Metal Oxide Nanorods-Based Sensor Array for Selective Detection of Biomarker Gases
Abstract
1. Introduction
2. Materials and Methods
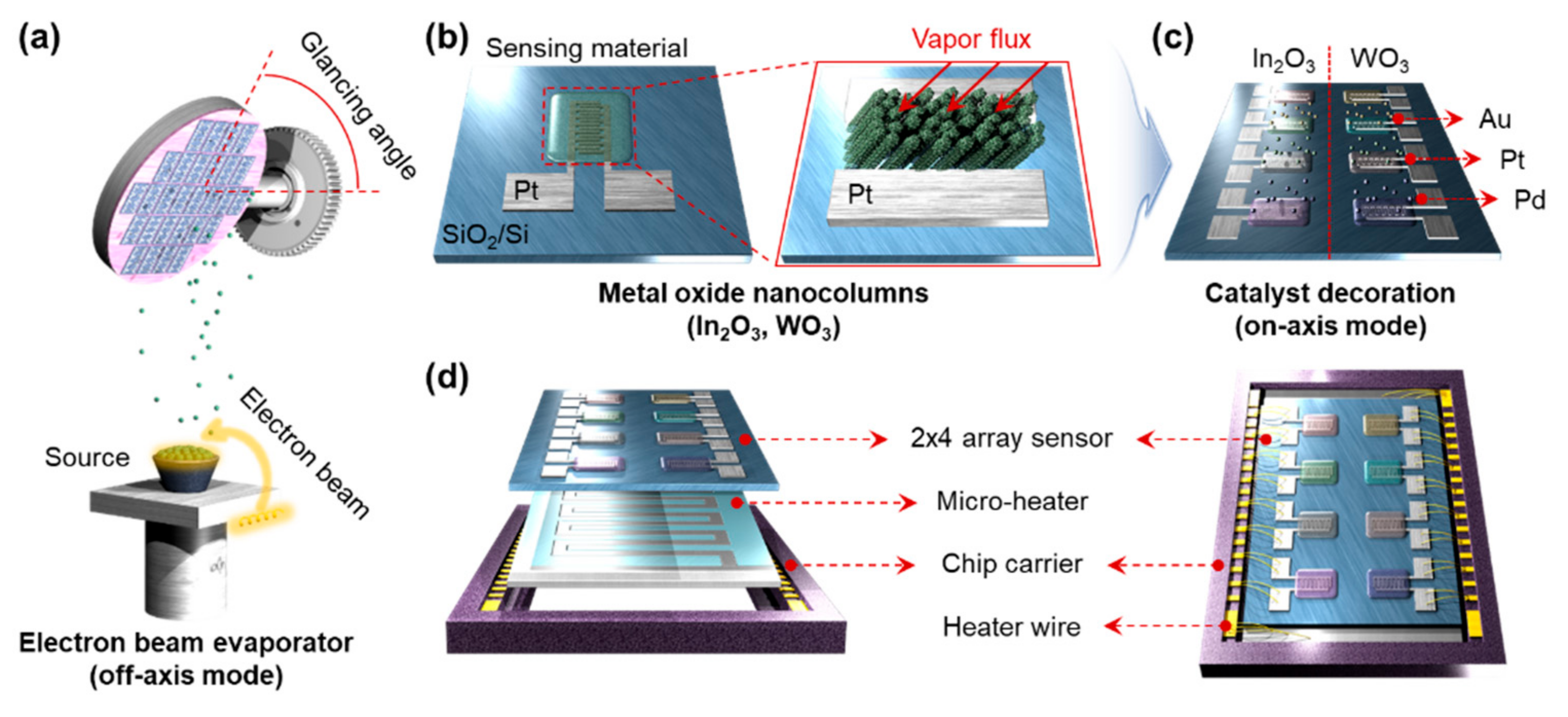
2.1. Device Fabrication
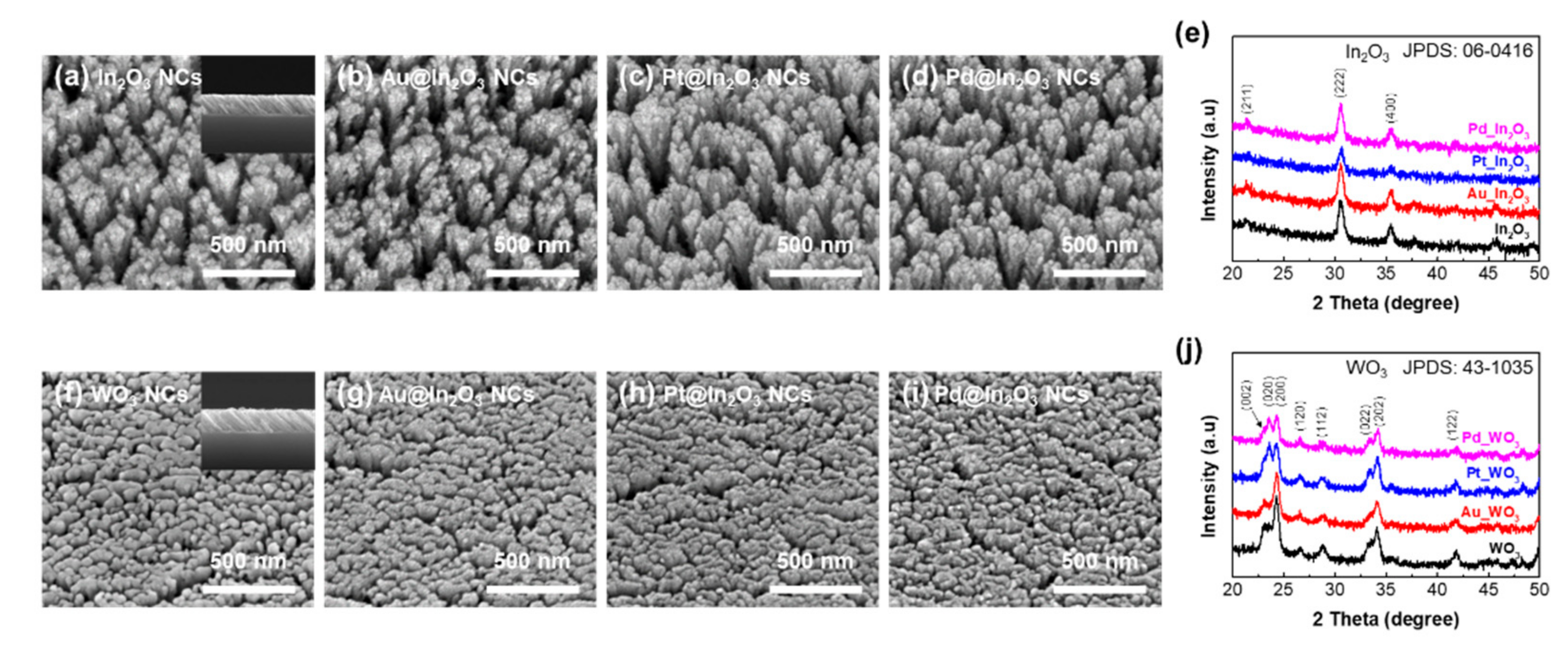
2.2. Characterization
2.3. Sensor Property Measurement
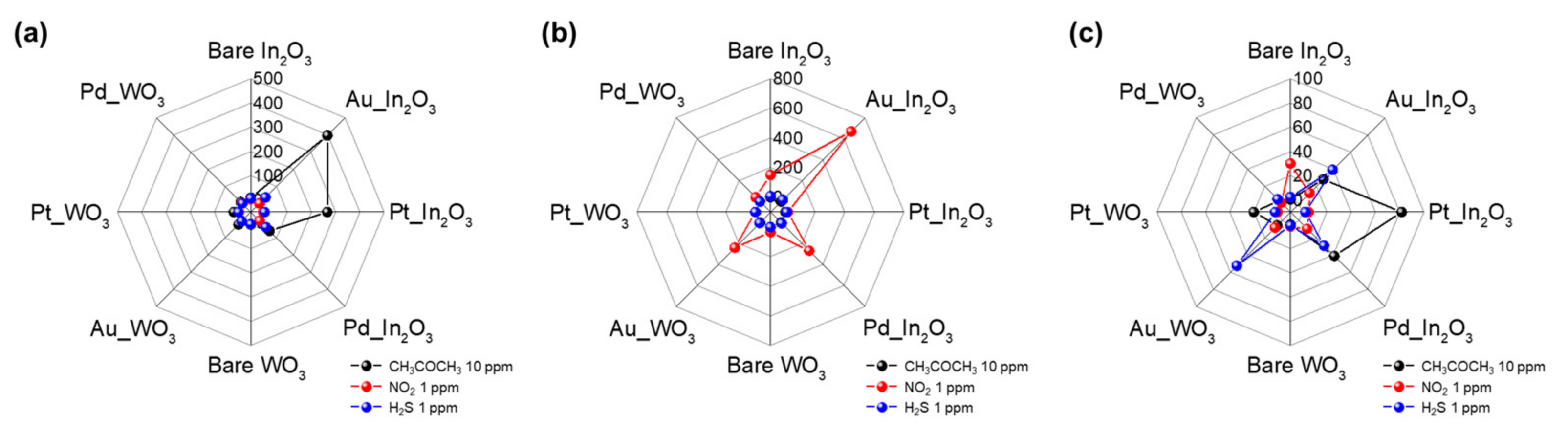
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhawan, A.P.; Heetderks, W.J.; Pavel, M.; Acharya, S.; Akay, M.; Mairal, A.; Wheeler, B.; Dacso, C.C.; Sunder, T.; Lovell, N.H.; et al. Current and future challenges in point-of-care technologies: A paradigm-shift in affordable global healthcare with personalized and preventive medicine. IEEE J. Transl. Eng. Health Med. 2015, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van Netten, J.J.; Woodburn, J.; Bus, S.A. The future for diabetic foot ulcer prevention: A paradigm shift from stratified healthcare towards personalized medicine. Diabetes Metab. Res. Rev. 2020, 36, e3234. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Glusman, G.; Brogaard, K.; Price, N.D.; Hood, L. P4 medicine: How systems medicine will transform the healthcare sector and society. Pers. Med. 2013, 10, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, D.N.; Motaung, D.E.; Swart, H.C. Selective detection of CO at room temperature with CuO nanoplatelets sensor for indoor air quality monitoring manifested by crystallinity. Appl. Surf. Sci. 2019, 466, 545–553. [Google Scholar] [CrossRef]
- Stefanov, B.I.; Lebrun, D.; Mattsson, A.; Granqvist, C.G.; Österlund, L. Demonstrating online monitoring of air pollutant photodegradation in a 3D printed gas-phase photocatalysis reactor. J. Chem. Educ. 2015, 92, 678–682. [Google Scholar] [CrossRef]
