Effect of a Brain–Computer Interface Based on Pedaling Motor Imagery on Cortical Excitability and Connectivity
Abstract
1. Introduction
2. Materials and Methods
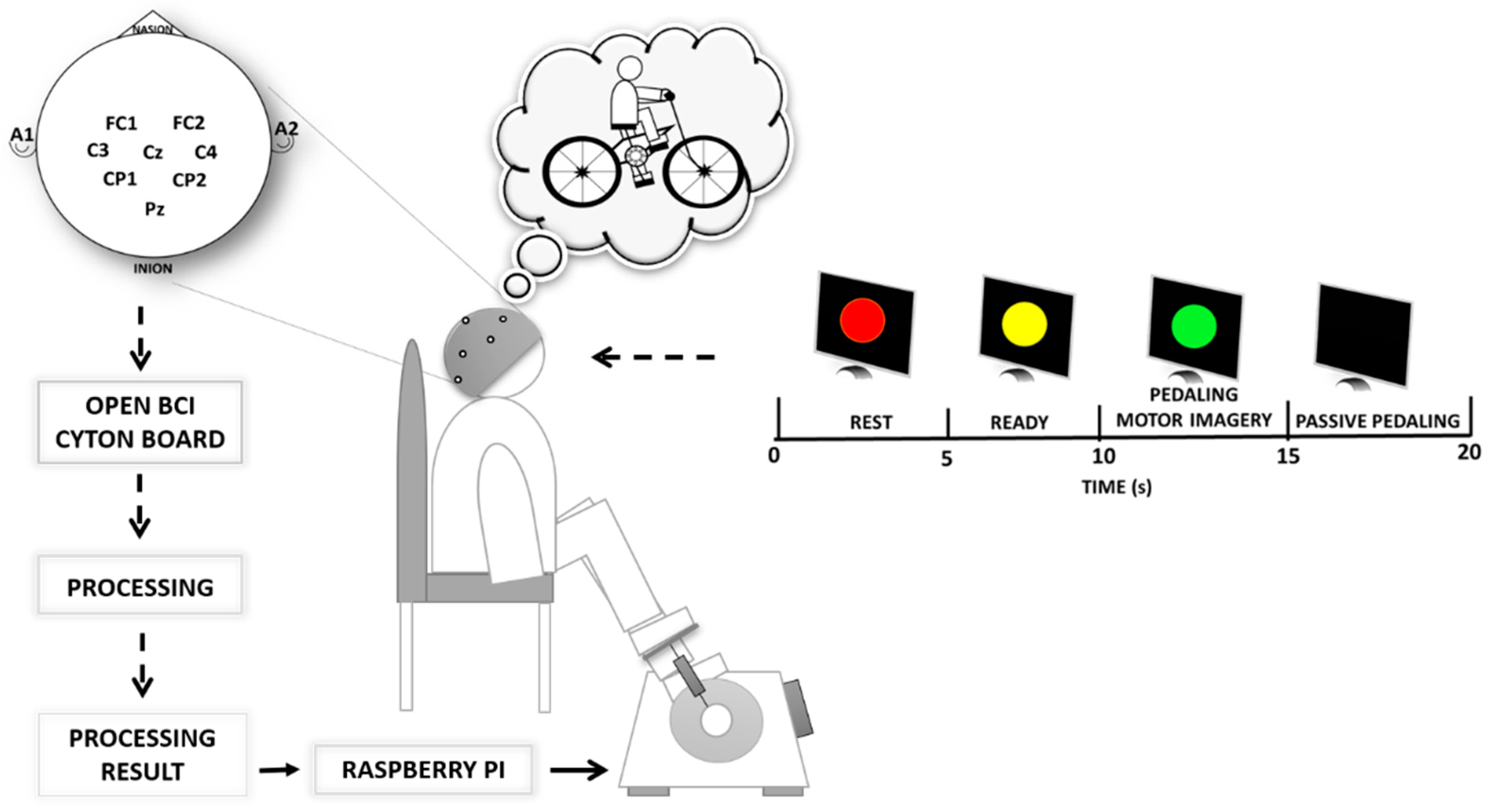
2.1. Brain—Computer Interface
2.2. Data Recording and Signal Processing
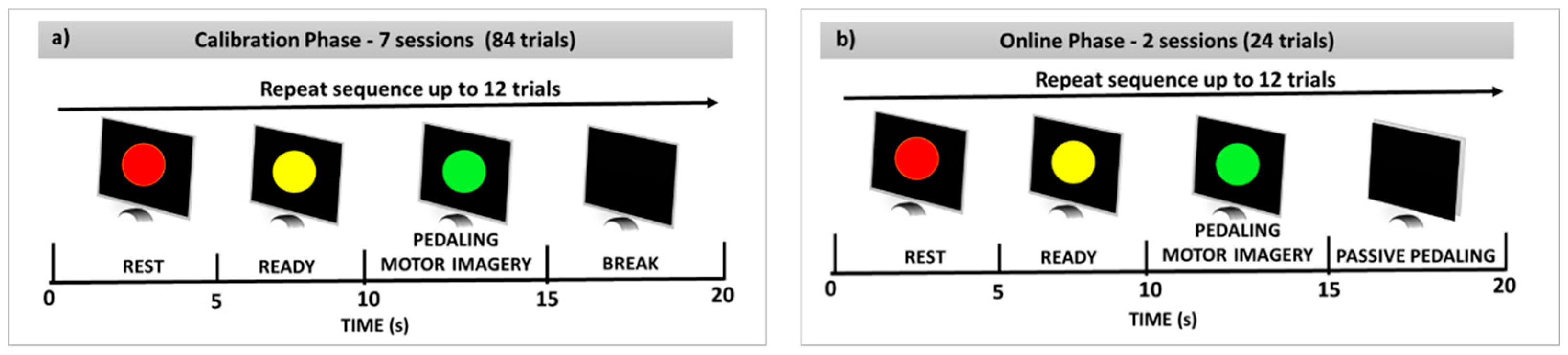
2.3. Protocol
2.4. Data Processing and Statistical Analysis
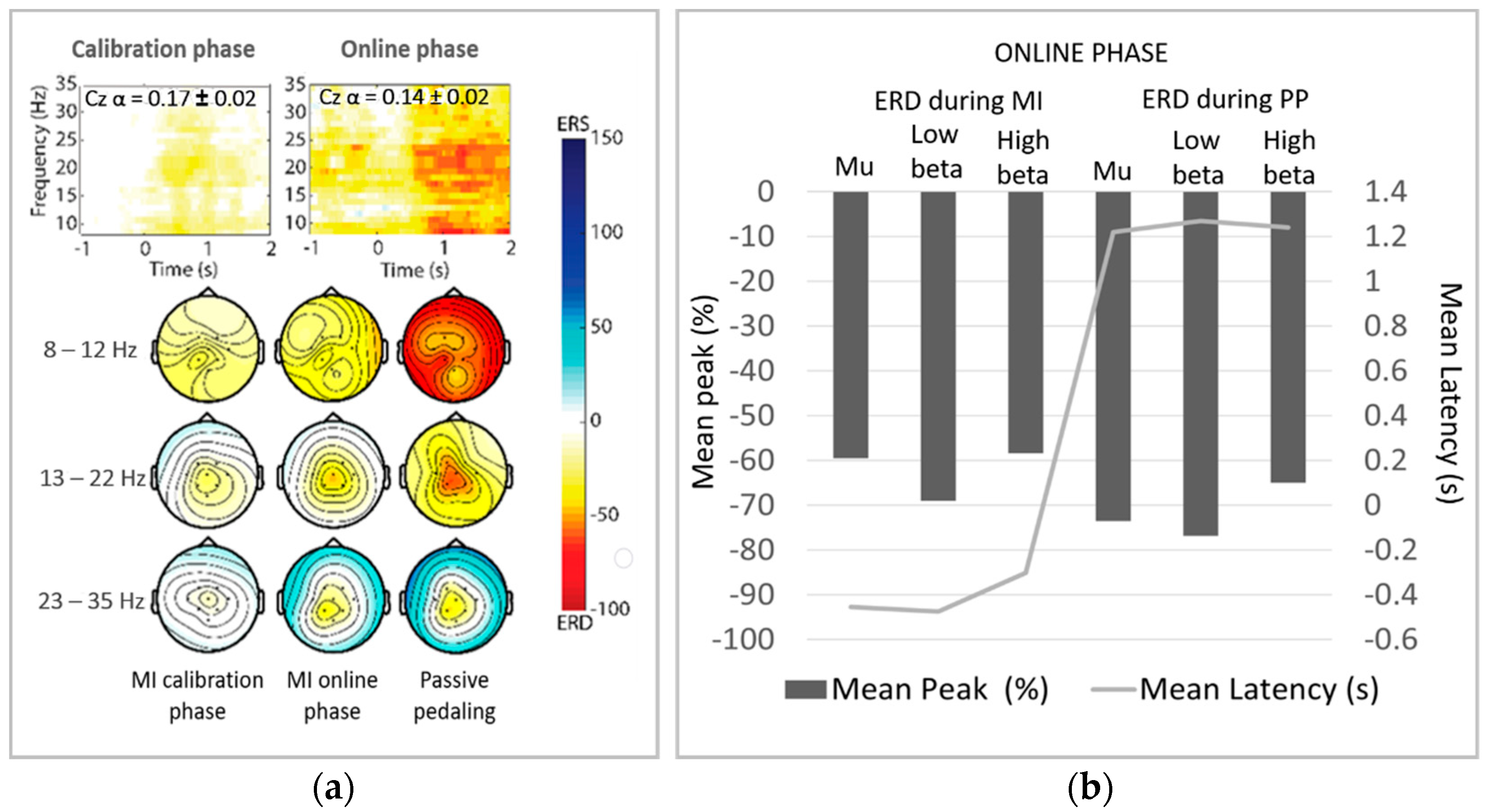
2.4.1. Significant ERD Patterns Analysis
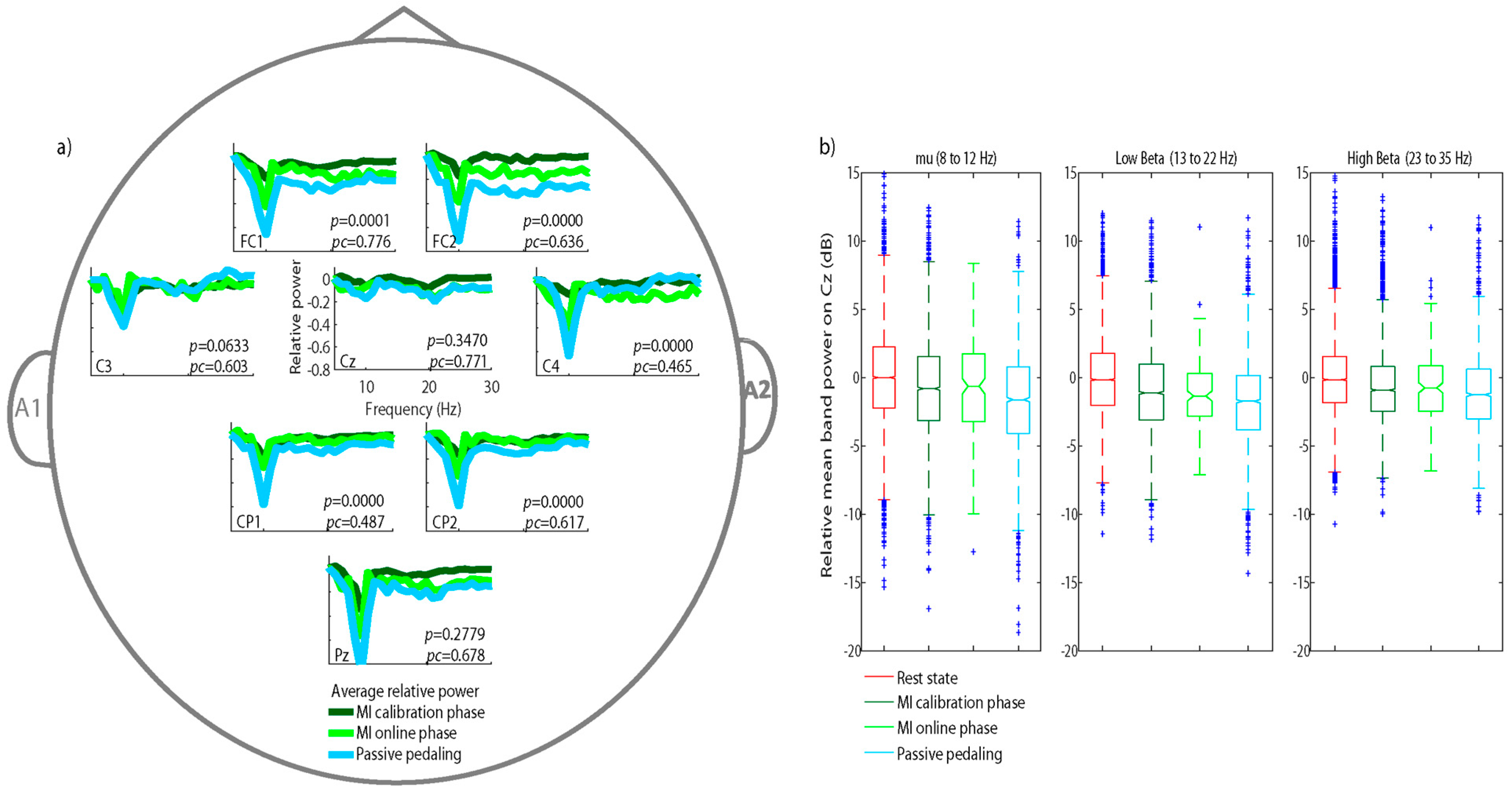
2.4.2. Relative Power
2.4.3. Connectivity
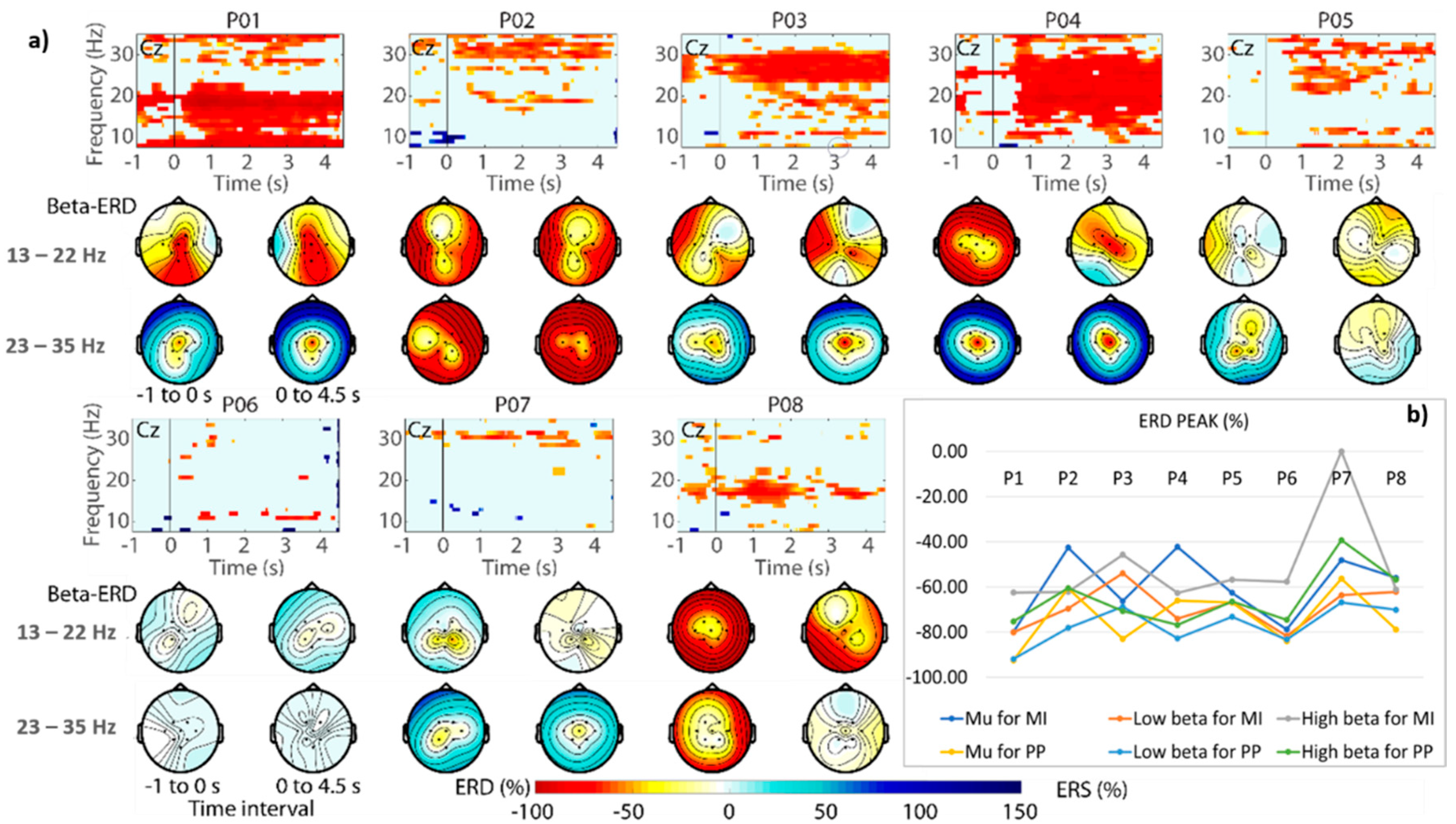
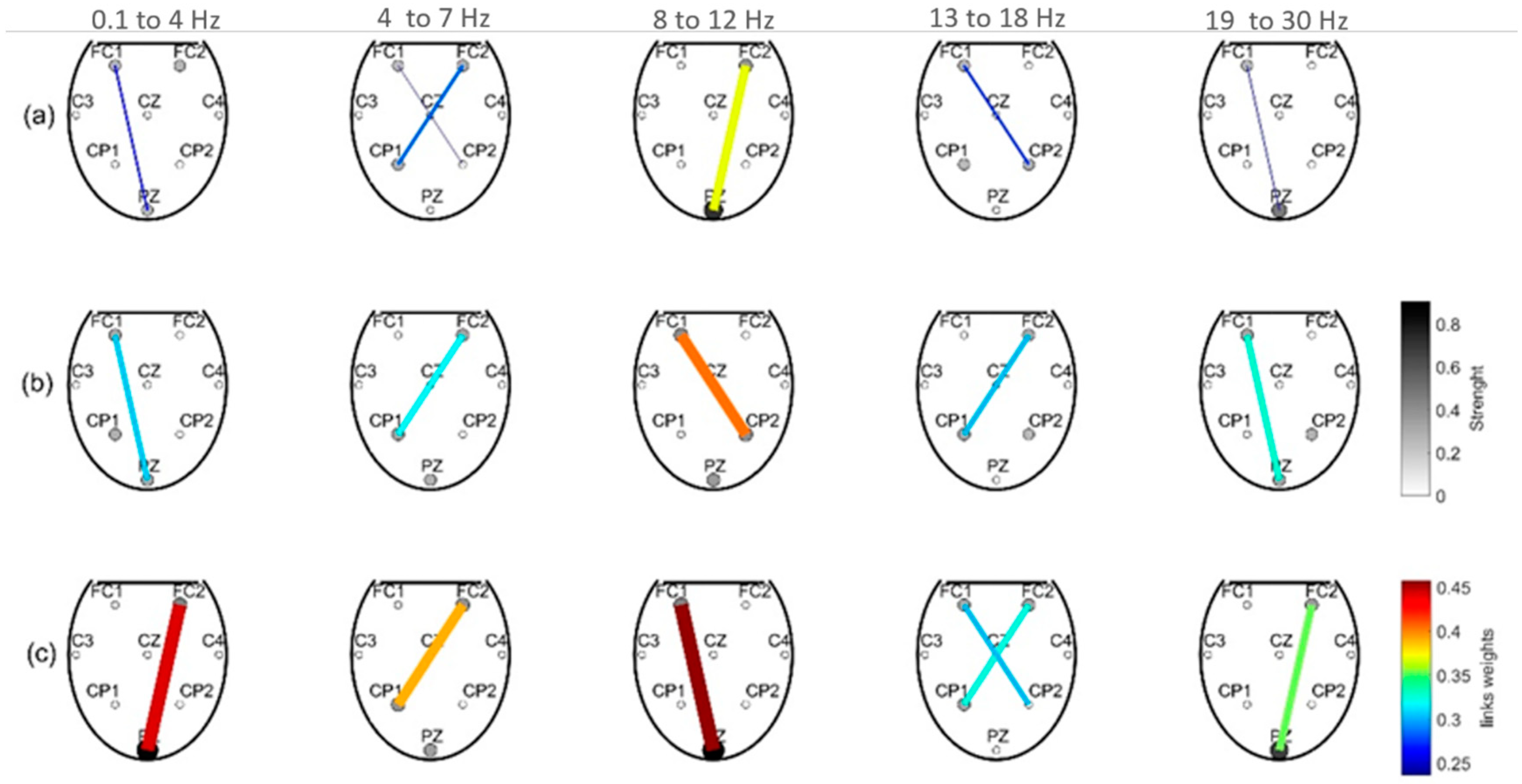
3. Results
3.1. Relative Power Analysis
3.2. ERD
3.3. Brain Connectivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bastos-Filho, T.F. Introduction to Non-Invasive EEG-Based Brain-Computer Interfaces for Assistive Technologies; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Takeuchi, N.; Izumi, S.-I. Rehabilitation with Poststroke Motor Recovery: A Review with a Focus on Neural Plasticity. Stroke Res. Treat. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Li, M.; Xu, G.; Xie, J.; Chen, C. A review: Motor rehabilitation after stroke with control based on human intent. Proc. Inst. Mech. Eng. Part H: J. Eng. Med. 2018, 232, 344–360. [Google Scholar] [CrossRef]
- Liu, D.; Chen, W.; Lee, K.; Pei, Z.; Millan, J.D.R. An EEG-based brain-computer interface for gait training. In Proceedings of the 2017 29th Chinese Control and Decision Conference (CCDC), Chongqing, China, 28–30 May 2017; pp. 6755–6760. [Google Scholar]
- Hashimoto, Y.; Ushiba, J. EEG-based classification of imaginary left and right foot movements using beta rebound. Clin. Neurophysiol. 2013, 124, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, W.; Pei, Z.; Wang, J. Detection of lower-limb movement intention from EEG signals. In Proceedings of the 2017 12th IEEE Conference on Industrial Electronics and Applications (ICIEA), Siem Reap, Cambodia, 18–20 June 2017; pp. 84–89. [Google Scholar]
