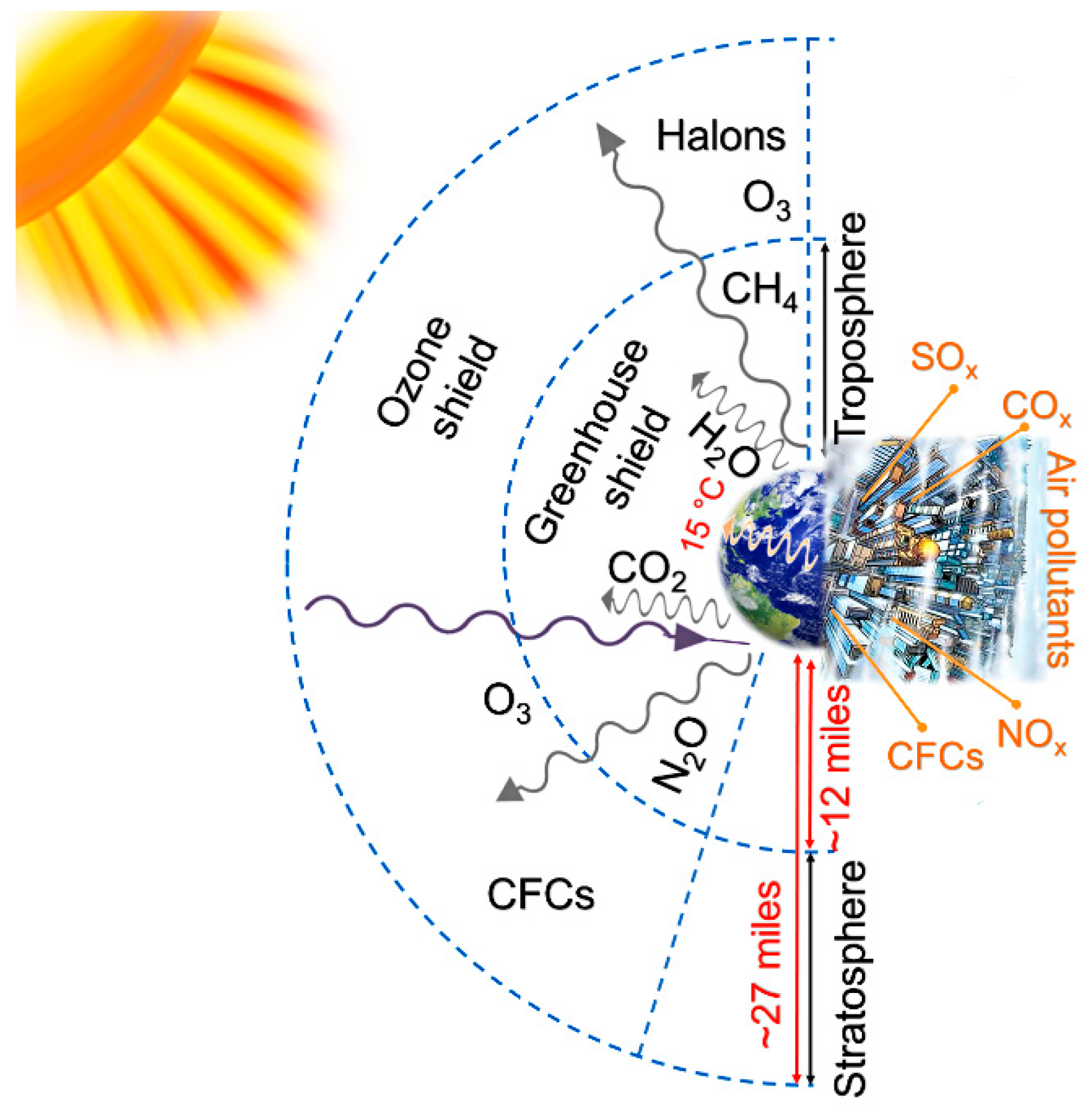
Recent Developments in Graphene-Based Toxic Gas Sensors: A Theoretical Overview
Abstract
1. Introduction
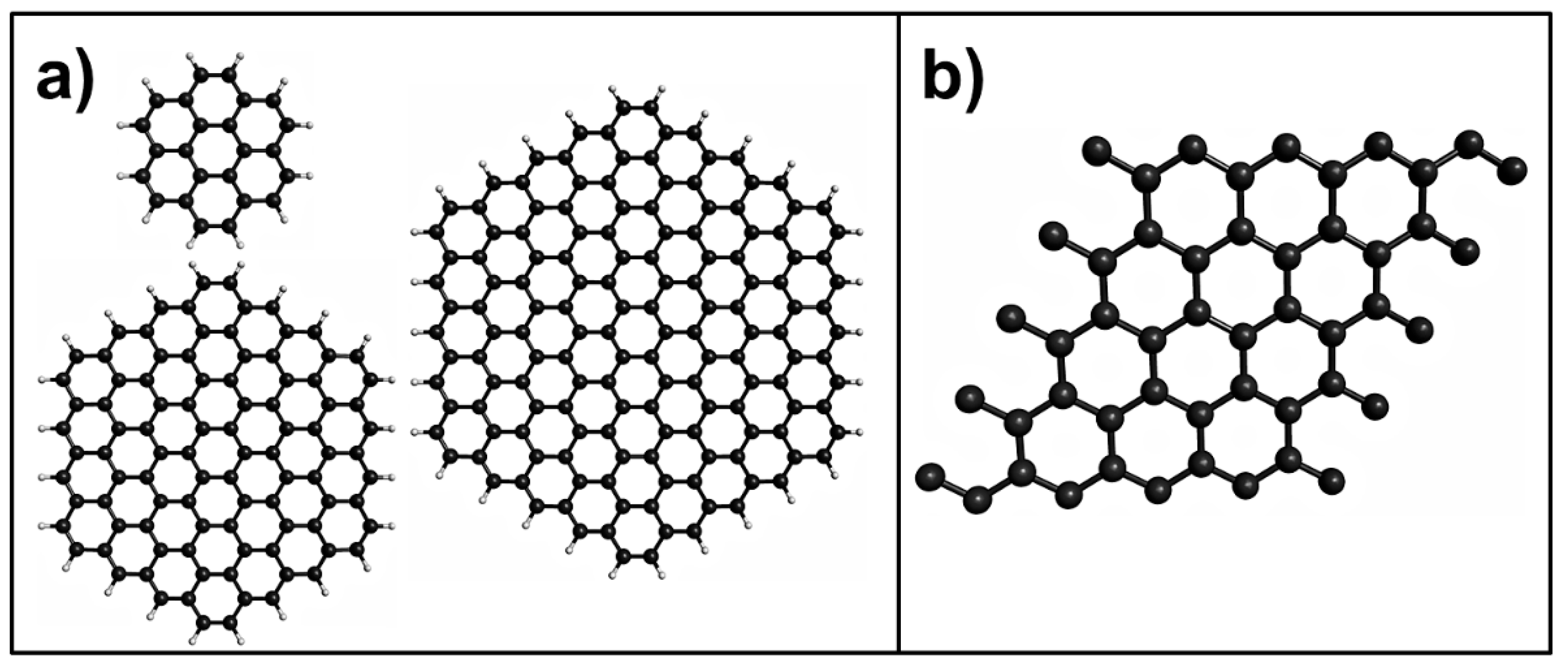
2. Pristine Graphene
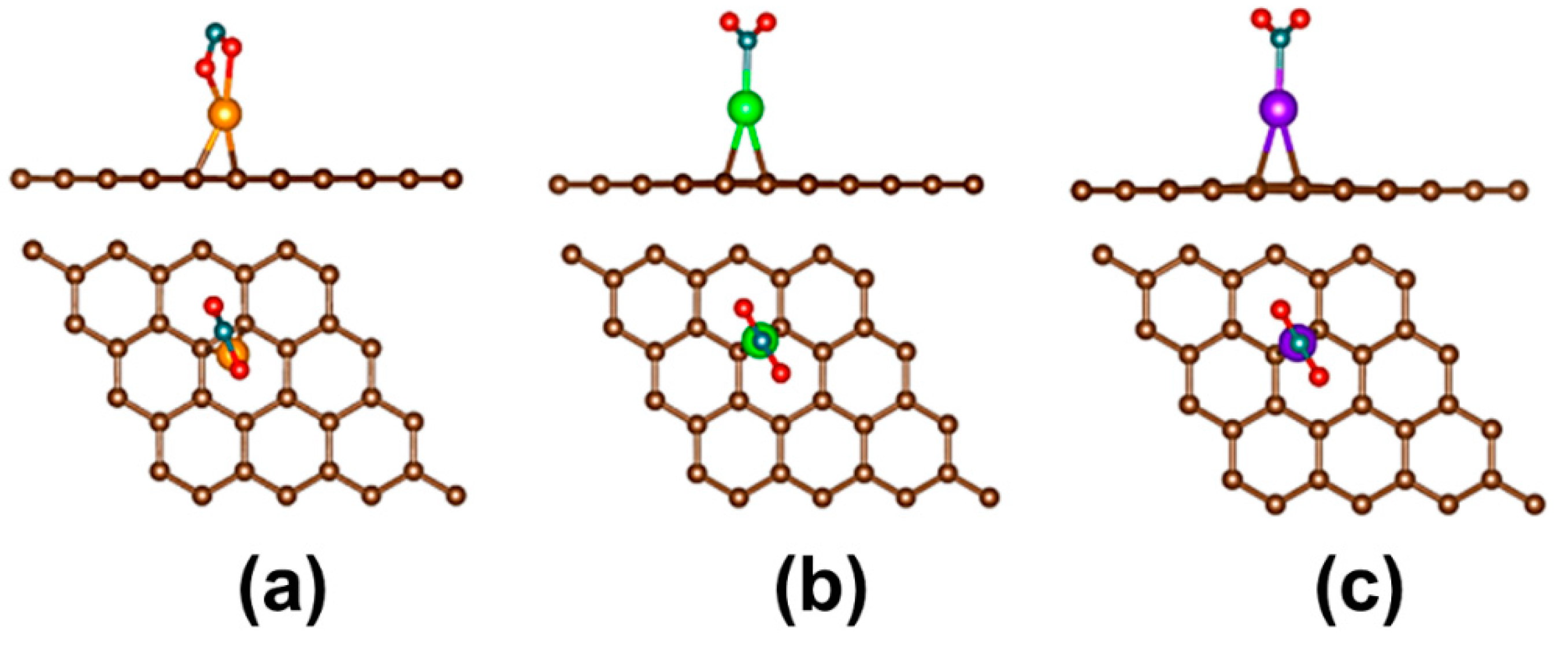
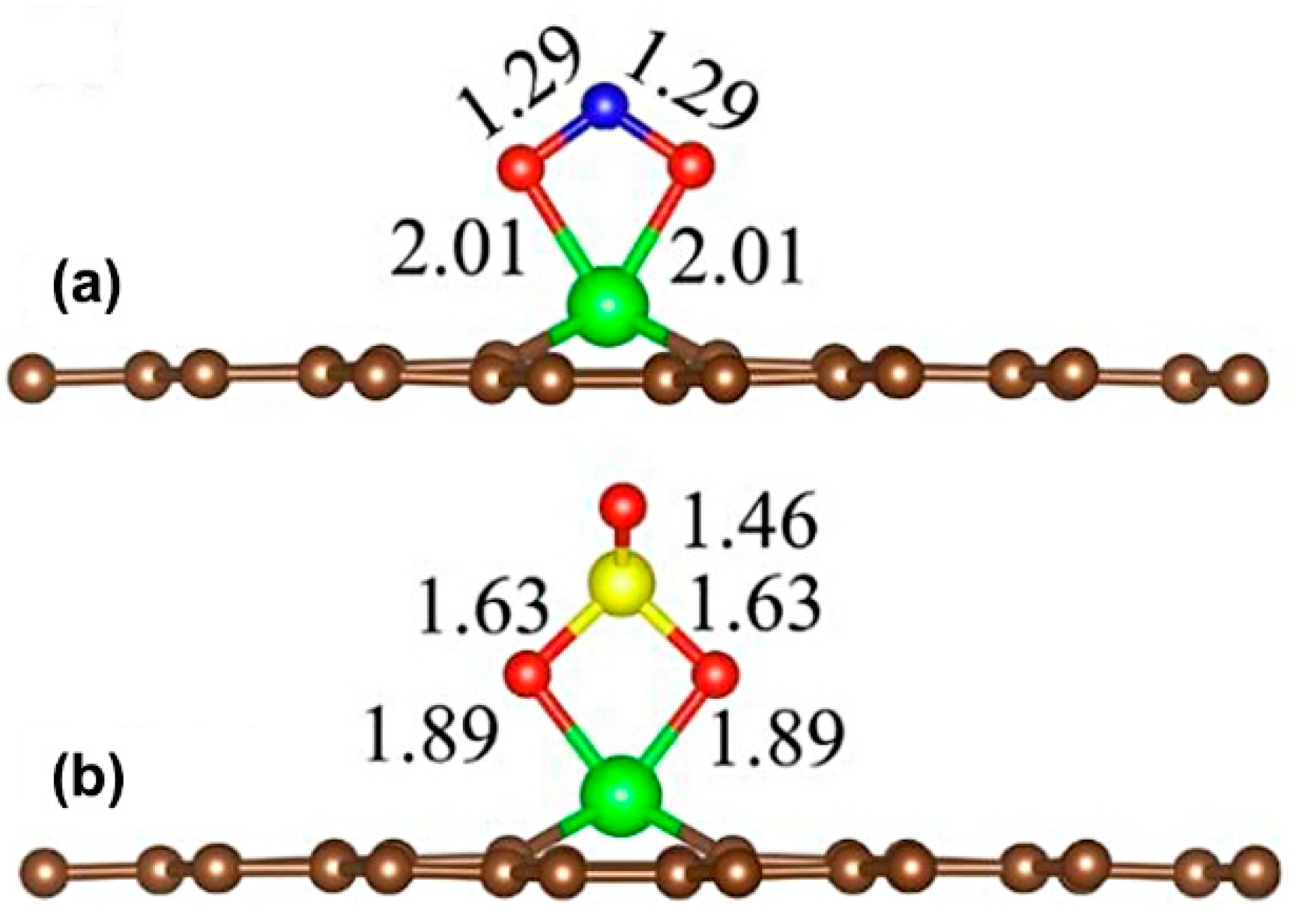
3. Pristine Graphene Decorated with Transition Metals
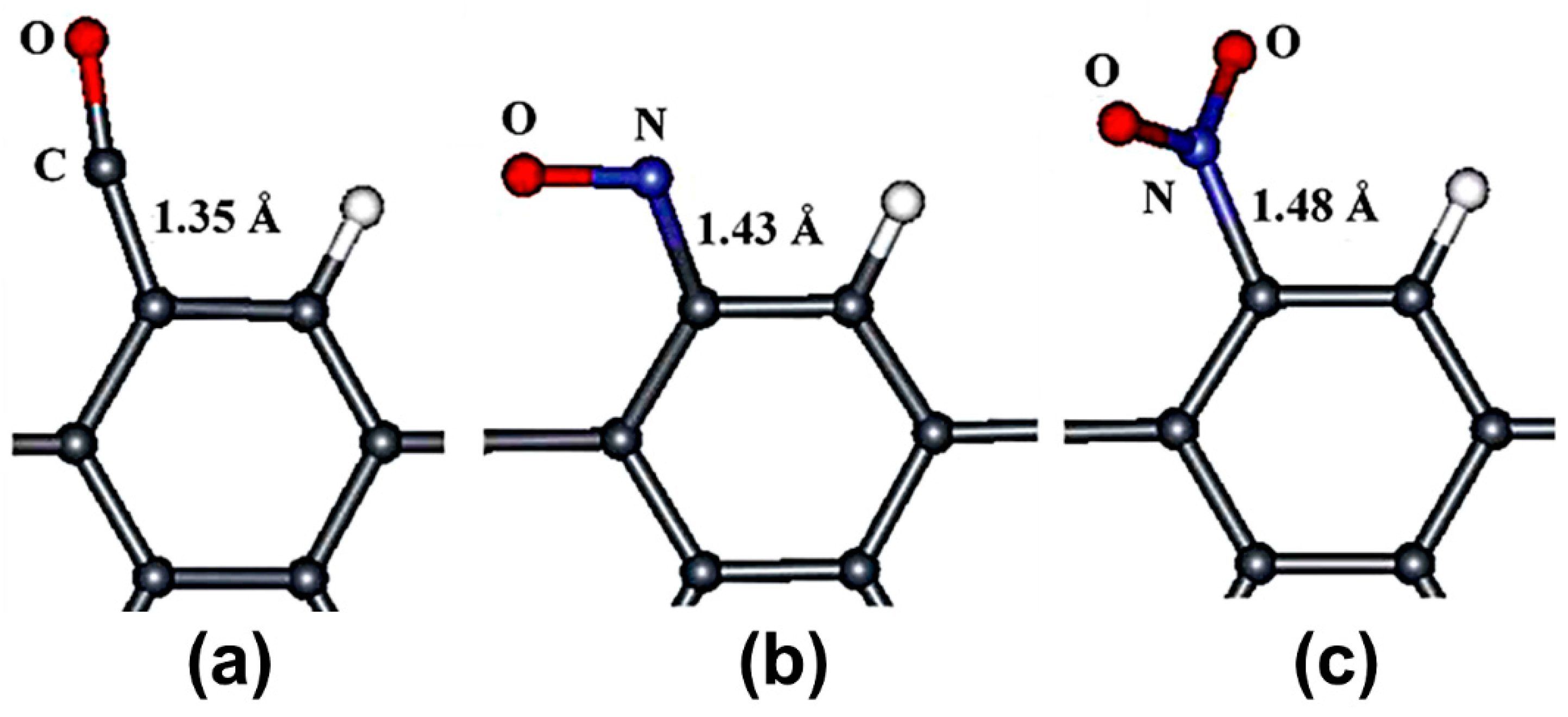
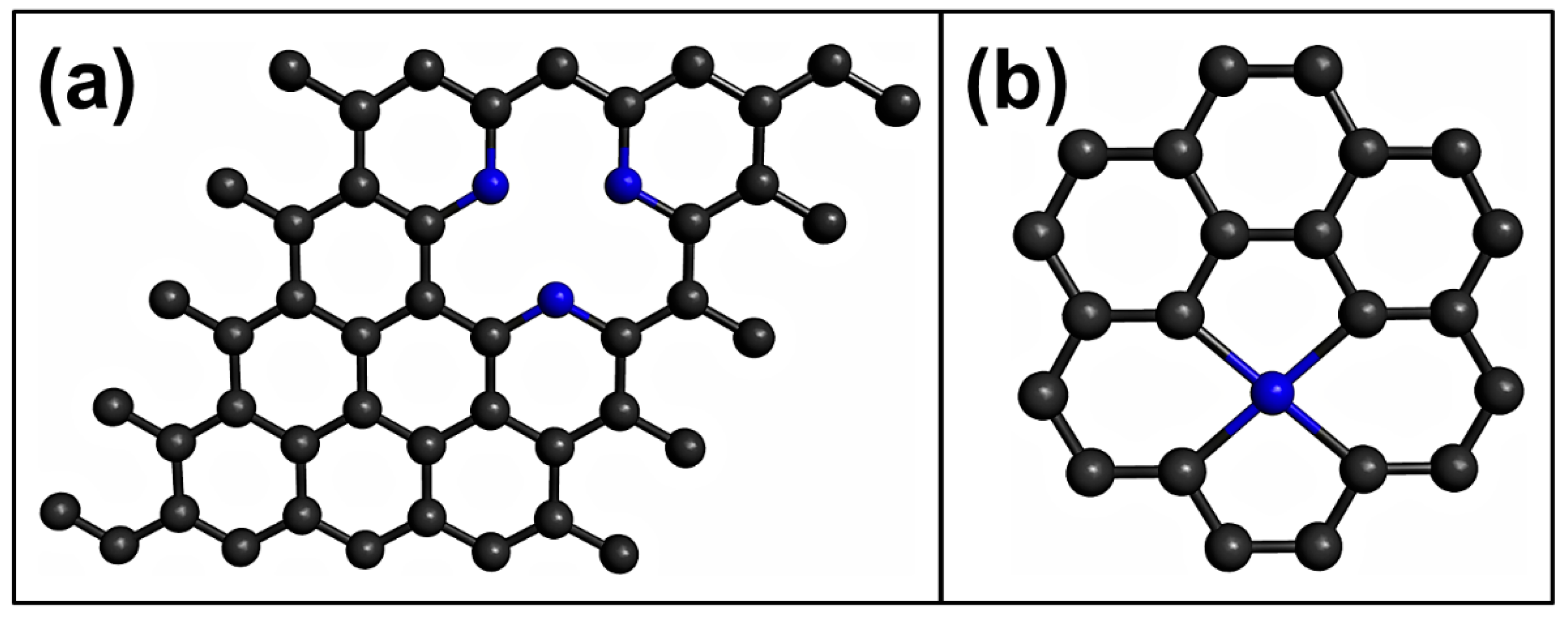
4. Defective Graphene
5. Doped Graphene
6. Conclusions and Perspectives
- (a)
- The interaction between toxic gases and pristine graphene is weak, which reduces the sensitivity and selectivity of pristine graphene toward the toxic gases.
- (b)
- The pristine graphene decorated with transition metals is a promising material for use in a toxic gas sensor. However, up to now these types of studies are still scarce; therefore, more theoretical studies on the sensitivity and selectivity of pristine graphene decorated with transition metals toward the toxic gases should be carried out.