- World Health Organization. Air Quality Guidelines for Europe, 2nd ed.; WHO Regional Publications; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- Spergel, J.M.; Fogg, M.I.; Bokszczanin-Knosala, A. Correlation of exhaled nitric oxide, spirometry and asthma symptoms. J. Asthma 2005, 42, 879–883. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C. Is breath acetone a biomarker of diabetes? A historical review on breath acetone measurements. J. Breath Res. 2013, 7, 037109. [Google Scholar] [CrossRef]
- Probert, C.S.; Khalid, T.; Ahmed, I.; Johnson, E.; Smith, S.; Ratcliffe, N.M. Volatile organic compounds as diagnostic biomarkers in gastrointestinal and liver diseases. J. Gastrointestin. Liver Dis. 2009, 18, 337–343. [Google Scholar] [PubMed]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Yu, H.Y.; Baek, I.B.; Ahn, C.G.; Lee, B.K.; Kim, Y.; Yoon, Y.S.; Lim, J.E.; Lee, B.J.; Jang, W.I.; et al. Use of gas-sensor array technology in lung cancer diagnosis. J. Sens. Sci. Technol. 2013, 22, 249–255. [Google Scholar] [CrossRef]
- Yoon, J.W.; Lee, J.H. Toward breath analysis on a chip for disease diagnosis using semiconductor-based chemiresistors: Recent progress and future perspectives. Lab Chip 2017, 17, 3537–3557. [Google Scholar] [CrossRef]
- Song, Y.G.; Shim, Y.S.; Suh, J.M.; Noh, M.S.; Kim, G.S.; Choi, K.S. Ionic-activated chemiresistive gas sensors for room-temperature operation. Small 2019, 15, 1902065. [Google Scholar] [CrossRef]
- Song, Y.G.; Shim, Y.S.; Han, S.D.; Lee, H.R.; Ju, B.K.; Kang, C.Y. Metal oxide nanocolumns for extremely sensitive gas sensors. J. Sens. Sci. Technol. 2016, 25, 184–188. [Google Scholar] [CrossRef]