- Mrachacz-Kersting, N.; Kristensen, S.R.; Niazi, I.K.; Farina, D. Precise temporal association between cortical potentials evoked by motor imagination and afference induces cortical plasticity. J. Physiol. 2012, 590, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; da Silva, F.L. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Jiang, N.; Gizzi, L.; Mrachacz-Kersting, N.; Dremstrup, K.; Farina, D. A brain–computer interface for single-trial detection of gait initiation from movement related cortical potentials. Clin. Neurophysiol. 2015, 126, 154–159. [Google Scholar] [CrossRef]
- Niazi, I.K.; Mrachacz-Kersting, N.; Jiang, N.; Dremstrup, K.; Farina, D. Peripheral Electrical Stimulation Triggered by Self-Paced Detection of Motor Intention Enhances Motor Evoked Potentials. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 595–604. [Google Scholar] [CrossRef]
- Belda-Lois, J.M.; Horno, S.M.-D.; Bermejo-Bosch, I.; Moreno, J.C.; Pons, J.L.; Farina, D.; Iosa, M.; Molinari, M.; Tamburella, F.; Ramos-Murguialday, A.; et al. Rehabilitation of gait after stroke: A review towards a top-down approach. J. Neuroeng. Rehabil. 2011, 8, 66. [Google Scholar] [CrossRef]
- Wolbrecht, E.T.; Chan, V.; Reinkensmeyer, D.J.; Bobrow, J.E. Optimizing compliant, model-based robotic assistance to promote neurorehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 286–297. [Google Scholar] [CrossRef]
- Donati, A.R.C.; Shokur, S.; Morya, E.; Campos, D.S.F.; Moioli, R.C.; Gitti, C.M.; Augusto, P.B.; Tripodi, S.; Pires, C.G.; Pereira, G.A.; et al. Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci. Rep. 2016, 6, 30383. [Google Scholar] [CrossRef] [PubMed]
