Pentacyanoammineferrate-Based Non-Enzymatic Electrochemical Biosensing Platform for Selective Uric Acid Measurement
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
2.2. Electrochemical Measurements
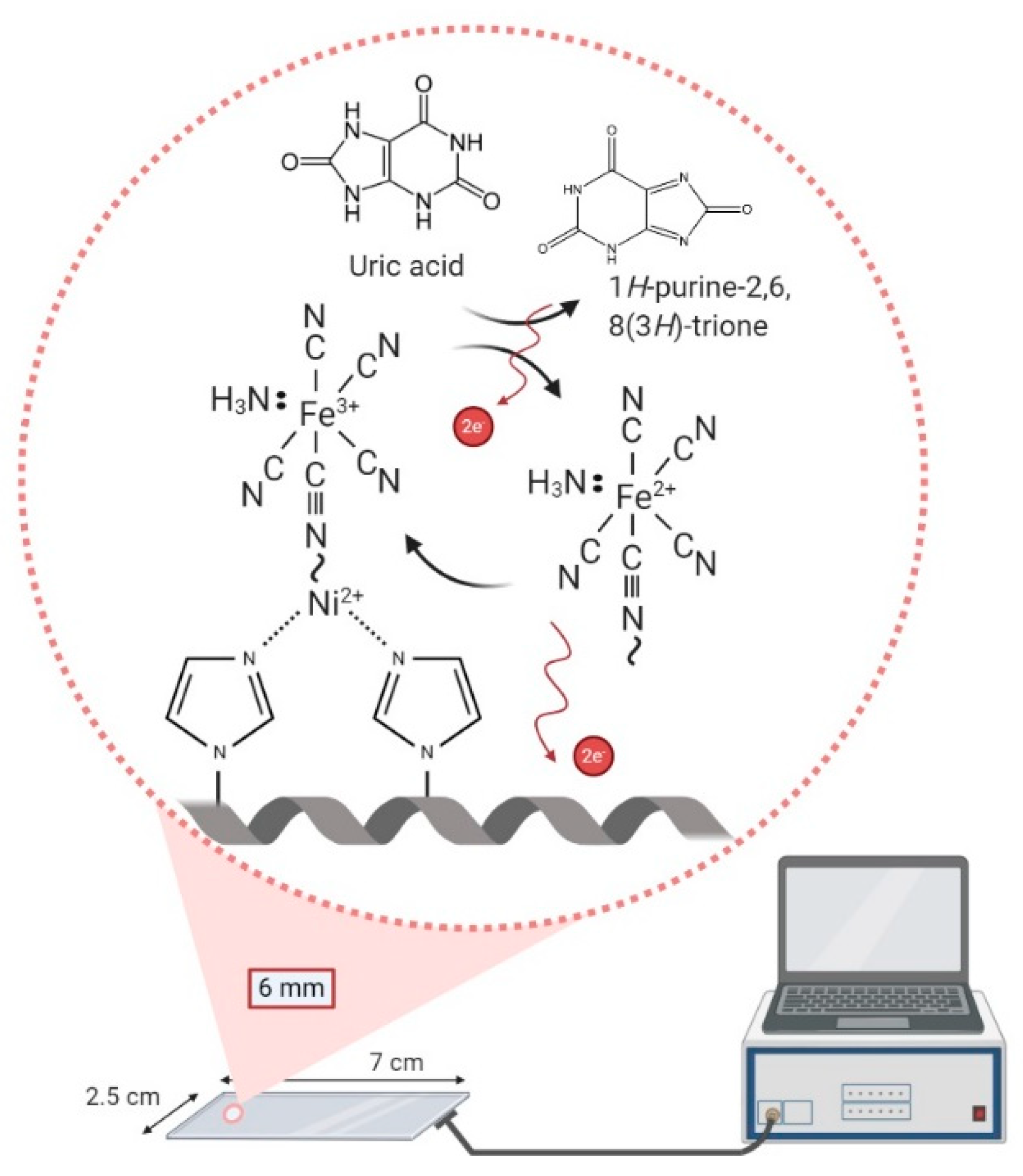
2.3. Fabrication of Nickel (II) Ions- and PVI-Modified ITO Electrode (Ni-PVI-ITO Electrode)
2.4. Optimization of Ni-PVI-ITO Electrode Modification Procedures
2.5. Uric Acid Determination
3. Results and Discussion
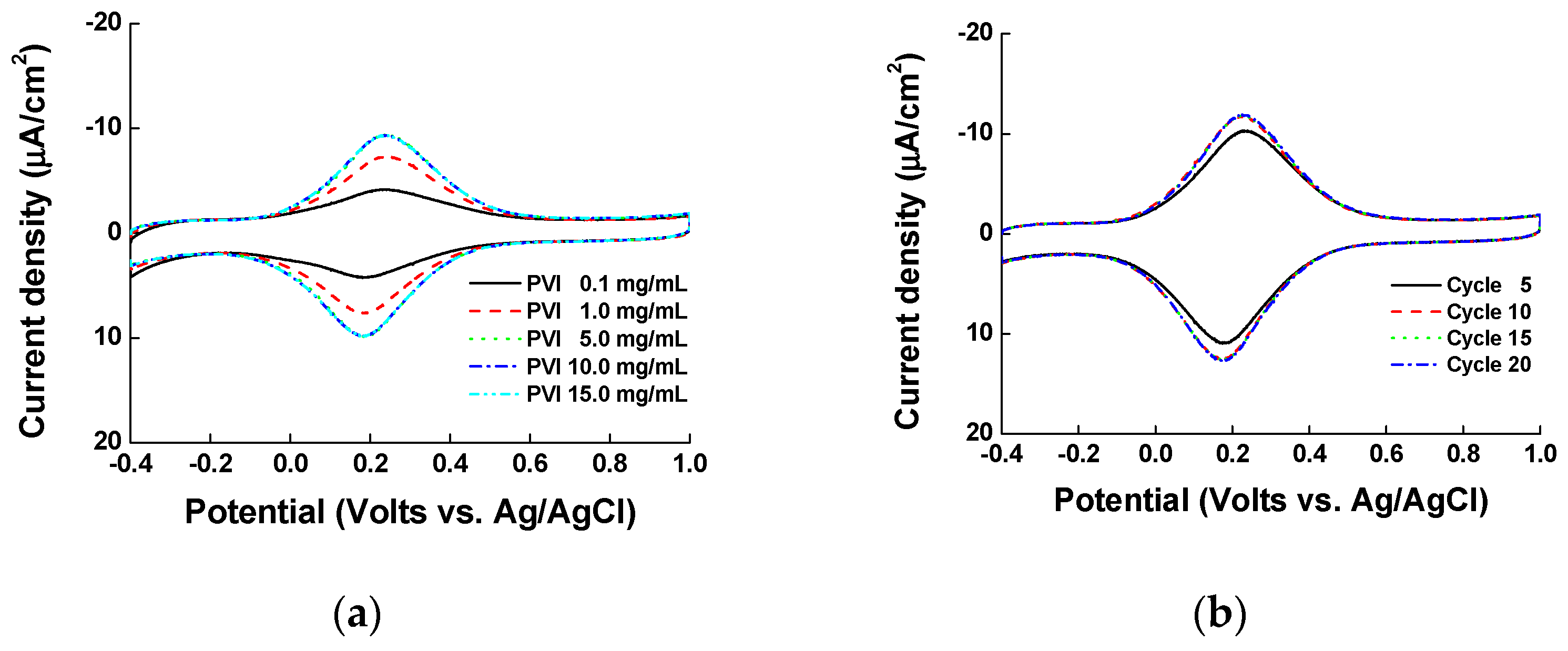
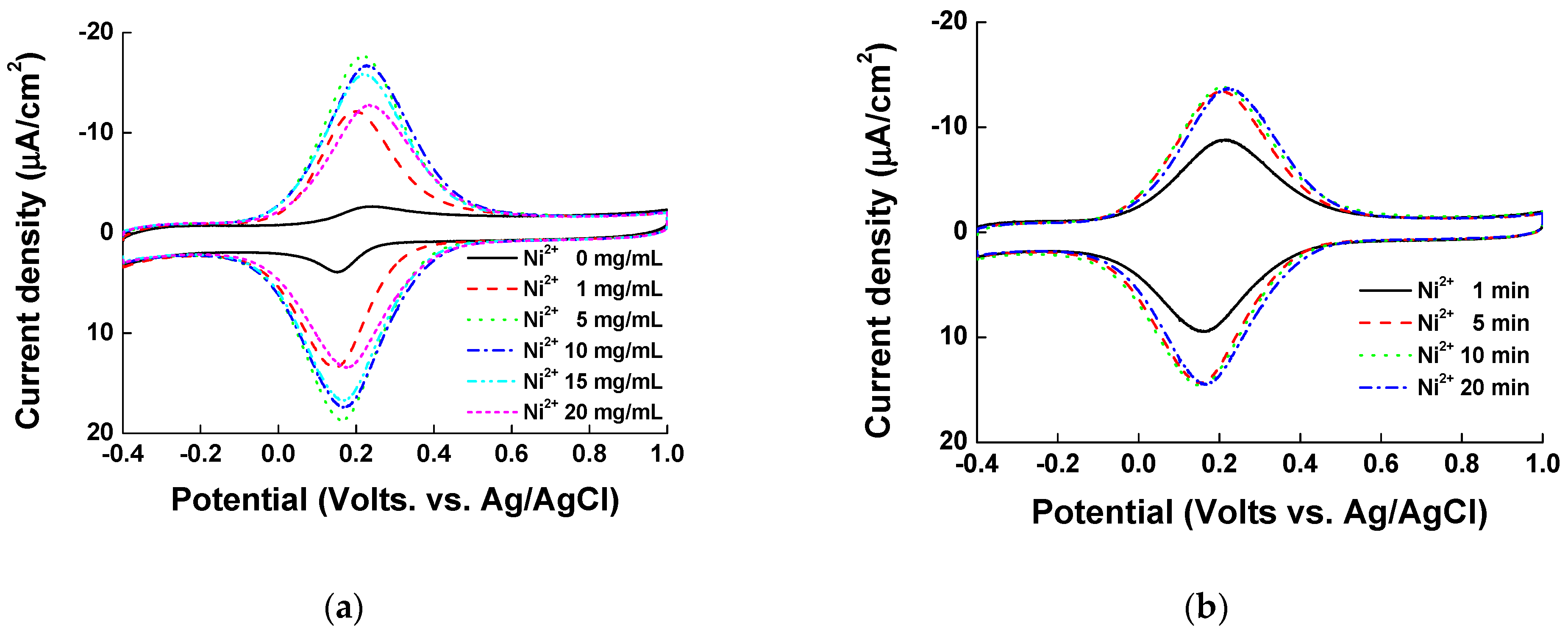
3.1. Optimization of ITO Modification with Ni and PVI; Ni-PVI-ITO
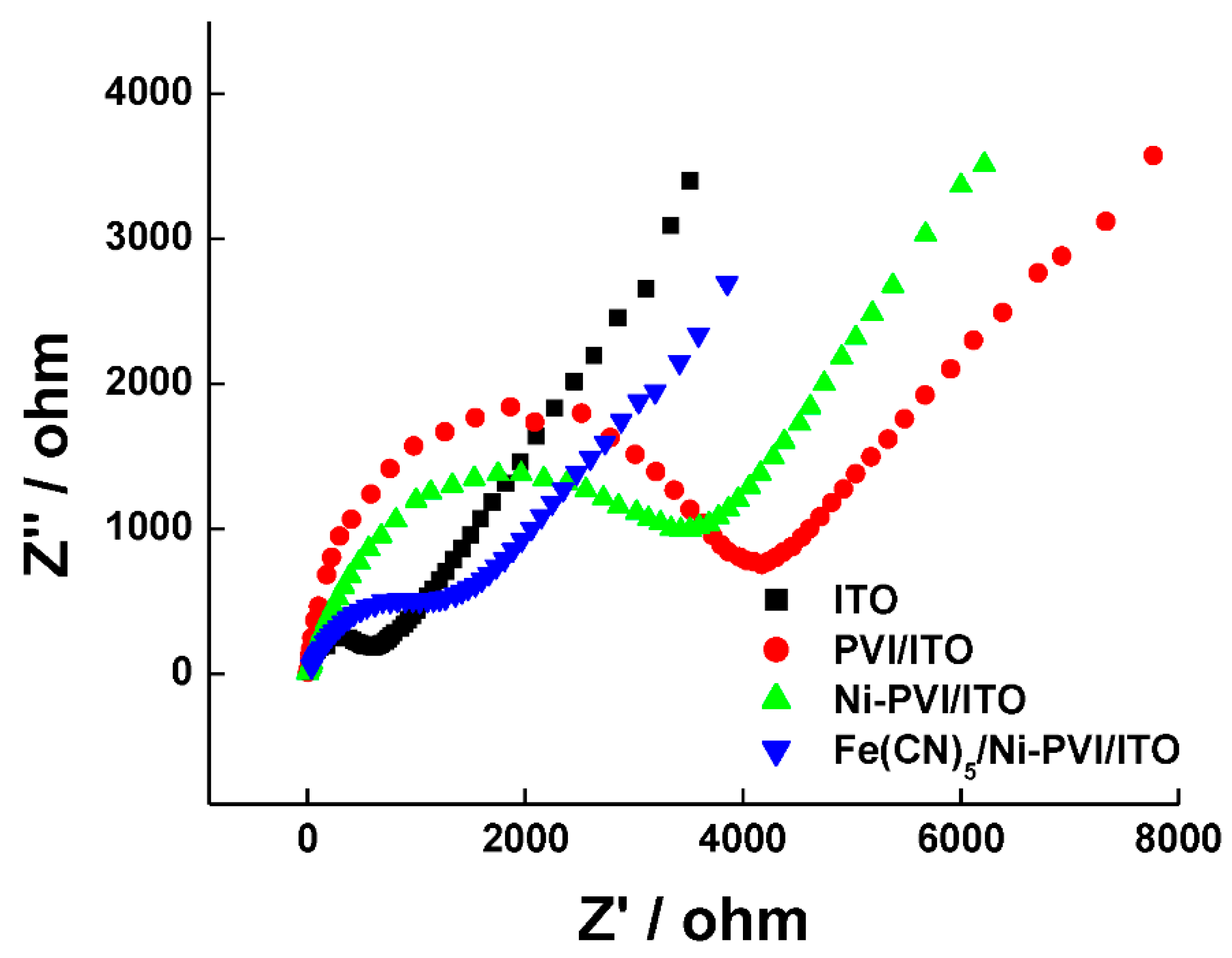
3.2. Electrochecmial Characterization of Ni-PVI-ITO Electorde
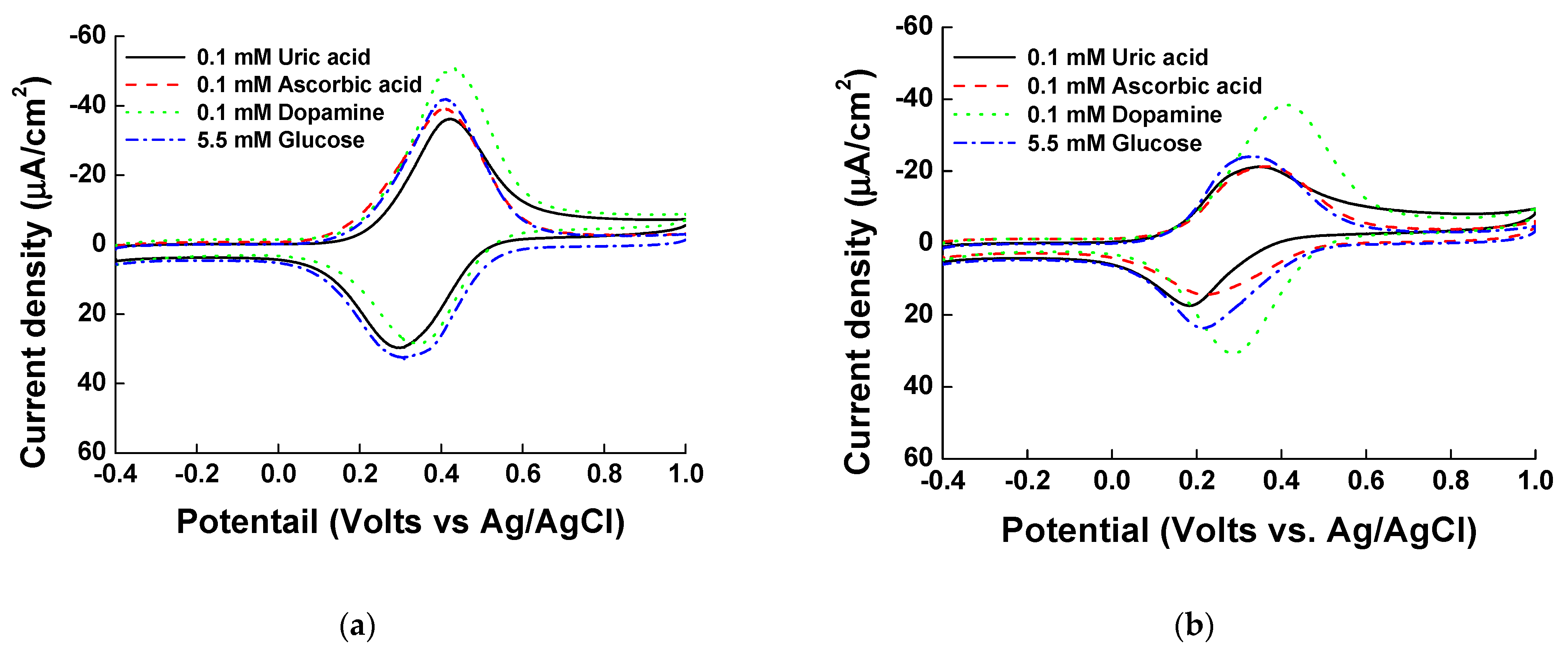
3.3. Interference Species Testing
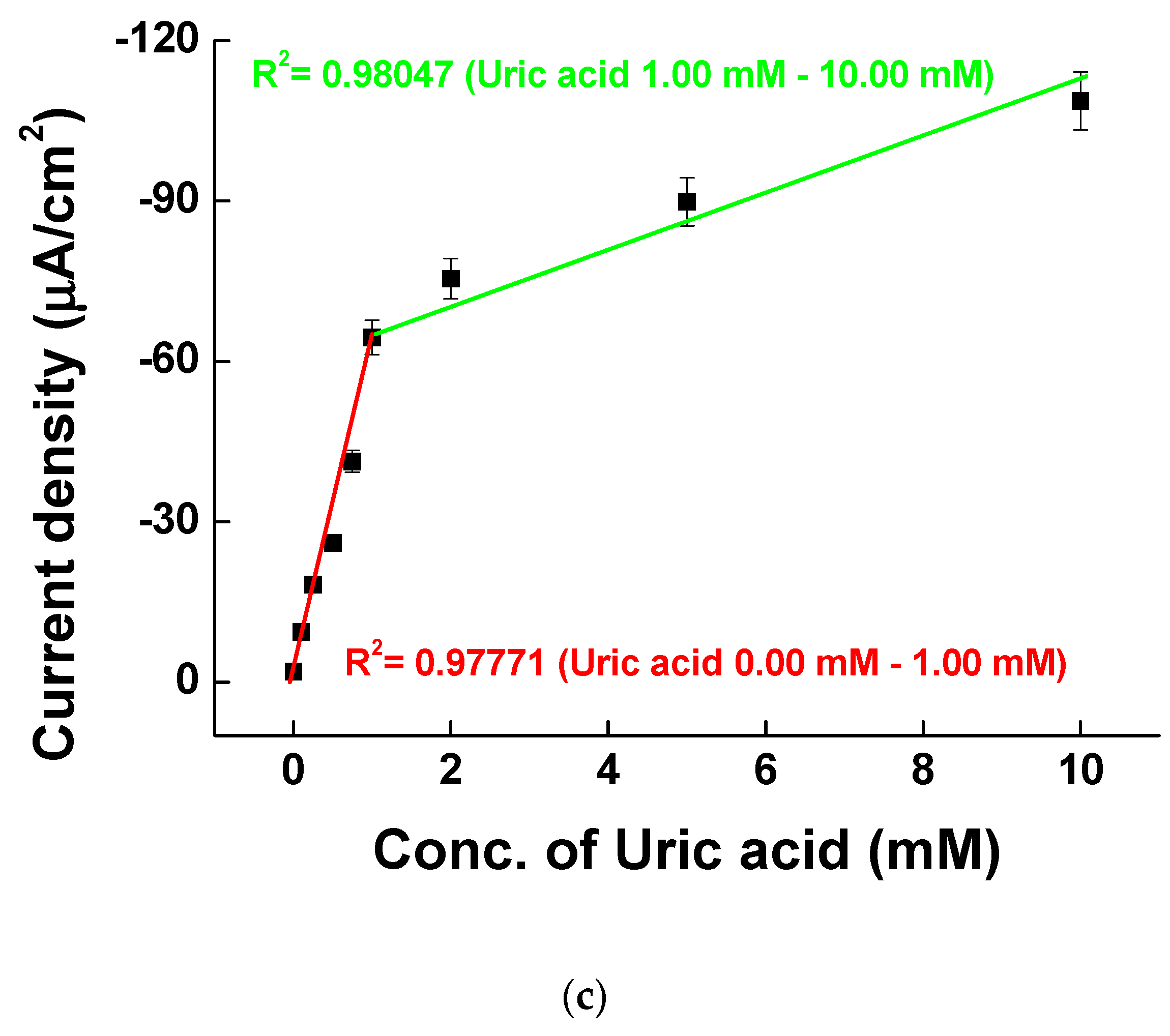
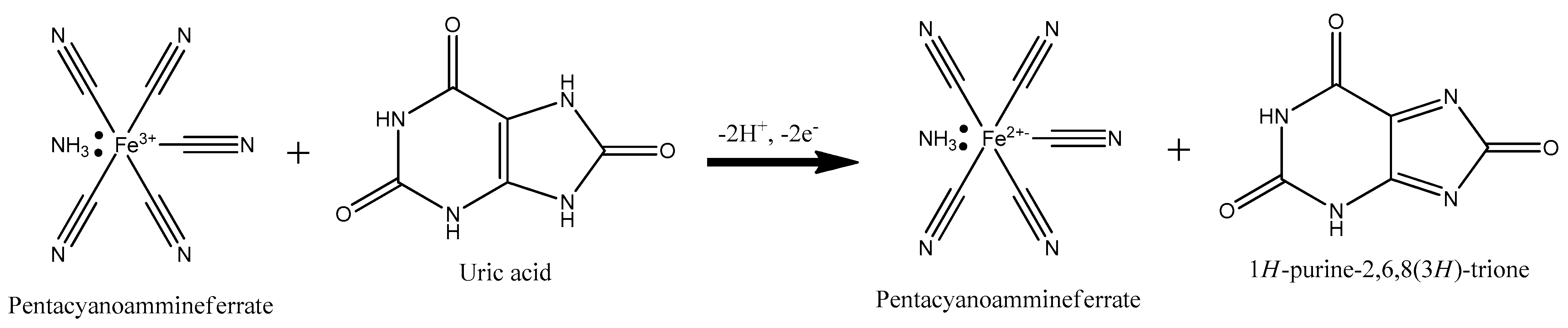
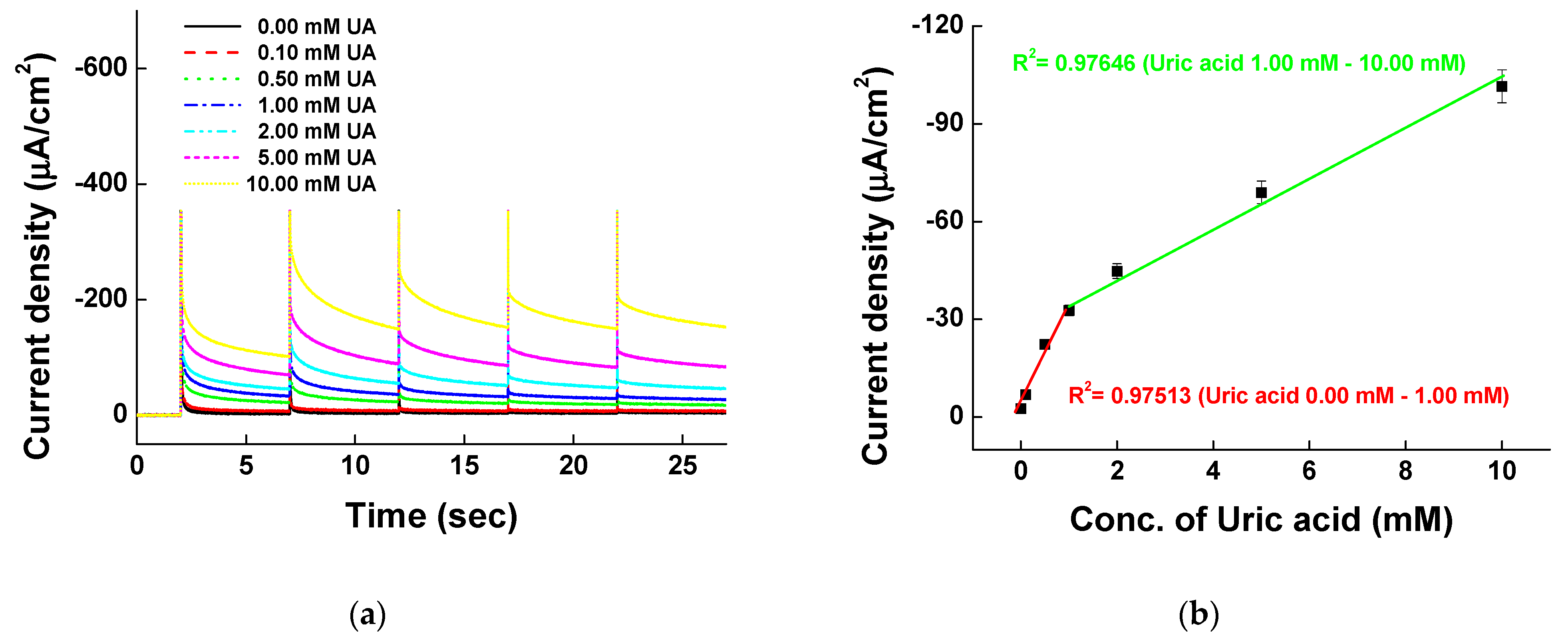
3.4. Uric Acid Measurement on Ni-PVI-ITO Electrode
3.5. Uric Acid Measurement on Ni-PVI-ITO Electrode in the Presence of Interferences
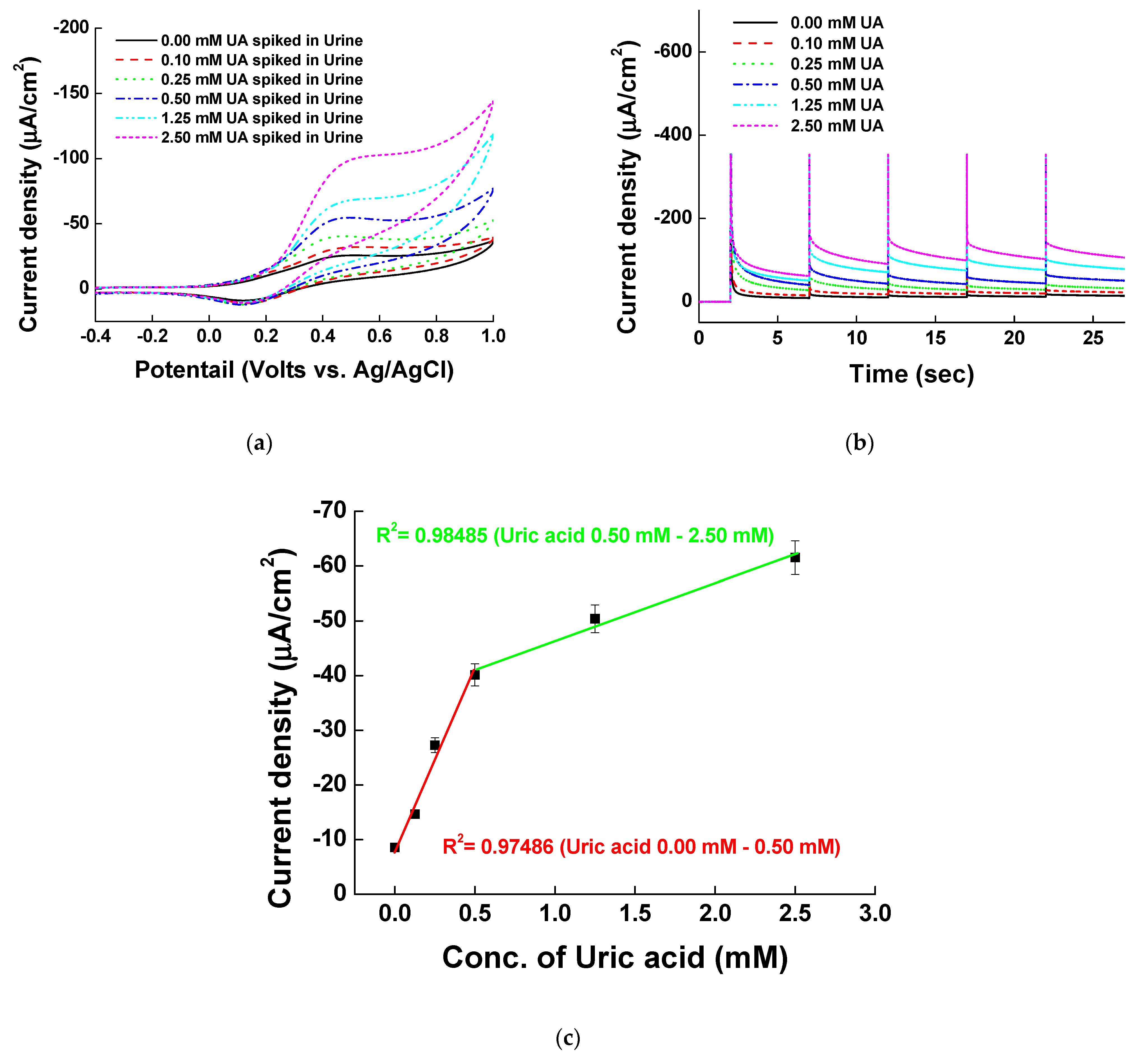
3.6. Uric Acid Measurement in Urine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Perkins, B.A.; Cherney, D.Z. Uric acid as a biomarker and a therapeutic target in diabetes. Can. J. Diabetes 2015, 39, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Pachla, L.A.; Reynolds, D.L.; Wright, D.S.; Kissinger, P.T. Analytical methods for measuring uric acid in biological samples and food products. J. Assoc. Off. Anal. Chem. 1987, 70, 1–14. [Google Scholar] [CrossRef]