- (c)
- It was observed that graphene with single-vacancy defects interacts stronger with the toxic gases compared to pristine graphene. Therefore, it is a promising material for use in toxic gas sensors. In addition to point defects, line or multivacancy defects should be investigated at the DFT level, to enrich graphene functionalities.
- (d)
- Bilayer and multilayer graphene exhibit higher different dimensionalities than single-layer graphene, which can increase the number of possible defect types, namely, point defects, line defects, and so on. At the theoretical level, more attention should be paid to understanding stable bilayer and multilayer graphene with randomly distributed defects.
- (e)
- A large number of theoretical studies have addressed the use of doped graphene as a toxic gas sensor. The evidence indicates that doped-graphene sheets are good candidate materials. However, up to date, DFT studies on the selectivity of doped graphene toward the toxic gases are limited. Therefore, more theoretical studies on the selectivity of doped graphene toward the toxic gases should be carried out. In addition, feasible approaches to facilitate the desorption of toxic gas on the doped graphene surface should be investigated.
- (f)
- The pyridinic-type N-doped graphene and doped vacancy-defected graphene are good materials for use in toxic gases sensors. However, more DFT-based studies on pyridinic-type N-doped graphene and doped vacancy-defected graphene as toxic gas sensors are needed.
- (g)
- The reasons for the difference of adsorption energy obtained by using different functionals (e.g., GGA, LDA, PBE, and vdW-DF2) in the calculation methods should be compared and analyzed.