- Prochnow, D.; Badia, S.B.I.; Schmidt, J.; Duff, A.; Brunheim, S.; Kleiser, R.; Seitz, R.J.; Verschure, P.F.M.J. A functional magnetic resonance imaging study of visuomotor processing in a virtual reality-based paradigm: Rehabilitation Gaming System. Eur. J. Neurosci. 2013, 37, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Freedman, D.O. Isolation, quarantine, social distancing and community containment: Pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J. Travel Med. 2020, 27, taaa020. [Google Scholar] [CrossRef] [PubMed]
- Prem, K.; Liu, Y.; Russell, T.W.; Kucharski, A.J.; Eggo, R.M.; Davies, N.; Centre for the Mathematical Modelli Group; Jit, M.; Klepac, P. The Effect of Control Strategies that Reduce Social Mixing on Outcomes of the COVID-19 Epidemic in Wuhan, China. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Nath, A.; Smith, B. Neurological issues during COVID-19: An overview. Neurosci. Lett. 2021, 742, 135533. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Katsanos, A.H.; Ornello, R.; Sacco, S. Ischemic stroke epidemiology during the COVID-19 pandemic: Navigating uncharted waters with changing tides. Am. Heart Assoc. 2020, 51, 1924–1926. [Google Scholar] [CrossRef]
- Hess, D.C.; Eldahshan, W.; Rutkowski, E. COVID-19-Related Stroke. Transl. Stroke Res. 2020, 11, 322–325. [Google Scholar] [CrossRef]
- Bhatia, R.; Srivastava, M.P. COVID-19 and Stroke: Incidental, Triggered or Causative. Ann. Indian Acad. Neurol. 2020, 23, 318–324. [Google Scholar]
- Hancock, N.J.; Shepstone, L.; Winterbotham, W.; Pomeroy, V. Effects of Lower Limb Reciprocal Pedalling Exercise on Motor Function after Stroke: A Systematic Review of Randomized and Nonrandomized Studies. Int. J. Stroke 2011, 7, 47–60. [Google Scholar] [CrossRef]
- Melinscak, F.; Montesano, L.; Minguez, J. Asynchronous detection of kinesthetic attention during mobilization of lower limbs using EEG measurements. J. Neural Eng. 2016, 13, 016018. [Google Scholar] [CrossRef]