- Hausen, A.; Fuchs, D.; König, K.; Wachter, H. Quantitation of urinary uric acid by reversed-phase liquid chromatography. Clin. Chem. 1981, 27, 1455–1456. [Google Scholar] [CrossRef] [PubMed]
- Wijemanne, N.; Soysa, P.; Wijesundara, S.; Perera, H. Development and validation of a simple high performance liquid chromatography/UV method for simultaneous determination of urinary uric acid, hypoxanthine, and creatinine in human urine. Int. J. Anal. Chem. 2018, 2018, 1647923. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Luo, Y.; Zhou, C.-Y.; Peng, A.; Liu, J.-Y. A sensitive and accurate method to simultaneously measure uric acid and creatinine in human saliva by using LC–MS/MS. Bioanalysis 2017, 9, 1751–1760. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, Y.; Han, W.; Zheng, Y.; Wang, X.; Xiao, D.; Mao, M.; Li, Q. Determination of uric acid in biological samples by high performance liquid chromatography-electrospray ionization-tandem mass spectrometry and study on pathogenesis of pulmonary arterial hypertension in pulmonary artery endothelium cells. RSC Adv. 2018, 8, 25808–25814. [Google Scholar] [CrossRef]
- Liu, M.; He, Y.; Zhou, J.; Ge, Y.; Zhou, J.; Song, G. A “naked-eye” colorimetric and ratiometric fluorescence probe for uric acid based on Ti3C2 MXene quantum dots. Anal. Chim. Acta 2020, 1103, 134–142. [Google Scholar] [CrossRef]