- (h)
- This review shows the importance of theoretical studies for the design of novel and efficient toxic gas sensors. The theoretical results obtained up to now can help and motivate experimental groups to design novel and efficient graphene-based toxic gas sensors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, W.; Liu, X.; Yu, W. Research progress of gas sensor based on graphene and its derivatives: A review. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef]
- Mahajan, S.; Jagtap, S. Metal-oxide semiconductors for carbon monoxide (CO) gas sensing: A review. Appl. Mater. Today 2020, 18, 100483. [Google Scholar] [CrossRef]
- Rose, J.J.; Wang, L.; Xu, Q.; McTiernan, C.F.; Shiva, S.; Tejero, J.; Gladwin, M.T. Carbon monoxide poisoning: Pathogenesis, management, and future directions of therapy. Am. J. Respir. Crit. Care Med. 2017, 195, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Hampson, N.B.; Bodwin, D. Toxic CO-ingestions in intentional carbon monoxide poisoning. J. Emerg. Med. 2013, 44, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Raub, J.A.; Mathieu-Nolf, M.; Hampson, N.B.; Thom, S.R. Carbon monoxide poisoning—a public health perspective. Toxicology 2000, 145, 1–14. [Google Scholar] [CrossRef]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total Environ. 2020, 714, 136712. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Li, C.; Tang, T.; Liu, X.; Han, S.; Lei, B.; Zhou, C. Detection of NO2 down to ppb levels using individual and multiple In2O3 nanowire devices. Nano Lett. 2004, 4, 1919–1924. [Google Scholar] [CrossRef]
- Niu, H.; Leung, D.Y.C. A review on the removal of nitrogen oxides from polluted flow by bioreactors. Environ. Rev. 2010, 18, 175–189. [Google Scholar] [CrossRef]
- Su, Y.; Wang, J.; Wang, B.; Yang, T.; Yang, B.; Xie, G.; Zhou, Y.; Zhang, S.; Tai, H.; Cai, Z.; et al. Alveolus-inspired active membrane sensors for self-powered wearable chemical sensing and breath analysis. ACS Nano 2020, 14, 6067–6075. [Google Scholar] [CrossRef]
- Nascimento, A.P.; Santos, J.M.; Mill, J.G.; de Almeida Albuquerque, T.T.; Júnior, N.C.R.; Reisen, V.A.; Pagel, É.C. Association between the incidence of acute respiratory diseases in children and ambient concentrations of SO2, PM10 and chemical elements in fine particles. Environ. Res. 2020, 188, 109619. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Omaye, S.T. Air pollutants, oxidative stress and human health. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 674, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Springer, K.J. Global What? Control Possibilities of CO2 and Other Greenhouse Gases. J. Eng. Gas Turbines Power 1991, 113, 440–447. [Google Scholar] [CrossRef]
- Khan, M.; Rao, M.; Li, Q. Recent advances in electrochemical sensors for detecting toxic gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, D.; Zong, X.; Yang, Z. Enhanced SO2 gas sensing properties of metal organic frameworks-derived titanium dioxide/reduced graphene oxide nanostructure. J. Mater. Sci. Mater. Electron. 2019, 30, 11070–11078. [Google Scholar] [CrossRef]
- Lins, V.F.C.; Guimarães, E.M. Failure of a heat exchanger generated by an excess of SO2 and H2S in the Sulfur Recovery Unit of a petroleum refinery. J. Loss Prev. Process. Ind. 2007, 20, 91–97. [Google Scholar] [CrossRef]
- Najjar, Y.S.H. Gaseous Pollutants Formation and Their Harmful Effects on Health and Environment. Innov. Energy Policies 2011, 1, 1–9. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, J.; Li, X.; He, C.; Lu, Z.; Lu, Z.; Lu, Z.; Yang, Z.; Wang, Y. C3N monolayers as promising candidates for NO2 sensors. Sens. Actuators B Chem. 2018, 266, 664–673. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, W.; Hong, Y.; Lee, G.; Yoon, D.S. Recent advances in carbon material-based NO2 gas sensors. Sens. Actuators B Chem. 2018, 255, 1788–1804. [Google Scholar] [CrossRef]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, L.B.; Ruan, S.; Li, X.; Yu, S.; Li, X.; Jiang, Y.; Yao, Z.; Ye, S.; Wang, C.; et al. Recent development in nanocarbon materials for gas sensor applications. Sens. Actuators B Chem. 2018, 274, 235–267. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of graphene and its applications: A review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Montejo-Alvaro, F.; Oliva, J.; Zarate, A.; Herrera-Trejo, M.; Hdz-García, H.M.; Mtz-Enriquez, A.I. Icosahedral transition metal clusters (M13, M = Fe, Ni, and Cu) adsorbed on graphene quantum dots, a DFT study. Phys. E Low Dimens. Syst. Nanostruct. 2019, 110, 52–58. [Google Scholar] [CrossRef]
- Milowska, K.Z.; Majewski, J.A. Graphene-based sensors: Theoretical study. J. Phys. Chem. C 2014, 118, 17395–17401. [Google Scholar] [CrossRef]
- Imamura, G.; Minami, K.; Shiba, K.; Mistry, K.; Musselman, K.P.; Yavuz, M.; Yoshikawa, G.; Saiki, K.; Obata, S. Graphene oxide as a sensing material for gas detection based on nanomechanical sensors in the static mode. Chemosensors 2020, 8, 82. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Mandeep, J.; Kakkar, R. Recent Advances on Graphene-Based Gas Sensors. Russ. J. Phys. Chem. A 2020, 94, 2115–2120. [Google Scholar] [CrossRef]
- Noei, M. DFT study on the sensitivity of open edge graphene toward CO2 gas. Vacuum 2016, 131, 194–200. [Google Scholar] [CrossRef]
- Varghese, S.S.; Swaminathan, S.; Singh, K.K.; Mittal, V. Ab initio study on gas sensing properties of group III (B, Al and Ga) doped graphene. Comput. Condens. Matter 2016, 9, 40–55. [Google Scholar] [CrossRef]
- Lv, R.; Li, Q.; Botello-Méndez, A.R.; Hayashi, T.; Wang, B.; Berkdemir, A.; Hao, Q.; Elías, A.L.; Cruz-Silva, R.; Gutiérrez, H.R.; et al. Nitrogen-doped graphene: Beyond single substitution and enhanced molecular sensing. Sci. Rep. 2012, 2, 586. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.; Kaur, M.; Kaur, N.; Kumari, V.; Singh, P.P. Heteroatom-doped graphene as sensing materials: A mini review. RSC Adv. 2020, 10, 28608–28629. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Yao, X. Defects on carbons for electrocatalytic oxygen reduction. Chem. Soc. Rev. 2018, 47, 7628–7658. [Google Scholar] [CrossRef] [PubMed]