- Song, Y.G.; Park, J.Y.; Suh, J.M.; Shim, Y.-S.; Yi, S.Y.; Jang, H.W.; Kim, S.; Yuk, J.M.; Ju, B.-K.; Kang, C.-Y. Heterojunction based on Rh-decorated WO3 nanorods for morphological change and gas sensor application using the transition effect. Chem. Mater. 2018, 31, 207–215. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Gong, B.; Shi, T.; Zhu, W.; Liao, G.; Li, X.; Huang, J.; Zhou, T.; Tang, Z. UV irradiation-assisted ethanol detection operated by the gas sensor based on ZnO nanowires/optical fiber hybrid structure. Sens. Actuators B Chem. 2017, 245, 821–827. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Cai, L.; Qi, W.; Sun, Y.; Xu, J.L.; Sun, M.; Zhu, H.; Xiang, L.; Xie, D.; et al. UV light irradiation enhanced gas sensor selectivity of NO2 and SO2 using rGO functionalized with hollow SnO2 nanofibers. Sens. Actuators B Chem. 2019, 290, 443–452. [Google Scholar] [CrossRef]
- Jeon, J.M.; Shim, Y.S.; Han, S.D.; Kim, Y.H.; Kang, C.Y.; Kim, J.S.; Kim, M.; Jang, H.W. Vertically ordered SnO2 nanobamboos for substantially improved detection of volatile reducing gases. J. Mater. Chem. A 2015, 3, 17939–17945. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, J.S.; Lee, H.J. Pt-doped SnO2 thin film based micro gas sensors with high selectivity to toluene and HCHO. Sens. Actuators B Chem. 2017, 248, 1011–1016. [Google Scholar] [CrossRef]
- Suh, J.M.; Shim, Y.-S.; Kwon, K.C.; Jeon, J.-M.; Lee, T.H.; Shokouhimehr, M.; Jang, H.W. Pd-and Au-decorated MoS2 gas sensors for enhanced selectivity. Electron. Mater. Lett. 2019, 15, 368–376. [Google Scholar] [CrossRef]
- Choi, S.J.; Ku, K.H.; Kim, B.J.; Kim, I.D. Novel templating route using Pt infiltrated block copolymer microparticles for catalytic Pt functionalized macroporous WO3 nanofibers and its application in breath pattern recognition. ACS Sens. 2016, 1, 1124–1131. [Google Scholar] [CrossRef]
- Moon, H.G.; Jung, Y.; Han, S.D.; Shim, Y.S.; Shin, B.; Lee, T.; Kim, J.S.; Lee, S.; Jun, S.C.; Park, H.H.; et al. Chemiresistive electronic nose toward detection of biomarkers in exhaled breath. ACS Appl. Mater. Interfaces 2016, 8, 20969–20976. [Google Scholar] [CrossRef]