- Garcia, M.B.Q.; Lima, J.; Silva, M.; Sousa, J. Automatic determination of uric acid in urine in a FIA system with a tubular amperometric detector. Port. Electrochim. Acta 2004, 22, 249–262. [Google Scholar] [CrossRef]
- Ernst, H.; Knoll, M. Electrochemical characterisation of uric acid and ascorbic acid at a platinum electrode. Anal. Chim. Acta 2001, 449, 129–134. [Google Scholar] [CrossRef]
- Nakaminami, T.; Ito, S.-i.; Kuwabata, S.; Yoneyama, H. Uricase-catalyzed oxidation of uric acid using an artificial electron acceptor and fabrication of amperometric uric acid sensors with use of a redox ladder polymer. Anal. Chem. 1999, 71, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
- Zen, J.-M.; Chen, P.-J. A selective voltammetric method for uric acid and dopamine detection using clay-modified electrodes. Anal. Chem. 1997, 69, 5087–5093. [Google Scholar] [CrossRef]
- Monošík, R.; Stred’anský, M.; Šturdík, E. Application of electrochemical biosensors in clinical diagnosis. J. Clin. Lab. Anal. 2012, 26, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Usman, F.; Dennis, J.O.; Ahmed, A.Y.; Meriaudeau, F.; Ayodele, O.B.; Rabih, A.A. A review of biosensors for non-invasive diabetes monitoring and screening in human exhaled breath. IEEE Access 2018, 7, 5963–5974. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, Z.; Zhou, H.S. Measurement and analysis of cancer biomarkers based on electrochemical biosensors. J. Electrochem. Soc. 2019, 167, 037525. [Google Scholar] [CrossRef]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.H. Enzyme-based glucose sensor: From invasive to wearable device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Su, F.-C.; Peng, P.-Y.; Young, H.-T.; Liao, S.; Wang, G.-J. Highly sensitive non-enzymatic electrochemical glucose biosensor using a photolithography fabricated micro/nano hybrid structured electrode. Sens. Actuators B Chem. 2016, 230, 559–565. [Google Scholar] [CrossRef]
- Sukhrobov, P.; Numonov, S.; Mamat, X.; Li, Y.; Wågberg, T.; Hu, G. A New Non-Enzymatic Amperometric Sensor Based on Nickel Decorated ZIF-8 Derived Carbon Nanoframe for the Glucose Determination in Blood Samples. Int. J. Electrochem. Sci. 2018, 13, 6550–6564. [Google Scholar] [CrossRef]
- Mullani, S.B.; Tawade, A.K.; Tayade, S.N.; Sharma, K.K.K.; Deshmukh, S.P.; Mullani, N.B.; Mali, S.S.; Hong, C.K.; Swamy, B.K.; Delekar, S.D. Synthesis of Ni2+ ion doped ZnO–MWCNTs nanocomposites using an in situ sol–gel method: An ultra sensitive non-enzymatic uric acid sensing electrode material. RSC Adv. 2020, 10, 36949–36961. [Google Scholar] [CrossRef]