- Dua, V.; Surwade, S.P.; Ammu, S.; Agnihotra, S.R.; Jain, S.; Roberts, K.E.; Park, S.; Ruoff, R.S.; Manohar, S.K. All-organic vapor sensor using inkjet-printed reduced graphene oxide. Angew. Chem. Int. Ed. 2010, 49, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Park, S.; Yu, K.; Ruoff, R.S.; Ocola, L.E.; Rosenmann, D.; Chen, J. Toward practical gas sensing with highly reduced graphene oxide: A new signal processing method to circumvent run-to-run and device-to-device variations. ACS Nano 2011, 5, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density functional theory of electronic structure. J. Phys. Chem. 1996, 100, 12974–12980. [Google Scholar] [CrossRef]
- Zeng, Y.; Lin, S.; Gu, D.; Li, X. Two-dimensional nanomaterials for gas sensing applications: The role of theoretical calculations. Nanomaterials 2018, 8, 851. [Google Scholar] [CrossRef]
- Tang, X.; Du, A.; Kou, L. Gas sensing and capturing based on two-dimensional layered materials: Overview from theoretical perspective. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1361. [Google Scholar] [CrossRef]
- Singh, E.; Meyyappan, M.; Nalwa, H.S. Flexible graphene-based wearable gas and chemical sensors. ACS Appl. Mater. Interfaces 2017, 9, 34544–34586. [Google Scholar] [CrossRef] [PubMed]
- Yavari, F.; Koratkar, N. Graphene-based chemical sensors. J. Phys. Chem. Lett. 2012, 3, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Wehling, T.O.; Novoselov, K.S.; Morozov, S.V.; Vdovin, E.E.; Katsnelson, M.I.; Geim, A.K.; Lichtenstein, A.I. Molecular doping of graphene. Nano Lett. 2008, 8, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ni, J.; Fang, C. Adsorption capacity of H2O, NH3, CO, and NO2 on the pristine graphene. J. Appl. Phys. 2013, 113, 034306. [Google Scholar] [CrossRef]
- Silvestrelli, P.L.; Ambrosetti, A. Including screening in van der Waals corrected density functional theory calculations: The case of atoms and small molecules physisorbed on graphene. J. Chem. Phys. 2014, 140, 124107. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, W.; Li, C.; Pan, L.; Dai, X.; Ma, D. Adsorption behavior of Co anchored on graphene sheets toward NO, SO2, NH3, CO and HCN molecules. Appl. Surf. Sci. 2015, 342, 191–199. [Google Scholar] [CrossRef]
- Rad, A.S.; Abedini, E. Chemisorption of NO on Pt-decorated graphene as modified nanostructure media: A first principles study. Appl. Surf. Sci. 2016, 360, 1041–1046. [Google Scholar] [CrossRef]
- Rad, A.S.; Zareyee, D. Adsorption properties of SO2 and O3 molecules on Pt-decorated graphene: A theoretical study. Vacuum 2016, 130, 113–118. [Google Scholar] [CrossRef]
- Song, Y.D.; Wang, L.; Wu, L.M. Theoretical study of the CO, NO, and N2 adsorptions on Li-decorated graphene and boron-doped graphene. Can. J. Chem. 2018, 96, 30–39. [Google Scholar] [CrossRef]
- Bo, Z.; Guo, X.; Wei, X.; Yang, H.; Yan, J.; Cen, K. Density functional theory calculations of NO2 and H2S adsorption on the group 10 transition metal (Ni, Pd and Pt) decorated graphene. Phys. E Low Dimens. Syst. Nanostruct. 2019, 109, 156–163. [Google Scholar] [CrossRef]
- Rajasekaran, G.; Narayanan, P.; Parashar, A. Effect of point and line defects on mechanical and thermal properties of graphene: A review. Crit. Rev. Solid State Mater. Sci. 2016, 41, 47–71. [Google Scholar] [CrossRef]
- Liu, L.; Qing, M.; Wang, Y.; Chen, S. Defects in graphene: Generation, healing, and their effects on the properties of graphene: A review. J. Mater. Sci. Technol. 2015, 31, 599–606. [Google Scholar] [CrossRef]
- Wang, B.; Pantelides, S.T. Controllable healing of defects and nitrogen doping of graphene by CO and NO molecules. Phys. Rev. B 2011, 83, 245403. [Google Scholar] [CrossRef]
- Hussain, T.; Panigrahi, P.; Ahuja, R. Sensing propensity of a defected graphane sheet towards CO, H2O and NO2. Nanotechnology 2014, 25, 325501. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, Z.; Liu, Z.; Zhou, G.; Hao, S.; Wu, J.; Gu, B.L.; Duan, W. Adsorption of gas molecules on graphene nanoribbons and its implication for nanoscale molecule sensor. J. Phys. Chem. C 2008, 112, 13442–13446. [Google Scholar] [CrossRef]
- Ma, C.; Shao, X.; Cao, D. Nitrogen-doped graphene as an excellent candidate for selective gas sensing. Sci. China Chem. 2014, 57, 911–917. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chen, Y.B.; Zhou, K.G.; Liu, C.H.; Zeng, J.; Zhang, H.L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [PubMed]
- Tit, N.; Said, K.; Mahmoud, N.M.; Kouser, S.; Yamani, Z.H. Ab-initio investigation of adsorption of CO and CO2 molecules on graphene: Role of intrinsic defects on gas sensing. Appl. Surf. Sci. 2017, 394, 219–230. [Google Scholar] [CrossRef]
- Shukri, M.S.M.; Saimin, M.N.S.; Yaakob, M.K.; Yahya, M.Z.A.; Taib, M.F.M. Structural and electronic properties of CO and NO gas molecules on Pd-doped vacancy graphene: A first principles study. Appl. Surf. Sci. 2019, 494, 817–828. [Google Scholar] [CrossRef]
- Yang, S.; Lei, G.; Xu, H.; Xu, B.; Li, H.; Lan, Z.; Wang, Z.; Gu, H. A DFT study of CO adsorption on the pristine, defective, In-doped and Sb-doped graphene and the effect of applied electric field. Appl. Surf. Sci. 2019, 480, 205–211. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, H.; Zhang, Z.; An, L. Effect of doping and vacancy defects on the adsorption of CO on graphene. Mater. Chem. Phys. 2020, 123114. [Google Scholar] [CrossRef]
- Ali, M.; Tit, N. Adsorption of NO and NO2 molecules on defected-graphene and ozone-treated graphene: First-principles analysis. Surf. Sci. 2019, 684, 28–36. [Google Scholar] [CrossRef]
- Zhou, Q.; Ju, W.; Su, X.; Yong, Y.; Li, X. Adsorption behavior of SO2 on vacancy-defected graphene: A DFT study. J. Phys. Chem. Solids 2017, 109, 40–45. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, J.M.; Xu, K.W.; Ji, V. Improving SO2 gas sensing properties of graphene by introducing dopant and defect: A first-principles study. Appl. Surf. Sci. 2014, 313, 405–410. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.P.; Vargas-Hernández, C.N.; Cruz-Martínez, H.; Medina, D.I. Stability, magnetic, energetic, and reactivity properties of icosahedral M@Pd12 (M = Fe, Co, Ni, and Cu) core-shell nanoparticles supported on pyridinic N3-doped graphene. Solid State Sci. 2021, 112, 106483. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.P.; Luo, X.G.; Lin, X.Y.; Lu, X.; Leng, Y.; Song, H.T. Density functional theory calculations on the adsorption of formaldehyde and other harmful gases on pure, Ti-doped, or N-doped graphene sheets. Appl. Surf. Sci. 2013, 283, 559–565. [Google Scholar] [CrossRef]