- Han, S.D.; Noh, M.S.; Kim, S.; Shim, Y.S.; Song, Y.G.; Lee, K.; Lee, H.R.; Nahm, S.; Yoon, S.J.; Kim, J.S. Verstile approaches to tune a nanocolumnar structure for optimized electrical properties of In2O3 based gas sensor. Sens. Actuators B Chem. 2017, 248, 894–901. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, Z.; Chen, X.; Huang, C.; Bai, W.; Lu, Z.; Wang, T. Nanomaterial-based gas sensors used for breath diagnosis. J. Mater. Chem. B. 2020, 8, 3231. [Google Scholar] [CrossRef]
- Hoa, N.D.; Duy, N.V.; Hieu, N.V. Crystalline mesoporous tungsten oxide nanoplate monoliths synthesized by directed soft template method for highly sensitive NO2 gas sensor applications. Mater. Res. Bull. 2013, 48, 440–448. [Google Scholar] [CrossRef]
- Volanti, D.P.; Felix, A.A.; Orlandi, M.O.; Whitfield, G.; Yang, D.-J.; Longo, E.; Tuller, H.L.; Varela, J.A. The role of hierarchical morphologies in the superior gas sensing performance of CuO-based chemiresistors. Adv. Funct. Mater. 2013, 23, 1759–1766. [Google Scholar] [CrossRef]
- Nasiri, N.S.; Clarke, C.T. Nanostructured chemiresistive gas sensors for medical applications. Sensors 2019, 19, 462. [Google Scholar] [CrossRef] [PubMed]
- Daryakenari, A.A.; Apostoluk, A.; Delaunay, J.J. Effect of Pt decoration on the gas response of ZnO nanoparticles. Phys. Status Solidi C 2013, 10, 1297–1300. [Google Scholar] [CrossRef]
- Elias, M.R.; Rotem, V.; Stephane, R. Measurement of temperature and relative humidity in exhaled breath. Sens. Actuators B Chem. 2020, 304, 127371. [Google Scholar]
- Lawryk, N.J.; Lioy, P.J.; Weisel, C.P. Exposure to volatile organic compounds in the passenger compartment of automobiles during periods of normal and malfunctioning operation. J. Expos. Anal. Environ. Epidemiol. 1995, 5, 511–531. [Google Scholar]