- Yan, Q.; Zhi, N.; Yang, L.; Xu, G.; Feng, Q.; Zhang, Q.; Sun, S. A highly sensitive uric acid electrochemical biosensor based on a nano-cube cuprous oxide/ferrocene/uricase modified glassy carbon electrode. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Feng, Y.; Wang, G.; Zhang, C.; Gu, A.; Liu, M. A uric acid sensor based on electrodeposition of nickel hexacyanoferrate nanoparticles on an electrode modified with multi-walled carbon nanotubes. Microchim. Acta 2011, 173, 27–32. [Google Scholar] [CrossRef]
- Income, K.; Ratnarathorn, N.; Khamchaiyo, N.; Srisuvo, C.; Ruckthong, L.; Dungchai, W. Disposable nonenzymatic uric acid and creatinine sensors using pad coupled with screen-printed reduced graphene oxide-gold nanocomposites. Int. J. Anal. Chem. 2019, 2019. [Google Scholar]
- Reddy, Y.V.M.; Sravani, B.; Agarwal, S.; Gupta, V.K.; Madhavi, G. Electrochemical sensor for detection of uric acid in the presence of ascorbic acid and dopamine using the poly (DPA)/SiO2@ Fe3O4 modified carbon paste electrode. J. Electroanal. Chem. 2018, 820, 168–175. [Google Scholar] [CrossRef]
- Kulesza, P.J.; Miecznikowski, K.; Malik, M.A.; Galkowski, M.; Chojak, M.; Caban, K.; Wieckowski, A. Electrochemical preparation and characterization of hybrid films composed of Prussian blue type metal hexacyanoferrate and conducting polymer. Electrochim. Acta 2001, 46, 4065–4073. [Google Scholar] [CrossRef]
- Wang, G.; Meng, J.; Liu, H.; Jiao, S.; Zhang, W.; Chen, D.; Fang, B. Determination of uric acid in the presence of ascorbic acid with hexacyanoferrate lanthanum film modified electrode. Electrochim. Acta 2008, 53, 2837–2843. [Google Scholar] [CrossRef]