- Montejo-Alvaro, F.; Oliva, J.; Herrera-Trejo, M.; Hdz-García, H.M.; Mtz-Enriquez, A.I. DFT study of small gas molecules adsorbed on undoped and N-, Si-, B-, and Al-doped graphene quantum dots. Theor. Chem. Acc. 2019, 138, 37. [Google Scholar] [CrossRef]
- Dai, J.; Yuan, J.; Giannozzi, P. Gas adsorption on graphene doped with B, N, Al, and S: A theoretical study. Appl. Phys. Lett. 2009, 95, 232105. [Google Scholar] [CrossRef]
- Zhang, H.P.; Luo, X.G.; Lin, X.Y.; Zhang, Y.P.; Tang, P.P.; Lu, X.; Tang, Y. Band structure of graphene modulated by Ti or N dopants and applications in gas sensoring. J. Mol. Graph. Model. 2015, 61, 224–230. [Google Scholar] [CrossRef]
- Shao, L.; Chen, G.; Ye, H.; Wu, Y.; Qiao, Z.; Zhu, Y.; Niu, H. Sulfur dioxide adsorbed on graphene and heteroatom-doped graphene: A first-principles study. Eur. Phys. J. B 2013, 86, 54. [Google Scholar] [CrossRef]
- Shokuhi Rad, A.; Esfahanian, M.; Maleki, S.; Gharati, G. Application of carbon nanostructures toward SO2 and SO3 adsorption: A comparison between pristine graphene and N-doped graphene by DFT calculations. J. Sulfur Chem. 2016, 37, 176–188. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z.; Shen, Z.; Chen, W.; Ma, D.; Dai, X. Adsorption sensitivity of metal atom decorated bilayer graphene toward toxic gas molecules (CO, NO, SO2 and HCN). Sens. Actuators B Chem. 2017, 238, 182–195. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, Y.; Li, M.; Yang, W.; Ding, X. Adsorption sensitivity of Fe decorated different graphene supports toward toxic gas molecules (CO and NO). Appl. Surf. Sci. 2018, 456, 351–359. [Google Scholar] [CrossRef]
- Wanno, B.; Tabtimsai, C. A DFT investigation of CO adsorption on VIIIB transition metal-doped graphene sheets. Superlattices Microstruct. 2014, 67, 110–117. [Google Scholar] [CrossRef]
- Cortés-Arriagada, D.; Villegas-Escobar, N.; Ortega, D.E. Fe-doped graphene nanosheet as an adsorption platform of harmful gas molecules (CO, CO2, SO2 and H2S), and the co-adsorption in O2 environments. Appl. Surf. Sci. 2018, 427, 227–236. [Google Scholar] [CrossRef]
- Promthong, N.; Tabtimsai, C.; Rakrai, W.; Wanno, B. Transition metal-doped graphene nanoflakes for CO and CO2 storage and sensing applications: A DFT study. Struct. Chem. 2020, 31, 2237–2247. [Google Scholar] [CrossRef]
- Cortés-Arriagada, D.; Villegas-Escobar, N. A DFT analysis of the adsorption of nitrogen oxides on Fe-doped graphene, and the electric field induced desorption. Appl. Surf. Sci. 2017, 420, 446–455. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, W.; Ding, X.; Lv, G.; Yan, W. Support effects in single atom iron catalysts on adsorption characteristics of toxic gases (NO2, NH3, SO3 and H2S). Appl. Surf. Sci. 2018, 436, 585–595. [Google Scholar] [CrossRef]
- Rad, A.S.; Shabestari, S.S.; Mohseni, S.; Aghouzi, S.A. Study on the adsorption properties of O3, SO2, and SO3 on B-doped graphene using DFT calculations. J. Solid State Chem. 2016, 237, 204–210. [Google Scholar] [CrossRef]
- Ao, Z.M.; Yang, J.; Li, S.; Jiang, Q. Enhancement of CO detection in Al doped graphene. Chem. Phys. Lett. 2008, 461, 276–279. [Google Scholar] [CrossRef]
- Ao, Z.M.; Li, S.; Jiang, Q. Correlation of the applied electrical field and CO adsorption/desorption behavior on Al-doped graphene. Solid State Commun. 2010, 150, 680–683. [Google Scholar] [CrossRef]
- Rad, A.S.; Foukolaei, V.P. Density functional study of Al-doped graphene nanostructure towards adsorption of CO, CO2 and H2O. Synth. Met. 2015, 210, 171–178. [Google Scholar] [CrossRef]
- Rad, A.S. First principles study of Al-doped graphene as nanostructure adsorbent for NO2 and N2O: DFT calculations. Appl. Surf. Sci. 2015, 357, 1217–1224. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, J.M.; Xu, K.W.; Ji, V. A first-principles study on gas sensing properties of graphene and Pd-doped graphene. Appl. Surf. Sci. 2015, 343, 121–127. [Google Scholar] [CrossRef]
- Zhang, J.N.; Ma, L.; Zhang, M.; Zhang, J.M. Effects of gas adsorption on electronic and optical properties of palladium-doped graphene: First-principles study. Phys. E Low Dimens. Syst. Nanostruct. 2020, 118, 113879. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Q.; Wang, J.; Xu, L.; Zeng, W. Adsorption of SO2 molecule on Ni-doped and Pd-doped graphene based on first-principle study. Appl. Surf. Sci. 2020, 517, 146180. [Google Scholar] [CrossRef]
- Gao, Z.; Li, L.; Huang, H.; Xu, S.; Yan, G.; Zhao, M.; Ding, Z. Adsorption characteristics of acid gases (NO, NO2, SO2 and SO3) on different single-atom nickel adsorbent: A First-Principles Study. Appl. Surf. Sci. 2020, 527, 146939. [Google Scholar] [CrossRef]
- Ma, H.; Ma, L.; Ma, L.C. Tuning the electronic and magnetic properties of Mn-doped graphene by gas adsorption and effect of external electric field: First-principles study. Int. J. Mod. Phys. B 2019, 33, 1950166. [Google Scholar] [CrossRef]
- Jia, X.; An, L. The adsorption of nitrogen oxides on noble metal-doped graphene: The first-principles study. Mod. Phys. Lett. B 2019, 33, 1950044. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Hwang, Y.; Chung, Y.C. Modulating magnetic characteristics of Pt embedded graphene by gas adsorption (N2, O2, NO2, SO2). Appl. Surf. Sci. 2014, 289, 445–449. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, B.; Zhao, J.X.; Cai, Q.H.; Fu, H.G. Si-doped graphene: An ideal sensor for NO- or NO2-detection and metal-free catalyst for N2O-reduction. J. Mol. Model. 2012, 18, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Yuan, J. Modulating the electronic and magnetic structures of P-doped graphene by molecule doping. J. Phys. Condens. Matter 2010, 22, 225501. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.Y.; Ding, N.; Ng, S.P.; Wu, C.M.L. Adsorption of gas molecules on Ga-doped graphene and effect of applied electric field: A DFT study. Appl. Surf. Sci. 2017, 411, 11–17. [Google Scholar] [CrossRef]
- Liang, X.; Ng, S.P.; Ding, N.; Wu, C.M.L. Thermal stability of NO on Ga-doped graphene and effect of external electric field. Comput. Mater. Sci. 2018, 151, 214–221. [Google Scholar] [CrossRef]
- Shao, L.; Chen, G.; Ye, H.; Niu, H.; Wu, Y.; Zhu, Y.; Ding, B. Sulfur dioxide molecule sensors based on zigzag graphene nanoribbons with and without Cr dopant. Phys. Lett. A 2014, 378, 667–671. [Google Scholar] [CrossRef]
- Kumar, J.; Nemade, H.B.; Giri, P.K. Adsorption of small molecules on niobium doped graphene: A study based on density functional theory. IEEE Electron. Device Lett. 2018, 39, 296–299. [Google Scholar] [CrossRef]