- Rosecrance, J.C.; Giuliani, C.A. Kinematic analysis of lower-limb movement during ergometer pedaling in hemiplegic and nonhemiplegic subjects. Phys. Ther. 1991, 71, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Katz-Leurer, M.; Sender, I.; Keren, O.; Dvir, Z. The influence of early cycling training on balance in stroke patients at the subacute stage. Results of a preliminary trial. Clin. Rehabil. 2006, 20, 398–405. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chen, S.-C.; Chen, J.-J.J.; Fu, L.-L.; Wang, Y.L. Kinesiological and kinematical analysis for stroke subjects with asymmetrical cycling movement patterns. J. Electromyogr. Kinesiol. 2005, 15, 587–595. [Google Scholar] [CrossRef]
- Brown, D.A.; Nagpal, S.; Chi, S. Limb-Loaded Cycling Program for Locomotor Intervention Following Stroke. Phys. Ther. 2005, 85, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Abdar, H.; Ridgel, A.L.; Discenzo, F.M.; Loparo, K.A. Design and development of a smart exercise bike for motor rehabilitation in individuals with Parkinson’s disease. IEEE/ASME Trans. Mechatron. 2015, 21, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ugarte, M.D.l.S.; Iáñez, E.; Ortiz-Garcia, M.; Azorín, J.M. Effects of tDCS on real-time BCI detection of pedaling motor imagery. Sensors 2018, 18, 1136. [Google Scholar] [CrossRef]
- Rezk, M.; Darweesh, M.; ElTamawy, M.; Basheer, M. S186 Effect of reciprocal pedaling exercise on cortical reorganization and gait in stroke patients. Clin. Neurophysiol. 2017, 128, e238. [Google Scholar] [CrossRef]
- Jain, S.; Gourab, K.; Schindler-Ivens, S.; Schmit, B.D. EEG during pedaling: Evidence for cortical control of locomotor tasks. Clin. Neurophysiol. 2013, 124, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Storzer, L.; Butz, M.; Hirschmann, J.; Abbasi, O.; Gratkowski, M.; Saupe, D.; Schnitzler, A.; Dalal, S.S. Bicycling and Walking are Associated with Different Cortical Oscillatory Dynamics. Front. Hum. Neurosci. 2016, 10, 61. [Google Scholar] [CrossRef]