- Antuch, M.; Matos-Peralta, Y.; Llanes, D.; Echevarría, F.; Rodríguez-Hernández, J.; Marin, M.H.; Díaz-García, A.M.; Reguera, L. Bimetallic Co2+ and Mn2+ hexacyanoferrate for hydrogen peroxide electrooxidation and its application in a highly sensitive cholesterol biosensor. ChemElectroChem 2019, 6, 1567–1573. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Qu, F.; Lu, Y.; Shen, G.; Yu, R. Attachment of nickel hexacyanoferrates nanoparticles on carbon nanotubes: Preparation, characterization and bioapplication. Anal. Chim. Acta 2006, 571, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Hexa-Cyano, H.U.U.N. Electrochemical Determination of Uric Acid in Human Urine Using Nickel Hexa-Cyano Ferrate Modified Carbon Paste Electrode. Int. J. Pure Appl. Res. 2015, 1, 43–60. [Google Scholar]
- Ohara, T.J.; Rajagopalan, R.; Heller, A. Glucose electrodes based on cross-linked bis (2, 2′-bipyridine) chloroosmium (+/2+) complexed poly (1-vinylimidazole) films. Anal. Chem. 1993, 65, 3512–3517. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Abarghoui, M.M.; Rezaei, B. A new non-enzymatic glucose sensor based on copper/porous silicon nanocomposite. Electrochim. Acta 2014, 123, 219–226. [Google Scholar] [CrossRef]
- Tucceri, I.R. Poly (o-aminophenol) as material of biosensors. Res. Open Access 2014, 2014, 884–900. [Google Scholar] [CrossRef]