- Wang, N.; Yang, S.; Lan, Z.; Xu, H.; Wang, Z.; Hu, Y.; Gu, H. A DFT study of the selective adsorption of XO2 (X = C, S or N) on Ta-doped graphene. Comput. Theor. Chem. 2020, 1190, 113003. [Google Scholar] [CrossRef]
- Liu, X.-J.; Cao, W.-Q.; Huang, Z.-H.; Yuan, J.; Fang, X.-Y.; Cao, M.-S. Electronic structures and adsorption of Li-doped graphenes for CO. Chin. Phys. Lett. 2015, 32, 036802. [Google Scholar] [CrossRef]
- Braubach, M.; Algoet, A.; Beaton, M.; Lauriou, S.; Héroux, M.E.; Krzyzanowski, M. Mortality associated with exposure to carbon monoxide in WHO European Member States. Indoor Air 2013, 23, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.D.; Wang, L.; Wang, Q.T. Computational study of the NO, SO2, and NH3 adsorptions on fragments of 3N-graphene and Al/3N graphene. J. Mol. Model. 2018, 24, 210. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, T. Sub-ppb and ultra selective nitrogen dioxide sensor based on sulfur doped graphene. Sens. Actuators B Chem. 2018, 255, 2258–2263. [Google Scholar] [CrossRef]
- Lv, R.; Chen, G.; Li, Q.; McCreary, A.; Botello-Méndez, A.; Morozov, S.V.; Liang, L.; Declerck, X.; Perea-Lopez, N.; Cullen, D.A.; et al. Ultrasensitive gas detection of large-area boron-doped graphene. Proc. Natl. Acad. Sci. USA 2015, 112, 14527–14532. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Yan, W.; Long, H.; Nelson, A.J.; Baker, A.; Lee, J.R.; Carraro, C.; Worsley, M.A.; Maboudian, R.; Zettl, A. Boron doping and defect engineering of graphene aerogels for ultrasensitive NO2 detection. J. Phys. Chem. C 2018, 122, 20358–20365. [Google Scholar] [CrossRef]
- Shaik, M.; Rao, V.K.; Gupta, M.; Murthy, K.S.R.C.; Jain, R. Chemiresistive gas sensor for the sensitive detection of nitrogen dioxide based on nitrogen doped graphene nanosheets. RSC Adv. 2016, 6, 1527–1534. [Google Scholar] [CrossRef]
- Srivastava, S.; Kashyap, P.K.; Singh, V.; Senguttuvan, T.D.; Gupta, B.K. Nitrogen doped high quality CVD grown graphene as a fast responding NO2 gas sensor. New J. Chem. 2018, 42, 9550–9556. [Google Scholar] [CrossRef]
- Niu, F.; Shao, Z.W.; Gao, H.; Tao, L.M.; Ding, Y. Si-doped graphene nanosheets for NOx gas sensing. Sens. Actuators B Chem. 2021, 328, 129005. [Google Scholar] [CrossRef]
| Gas or Vapor | Irritate | Odor | Signs and Symptoms | Refs. |
|---|---|---|---|---|
| Carbon monoxide | No | No | Tissue hypoxia, hypoxic cardiac dysfunction, subtle cardiovascular, unconsciousness, and death after prolonged exposures or after acute exposures to high concentrations of CO. | [3,4,5] |
| Nitrogen oxides | Yes | No | Nausea, headache, respiratory illness (cough and irritation of the respiratory tract), asthma, pneumonia, possibly tuberculosis, and Parkinson’s disease. | [6,7,8,9] |
| Sulfur oxides | Yes | Yes | Neurological damage, bronchitis, bronchial asthma, emphysema, bronchoconstriction and mucus. | [3,4,10,11] |
| Material | Gas | Eads (in eV) | Functional | Approach | Refs. |
|---|---|---|---|---|---|
| Co-decorated graphene | CO | −2.19 | PBE | Supercell | [47] |
| Co-decorated graphene | NO | −4.04 | PBE | Supercell | [47] |
| Co-decorated graphene | SO2 | −2.35 | PBE | Supercell | [47] |
| Pt-decorated graphene | NO | −2.06 | B3LYP | Supercell | [48] |
| Pt-decorated graphene | NO2 | −2.00 | PBE | Supercell | [51] |
| Pt-decorated graphene | SO2 | −1.58 | B3LYP | Supercell | [49] |
| Li- decorated graphene | CO | −0.55 | B3LYP | Finite system | [50] |
| Li-decorated graphene | NO | −0.14 | B3LYP | Finite system | [50] |
| Ni-decorated graphene | NO2 | −2.63 | PBE | Supercell | [51] |
| Pd-decorated graphene | NO2 | −1.59 | PBE | Supercell | [51] |
| Material | Gas | Eads (in eV) | Functional | Approach | Refs. |
|---|---|---|---|---|---|
| Single vacancy | CO | −6.05 | GGA | Supercell | [57] |
| Single vacancy | CO | −2.33 | CA-PZ | Supercell | [58] |
| Single vacancy | CO | −5.06 a, −5.15 | PBE | Supercell | [59] |
| Single vacancy | CO | −0.07 | PBE | Supercell | [60] |
| Single vacancy | CO | −1.86 | PBE | Supercell | [61] |
| Single vacancy | CO | −0.18 | PBE | Supercell | [62] |
| Single vacancy | NO | −6.64 | GGA | Supercell | [57] |
| Single vacancy | NO | −3.04 | CA-PZ | Supercell | [58] |
| Single vacancy | NO | −1.20 | PBE | Supercell | [60] |
| Single vacancy | NO | −8.25 | PBE | Supercell | [63] |
| Single vacancy | NO2 | −3.04 | CA-PZ | Supercell | [58] |
| Single vacancy | NO2 | −6.41 b | PBE | Supercell | [63] |
| Single vacancy | SO2 | −2.38 | PBE | Supercell | [64] |
| Stone—Wales | CO | −1.30 a | Supercell | [59] | |
| Stone—Wales | SO2 | −0.19 | PBE | Supercell | [65] |
| Material | Gas | Eads (in eV) | Functional | Approach | Refs. |
|---|---|---|---|---|---|
| N-doped | CO | −0.14 | CA-PZ | Supercell | [58] |
| N-doped | CO | −0.03 | PBE | Supercell | [68] |
| N-doped | CO | −0.13 | PBE | Finite system | [69] |
| N-doped | CO | −0.01 | PBE | Supercell | [70] |
| N-doped | NO | −0.40 | CA-PZ | Supercell | [58] |
| N-doped | NO | −0.08 | PBE | Supercell | [68] |
| N-doped | NO | −0.09 | PBE | Supercell | [70] |
| N-doped | NO | 0.16 | PBE | Supercell | [71] |
| N-doped | NO2 | −0.98 | CA-PZ | Supercell | [58] |
| N-doped | NO2 | −0.26 | PBE | Supercell | [70] |
| N-doped | NO2 | −0.44 | PBE | Supercell | [71] |
| N-doped | SO2 | −0.29 | PBE | Supercell | [68] |
| N-doped | SO2 | −0.19 | PBE | Supercell | [70] |
| N-doped | SO2 | −0.17 | PW91 | Supercell | [72] |
| N-doped | SO2 | −0.29 | B3LYP | Finite system | [73] |
| N-doped | SO3 | −0.68 | B3LYP | Finite system | [73] |
| Fe-doped | CO | −1.71 | PBE | Supercell | [74] |
| Fe-doped | CO | −1.45 | PBE | Supercell | [75] |
| Fe-doped | CO | −1.46 | B3LYP | Finite system | [76] |
| Fe-doped | CO | −1.60 | PBE | Finite system | [77] |
| Fe-doped | CO | −1.50 | B3LYP | Finite system | [78] |
| Fe-doped | NO | −2.40 | PBE | Supercell | [74] |
| Fe-doped | NO | −2.24 | PBE | Supercell | [75] |
| Fe-doped | NO | −2.23 | PBE | Finite system | [79] |
| Fe-doped | NO2 | −2.19 | PBE | Finite system | [79] |
| Fe-doped | NO2 | −2.20 | PBE | Supercell | [80] |
| Fe-doped | SO2 | −1.68 | PBE | Supercell | [74] |
| Fe-doped | SO2 | −1.80 | PBE | Finite system | [77] |
| Fe-doped | SO3 | −1.81 | PBE | Supercell | [80] |
| B-doped | CO | −0.14 | CA-PZ | Supercell | [58] |
| B-doped | CO | −0.13 | PBE | Finite system | [69] |