- Christensen, L.; Johannsen, P.; Petersen, N.; Pyndt, H.; Nielsen, J. Cerebral activation during bicycle movements in man. Exp. Brain Res. 2000, 135, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.G.; Kim, M.-K.; Kim, S.-P. Inter-channel Connectivity of Motor Imagery EEG Signals for a Noninvasive BCI Application. In Proceedings of the 2011 International Workshop on Pattern Recognition in NeuroImaging, Seoul, Korea, 16–18 May 2011; pp. 49–52. [Google Scholar]
- Athanasiou, A.; Lithari, C.; Kalogianni, K.; Klados, M.A.; Bamidis, P.D. Source detection and functional connectivity of the sensorimotor cortex during actual and imaginary limb movement: A preliminary study on the implementation of econnectome in motor imagery protocols. Adv. Hum. Comput. Interact. 2012, 2012, 127627. [Google Scholar] [CrossRef]
- Astolfi, L.; Cincotti, F.; Mattia, D.; Marciani, M.G.; Baccala, L.A.; Fallani, F.D.V.; Salinari, S.; Ursino, M.; Zavaglia, M.; Ding, L.; et al. Comparison of different cortical connectivity estimators for high-resolution EEG recordings. Hum. Brain Mapp. 2007, 28, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ong, S.-H.; Pan, Y.; Ang, K.K. Connectivity pattern modeling of motor imagery EEG. In Proceedings of the 2013 IEEE Symposium on Computational Intelligence, Cognitive Algorithms, Mind, and Brain (CCMB), Singapore, 16–19 April 2013; pp. 94–100. [Google Scholar]
- Gonuguntla, V.; Wang, Y.; Veluvolu, K.C. Phase synchrony in subject-specific reactive band of EEG for classification of motor imagery tasks. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; Volume 2013, pp. 2784–2787. [Google Scholar]
- Jin, L.; Kim, E.Y. Interpretable Cross-Subject EEG-Based Emotion Recognition Using Channel-Wise Features. Sensors 2020, 20, 6719. [Google Scholar] [CrossRef] [PubMed]