- Clarkson, M.R.; Brenner, B.M.; Magee, C. Pocket Companion to Brenner and Rector’s the Kidney E-Book, 8th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2010. [Google Scholar]
- Chaudhari, R.; Joshi, A.; Srivastava, R. pH and urea estimation in urine samples using single fluorophore and ratiometric fluorescent biosensors. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Singh, R.K.; Devivaraprasad, R.; Kar, T.; Chakraborty, A.; Neergat, M. Electrochemical impedance spectroscopy of oxygen reduction reaction (ORR) in a rotating disk electrode configuration: Effect of ionomer content and carbon-support. J. Electrochem. Soc. 2015, 162, F489. [Google Scholar] [CrossRef]
- Wasko, R.; Frankenfield, B.A. Allopurinol dissolution of renal uric acid calculi. JAMA 1968, 205, 801. [Google Scholar] [CrossRef]
- Asplin, J.R. Uric acid stones. In Seminars in Nephrology; Elsevier: Philadelphia, PA, USA, 1996; pp. 412–424. [Google Scholar]
- Krishnan, R.G.; Rejithamol, R.; Saraswathyamma, B. Non-enzymatic electrochemical sensor for the simultaneous determination of adenosine, adenine and uric acid in whole blood and urine. Microchem. J. 2020, 155, 104745. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, X.; Wang, H.; Liu, X.; Guo, L.; Ji, X.; Wang, L.; Qiu, H.; Liu, X. Non-enzymatic sensing of uric acid using a carbon nanotube ionic-liquid paste electrode modified with poly (β-cyclodextrin). Microchim. Acta 2015, 182, 1877–1884. [Google Scholar] [CrossRef]