| B-doped | CO | −0.02 | PBE | Supercell | [70] |
| B-doped | NO | −1.07 | CA-PZ | Supercell | [58] |
| B-doped | NO | −0.34 | PBE | Supercell | [70] |
| B-doped | NO2 | −1.37 | CA-PZ | Supercell | [58] |
| B-doped | NO2 | −0.33 | PBE | Supercell | [70] |
| B-doped | SO2 | −0.03 | PBE | Supercell | [70] |
| B-doped | SO2 | −0.21 | PW91 | Supercell | [72] |
| B-doped | SO2 | −0.12 | B3LYP | Finite system | [81] |
| B-doped | SO3 | −0.18 | B3LYP | Finite system | [81] |
| Al-doped | CO | −0.77 | PBE | Finite system | [69] |
| Al-doped | CO | −0.66 | PBE | Supercell | [70] |
| Al-doped | CO | −4.98 | PBE | Supercell | [82] |
| Al-doped | CO | −0.57 a | PBE | Supercell | [83] |
| Al-doped | CO | −0.56 | B3LYP | Supercell | [84] |
| Al-doped | NO | −1.35 | PBE | Supercell | [70] |
| Al-doped | NO2 | −2.48 | PBE | Supercell | [70] |
| Al-doped | NO2 | −0.65 | B3LYP | Supercell | [85] |
| Al-doped | SO2 | −1.65 | PBE | Supercell | [65] |
| Al-doped | SO2 | −1.54 | PBE | Supercell | [70] |
| Al-doped | SO2 | −1.26 | PW91 | Supercell | [72] |
| Pd-doped | CO | −0.91 | PBE | Supercell | [60] |
| Pd-doped | CO | −0.92 | B3LYP | Finite system | [76] |
| Pd-doped | CO | −1.05 | PBE | Supercell | [86] |
| Pd-doped | CO | −1.07 | PBE | Supercell | [87] |
| Pd-doped | NO | −3.92 | PBE | Supercell | [60] |
| Pd-doped | NO | −1.33 | PBE | Supercell | [82] |
| Pd-doped | NO2 | −2.17 | PBE | Supercell | [87] |
| Pd-doped | NO2 | −2.19 | PBE | Supercell | [87] |
| Pd-doped | SO2 | −1.12 | PBE | Supercell | [87] |
| Pd-doped | SO2 | −5.78 | PBE | Supercell | [88] |
| Ni-doped | CO | −1.02 | B3LYP | Finite system | [76] |
| Ni-doped | CO | −0.96 | B3LYP | Finite system | [78] |
| Ni-doped | NO | −1.64 | PBE | Supercell | [89] |
| Ni-doped | NO2 | −1.83 | PBE | Supercell | [89] |
| Ni-doped | SO2 | −4.21 | PBE | Supercell | [88] |
| Ni-doped | SO2 | −0.92 | PBE | Supercell | [89] |
| Ni-doped | SO3 | −1.59 | PBE | Supercell | [89] |
| Ti-doped | CO | −0.45 | PBE | Supercell | [68] |
| Ti-doped | CO | −1.00 | B3LYP | Finite system | [78] |
| Ti-doped | NO | −1.72 | PBE | Supercell | [68] |
| Ti-doped | NO | −1.44 | PBE | Supercell | [71] |
| Ti-doped | NO2 | −2.98 | PBE | Supercell | [71] |
| Ti-doped | SO2 | −3.20 | PBE | Supercell | [68] |
| Mn-doped | CO | −1.50 | PBE | Supercell | [62] |
| Mn-doped | CO | −1.42 | B3LYP | Finite system | [78] |
| Mn-doped | NO | −2.14 | PBE | Supercell | [90] |
| Mn-doped | NO2 | −2.76 | PBE | Supercell | [90] |
| Mn-doped | SO2 | −1.73 | PW91 | Supercell | [72] |
| Mn-doped | SO2 | −1.83 | PBE | Supercell | [90] |
| Co-doped | CO | −0.94 | B3LYP | Finite system | [76] |
| Co-doped | CO | −0.94 | B3LYP | Finite system | [78] |
| Co-doped | CO | −0.62 | PBE | Supercell | [47] |
| Co-doped | NO | −1.51 | PBE | Supercell | [47] |
| Co-doped | SO2 | −1.07 | PBE | Supercell | [47] |
| Pt-doped | CO | −1.30 | B3LYP | Finite system | [76] |
| Pt-doped | NO | −6.22 | PBE | Supercell | [91] |
| Pt-doped | NO2 | −7.37 | PBE | Supercell | [91] |
| Pt-doped | NO2 | −2.21 | PBE | Supercell | [92] |
| Pt-doped | SO2 | −1.02 | PW91 | Supercell | [72] |
| Pt-doped | SO2 | −1.06 | PBE | Supercell | [92] |
| Si-doped | CO | −0.25 | PBE | Finite system | [69] |
| Si-doped | NO | −0.82 | PBE | Supercell | [93] |
| Si-doped | NO2 | −2.17 | PBE | Supercell | [93] |
| Si-doped | SO2 | −0.90 | PW91 | Supercell | [72] |
| P-doped | CO | −0.07 | PBE | Supercell | [94] |
| P-doped | NO | −0.51 | PBE | Supercell | [94] |
| P-doped | NO2 | −1.89 | PBE | Supercell | [94] |
| P-doped | SO2 | −0.32 | PBE | Supercell | [94] |
| S-doped | CO | −0.01 | PBE | Supercell | [70] |
| S-doped | NO | −0.12 | PBE | Supercell | [70] |
| S-doped | NO2 | −0.83 | PBE | Supercell | [70] |
| S-doped | SO2 | −0.09 | PBE | Supercell | [70] |
| Ga-doped | CO | −0.67 | PBE | Supercell | [95] |
| Ga-doped | NO | −0.78 | PBE | Supercell | [95] |
| Ga-doped | NO | −0.81 | PBE | Supercell | [96] |
| Ga-doped | NO2 | −1.93 | PBE | Supercell | [95] |
| Ag-doped | NO | −6.93 | PBE | Supercell | [91] |
| Ag-doped | NO2 | −7.83 | PBE | Supercell | [91] |
| Ag-doped | SO2 | −0.97 | PW91 | Supercell | [72] |
| Au-doped | NO | −8.47 | PBE | Supercell | [91] |
| Au-doped | NO2 | −9.34 | PBE | Supercell | [91] |
| Au-doped | SO2 | −1.28 | PW91 | Supercell | [72] |
| Cr-doped | CO | −1.63 | B3LYP | Finite system | [78] |
| Cr-doped | SO2 | −1.68 | PW91 | Supercell | [72] |
| Cr-doped | SO2 | −1.59 b | PW91 | Supercell | [97] |
| Nb-doped | CO | −0.53 | PBE | Supercell | [98] |
| Nb-doped | SO2 | −0.32 | PBE | Supercell | [98] |
| Ta-doped | NO2 | −2.31 | PBE | Supercell | [99] |
| Ta-doped | SO2 | −1.68 | PBE | Supercell | [99] |
| Li-doped | CO | −3.51 | PBE | Supercell | [100] |
| Sc-doped | CO | −0.35 | B3LYP | Finite system | [78] |
| V-doped | CO | −0.55 | B3LYP | Finite system | [78] |
| Cu-doped | CO | −1.20 | B3LYP | Finite system | [78] |
| Zn-doped | CO | −0.67 | B3LYP | Finite system | [78] |
| Ru-doped | CO | −1.22 | B3LYP | Finite system | [76] |
| Rh-doped | CO | −1.01 | B3LYP | Finite system | [76] |
| In-doped | CO | −0.02 | PBE | Supercell | [61] |
| Sb-doped | CO | −0.01 | PBE | Supercell | [61] |
| Os-doped | CO | −1.80 | B3LYP | Finite system | [76] |
| Ir-doped | CO | −1.57 | B3LYP | Finite system | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Martínez, H.; Rojas-Chávez, H.; Montejo-Alvaro, F.; Peña-Castañeda, Y.A.; Matadamas-Ortiz, P.T.; Medina, D.I. Recent Developments in Graphene-Based Toxic Gas Sensors: A Theoretical Overview. Sensors 2021, 21, 1992. https://doi.org/10.3390/s21061992
Cruz-Martínez H, Rojas-Chávez H, Montejo-Alvaro F, Peña-Castañeda YA, Matadamas-Ortiz PT, Medina DI. Recent Developments in Graphene-Based Toxic Gas Sensors: A Theoretical Overview. Sensors. 2021; 21(6):1992. https://doi.org/10.3390/s21061992
Chicago/Turabian StyleCruz-Martínez, Heriberto, Hugo Rojas-Chávez, Fernando Montejo-Alvaro, Yesica A. Peña-Castañeda, Pastor T. Matadamas-Ortiz, and Dora I. Medina. 2021. "Recent Developments in Graphene-Based Toxic Gas Sensors: A Theoretical Overview" Sensors 21, no. 6: 1992. https://doi.org/10.3390/s21061992
APA StyleCruz-Martínez, H., Rojas-Chávez, H., Montejo-Alvaro, F., Peña-Castañeda, Y. A., Matadamas-Ortiz, P. T., & Medina, D. I. (2021). Recent Developments in Graphene-Based Toxic Gas Sensors: A Theoretical Overview. Sensors, 21(6), 1992. https://doi.org/10.3390/s21061992