- Broetz, D.; Braun, C.; Weber, C.; Soekadar, S.R.; Caria, A.; Birbaumer, N. Combination of Brain-Computer Interface Training and Goal-Directed Physical Therapy in Chronic Stroke: A Case Report. Neurorehabilit. Neural Repair 2010, 24, 674–679. [Google Scholar] [CrossRef]
- Ang, K.K.; Guan, C.; Phua, K.S.; Wang, C.; Zhou, L.; Tang, K.Y.; Joseph, G.J.E.; Kuah, C.W.K.; Chua, K.S.G. Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: Results of a three-armed randomized controlled trial for chronic stroke. Front. Neuroeng. 2014, 7, 30. [Google Scholar] [CrossRef]
- Wang, W.; Collinger, J.L.; Perez, M.A.; Tyler-Kabara, E.C.; Cohen, L.G.; Birbaumer, N.; Brose, S.W.; Schwartz, A.B.; Boninger, M.L.; Weber, D.J. Neural Interface Technology for Rehabilitation: Exploiting and Promoting Neuroplasticity. Phys. Med. Rehabil. Clin. N. Am. 2010, 21, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.J.; Krusienski, D.J.; Wolpaw, J.R. Brain-Computer Interfaces in Medicine. Mayo Clin. Proc. 2012, 87, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Simmons, L.H.; Jones, P.S.; Day, D.J.; Carpenter, T.A.; Pomeroy, V.M.; Warburton, E.A.; Baron, J.-C. Motor imagery after subcortical stroke: A functional magnetic resonance imaging study. Stroke 2009, 40, 1315–1324. [Google Scholar] [CrossRef]
- Cho, H.; Ahn, M.; Ahn, S.; Kwon, M.; Jun, S.C. EEG datasets for motor imagery brain–computer interface. GigaScience 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Neuper, C.; Scherer, R.; Reiner, M.; Pfurtscheller, G. Imagery of motor actions: Differential effects of kinesthetic and visual–motor mode of imagery in single-trial EEG. Cogn. Brain Res. 2005, 25, 668–677. [Google Scholar] [CrossRef]