- Tom, A.; Tomas, E.; Ingemar, L.; Per, M. Drift correction for gas sensors using multivariate methods. J. Chemom. 2000, 14, 711–723. [Google Scholar]
- Alice, C.; Sabato, M.; Marco, C. An exploratory factor analysis of sensor-based physical capability assessment. Sensors 2019, 19, 2227. [Google Scholar]
- Alexander, S.; Vikram, J.; Sergei, S. Gas sensor array based on metal-decorated carbon nanotubes. J. Phys. Chem. B 2006, 110, 42. [Google Scholar]
- Sanchez, C.; Santos, P.; Lozano, J. Use of electronic noses for diagnosis of digestive and respiratory diseases through the breath. Biosensors 2019, 9, 35. [Google Scholar] [CrossRef]
- Wilson, A.D. Applications of electronic-nose technologies for noninvasive early detection of plant, animal and human diseases. Chemosensors 2018, 6, 45. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.S.; Park, Y.; Shin, J.; Song, Y.G.; Kang, C.-Y. Metal Oxide Nanorods-Based Sensor Array for Selective Detection of Biomarker Gases. Sensors 2021, 21, 1922. https://doi.org/10.3390/s21051922
Kim GS, Park Y, Shin J, Song YG, Kang C-Y. Metal Oxide Nanorods-Based Sensor Array for Selective Detection of Biomarker Gases. Sensors. 2021; 21(5):1922. https://doi.org/10.3390/s21051922
Chicago/Turabian StyleKim, Gwang Su, Yumin Park, Joonchul Shin, Young Geun Song, and Chong-Yun Kang. 2021. "Metal Oxide Nanorods-Based Sensor Array for Selective Detection of Biomarker Gases" Sensors 21, no. 5: 1922. https://doi.org/10.3390/s21051922
APA StyleKim, G. S., Park, Y., Shin, J., Song, Y. G., & Kang, C.-Y. (2021). Metal Oxide Nanorods-Based Sensor Array for Selective Detection of Biomarker Gases. Sensors, 21(5), 1922. https://doi.org/10.3390/s21051922




