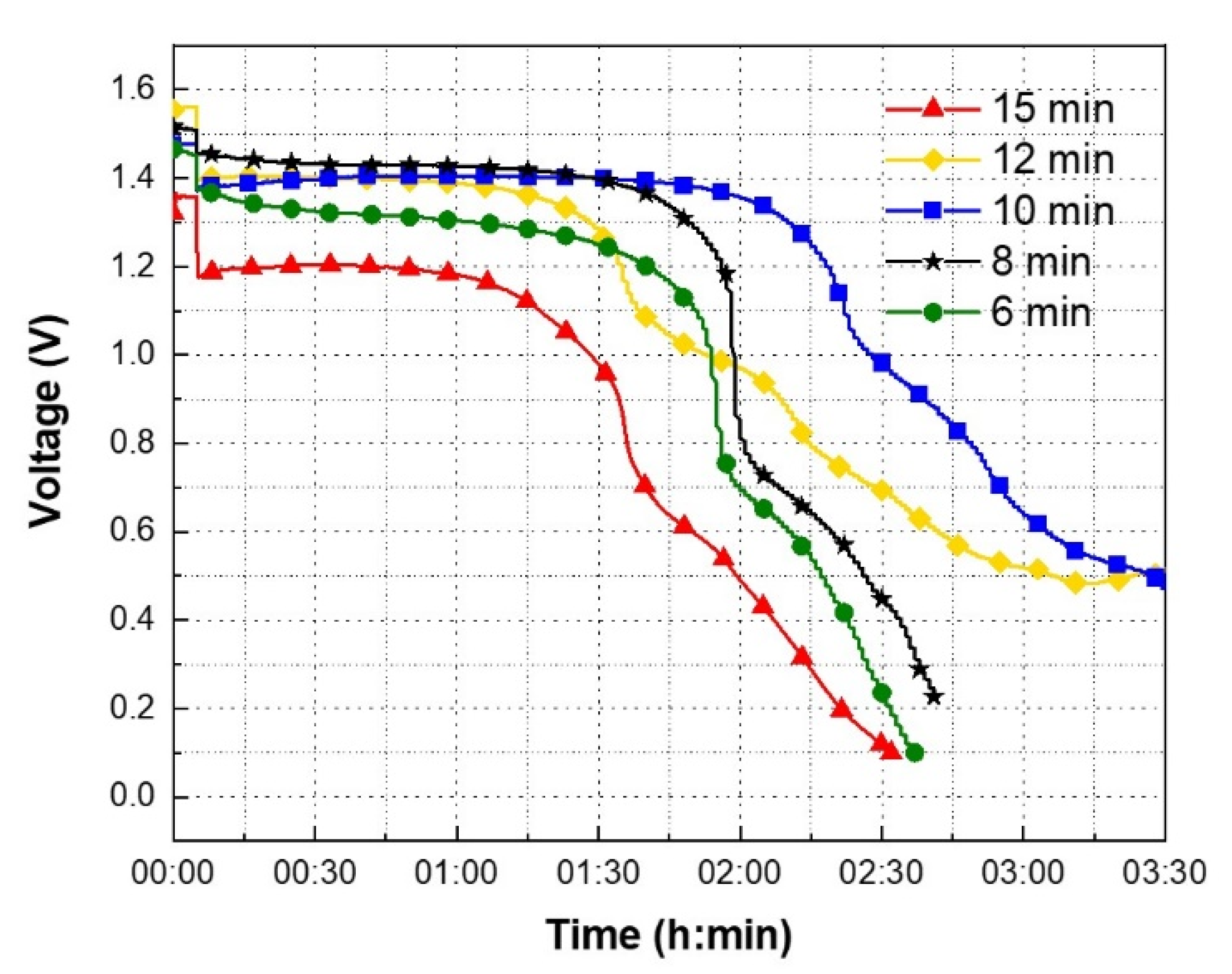
| Drying Time | OCV (V1) | Voltage at 6 min (V2) | Voltage at 135 min (V3) | ΔV (%) |
|---|---|---|---|---|
| 6 min | 1.45 V | 1.39 V | 0.44 V | 68.35% |
| 8 min | 1.51 V | 1.47 V | 0.58 V | 60.54% |
| 10 min | 1.47 V | 1.39 V | 1.18 V | 15.11% |
| 12 min | 1.56 V | 1. 42 V | 0.75 V | 47.18% |
| 15 min | 1.35 V | 1.24 V | 0.22 V | 82.26% |
| Battery | Separator Material | Binder Material | Printing Method | Aerial Capacity (mAh/cm2) |
|---|---|---|---|---|
| Ag2O-Zn | PEO | PVDF | Stencil printing | 0.6 [This work] |
| MnO2-Zn | PVA/Cellulose | Styrene-butadiene rubber | Stencil printing | 0.8 [26] |
| Ag2O-Zn | PAA | Polystyrene-block-polyisoprene-block-polystyrene (SIS) | Screen printing | 2.5 [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kota, A.; Gogia, A.; Neidhard-Doll, A.T.; Chodavarapu, V.P. Printed Textile-Based Ag2O–Zn Battery for Body Conformal Wearable Sensors. Sensors 2021, 21, 2178. https://doi.org/10.3390/s21062178
Kota A, Gogia A, Neidhard-Doll AT, Chodavarapu VP. Printed Textile-Based Ag2O–Zn Battery for Body Conformal Wearable Sensors. Sensors. 2021; 21(6):2178. https://doi.org/10.3390/s21062178
Chicago/Turabian StyleKota, Akash, Ashish Gogia, Amy T. Neidhard-Doll, and Vamsy P. Chodavarapu. 2021. "Printed Textile-Based Ag2O–Zn Battery for Body Conformal Wearable Sensors" Sensors 21, no. 6: 2178. https://doi.org/10.3390/s21062178
APA StyleKota, A., Gogia, A., Neidhard-Doll, A. T., & Chodavarapu, V. P. (2021). Printed Textile-Based Ag2O–Zn Battery for Body Conformal Wearable Sensors. Sensors, 21(6), 2178. https://doi.org/10.3390/s21062178