- Yang, Y.J.; Jeon, E.J.; Kim, J.S.; Chung, C.K. Characterization of kinesthetic motor imagery compared with visual motor imageries. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Rimbert, S.; Gayraud, N.; Bougrain, L.; Clerc, M.; Fleck, S. Can a subjective questionnaire be used as brain-computer interface performance predictor? Front. Hum. Neurosci. 2019, 12, 529. [Google Scholar] [CrossRef] [PubMed]
- Graimann, B.; Huggins, J.; Levine, S.; Pfurtscheller, G. Visualization of significant ERD/ERS patterns in multichannel EEG and ECoG data. Clin. Neurophysiol. 2002, 113, 43–47. [Google Scholar] [CrossRef]
- Mason, S.G.; Bashashati, A.; Fatourechi, M.; Navarro, K.F.; Birch, G.E. A Comprehensive Survey of Brain Interface Technology Designs. Ann. Biomed. Eng. 2006, 35, 137–169. [Google Scholar] [CrossRef] [PubMed]
- Vourvopoulos, A.; Badia, S.B.I. Motor priming in virtual reality can augment motor-imagery training efficacy in restorative brain-computer interaction: A within-subject analysis. J. Neuroeng. Rehabil. 2016, 13, 69. [Google Scholar] [CrossRef]
- Romero-Laiseca, M.A.; Delisle-Rodriguez, D.; Cardoso, V.; Gurve, D.; Loterio, F.; Nascimento, J.H.P.; Krishnan, S.; Frizera-Neto, A.; Bastos-Filho, T. A Low-Cost Lower-Limb Brain-Machine Interface Triggered by Pedaling Motor Imagery for Post-Stroke Patients Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 988–996. [Google Scholar] [CrossRef]
- Astolfi, L.; Bakardjian, H.; Cincotti, F.; Mattia, D.; Marciani, M.G.; Fallani, F.d.; Colosimo, A.; Salinari, S.; Miwakeichi, F.; Yamaguchi, Y. Estimate of causality between independent cortical spatial patterns during movement volition in spinal cord injured pa-tients. Brain Topogr. 2007, 19, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Solis-Escalante, T.; Grieshofer, P.; Neuper, C.; Müller-Putz, G.; Scherer, R. Level of participation in robotic-assisted treadmill walking modulates midline sensorimotor EEG rhythms in able-bodied subjects. NeuroImage 2012, 63, 1203–1211. [Google Scholar] [CrossRef]
- Grosse-Wentrup, M.; Mattia, D.; Oweiss, K. Using brain–computer interfaces to induce neural plasticity and restore function. J. Neural Eng. 2011, 8, 025004. [Google Scholar] [CrossRef]
- Xu, R.; Jiang, N.; Lin, C.; Mrachacz-Kersting, N.; Dremstrup, K.; Farina, D. Enhanced Low-Latency Detection of Motor Intention From EEG for Closed-Loop Brain-Computer Interface Applications. IEEE Trans. Biomed. Eng. 2013, 61, 288–296. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, V.F.; Delisle-Rodriguez, D.; Romero-Laiseca, M.A.; Loterio, F.A.; Gurve, D.; Floriano, A.; Valadão, C.; Silva, L.; Krishnan, S.; Frizera-Neto, A.; et al. Effect of a Brain–Computer Interface Based on Pedaling Motor Imagery on Cortical Excitability and Connectivity. Sensors 2021, 21, 2020. https://doi.org/10.3390/s21062020
Cardoso VF, Delisle-Rodriguez D, Romero-Laiseca MA, Loterio FA, Gurve D, Floriano A, Valadão C, Silva L, Krishnan S, Frizera-Neto A, et al. Effect of a Brain–Computer Interface Based on Pedaling Motor Imagery on Cortical Excitability and Connectivity. Sensors. 2021; 21(6):2020. https://doi.org/10.3390/s21062020
Chicago/Turabian StyleCardoso, Vivianne Flávia, Denis Delisle-Rodriguez, Maria Alejandra Romero-Laiseca, Flávia A. Loterio, Dharmendra Gurve, Alan Floriano, Carlos Valadão, Leticia Silva, Sridhar Krishnan, Anselmo Frizera-Neto, and et al. 2021. "Effect of a Brain–Computer Interface Based on Pedaling Motor Imagery on Cortical Excitability and Connectivity" Sensors 21, no. 6: 2020. https://doi.org/10.3390/s21062020
APA StyleCardoso, V. F., Delisle-Rodriguez, D., Romero-Laiseca, M. A., Loterio, F. A., Gurve, D., Floriano, A., Valadão, C., Silva, L., Krishnan, S., Frizera-Neto, A., & Freire Bastos-Filho, T. (2021). Effect of a Brain–Computer Interface Based on Pedaling Motor Imagery on Cortical Excitability and Connectivity. Sensors, 21(6), 2020. https://doi.org/10.3390/s21062020