- Durai, L.; Kong, C.Y.; Badhulika, S. One-step solvothermal synthesis of nanoflake-nanorod WS2 hybrid for non-enzymatic detection of uric acid and quercetin in blood serum. Mater. Sci. Eng. C 2020, 107, 110217. [Google Scholar] [CrossRef]
- Jesny, S.; Kumar, K.G. Poly (para amino benzene sulfonic acid) Modified Glassy Carbon Electrode for the Simultaneous as well as Individual Voltammetric Determination of Guanine, Adenine and Uric Acid. J. Electrochem. Soc. 2018, 165, B720. [Google Scholar] [CrossRef]



| Electrode | Technique | Electrolyte and pH | Potential Applied (V) | Linear Range (mM) | LOD (μM) | Ref. |
|---|---|---|---|---|---|---|
| PAMTA/PG | DPV | PB 7 | 0.92 | 0.005 to 1.2 | 2.74 | [39] |
| β-CD/MWNT-CILE | LSV | AB 5 | 0.5 | 0.0006 to 0.4 and 0.4 to 1.0 | 0.3 | [40] |
| WS2/GCE | DPV | PBS 7.2 | 0.24 | 0.005 to 1.0 | 1.2 | [41] |
| p (ABSA)/GCE | SWV | 0.1 M NaOH | −0.132 | 0.02 to 0.2 | 5.9 | [42] |
| Ni-PVI-ITO | MPS | BB 9.0 | 0.4 | 0.1 to 10.0 | 50 | Our report |
| Assay | Sample | Nominal Concentration (mM) | Calculated Concentration (mM) | RSD (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Intra | Borate buffer | 0.100 | 0.1243 | 0.0004 | 124.27 ± 0.0005 |
| 1.000 | 1.0491 | 0.0687 | 104.91 ± 0.068 | ||
| 10.000 | 11.6699 | 4.879 | 116.69 ± 4.88 | ||
| Spiking in Urine | 0.125 | 0.113 | 0.031 | 90.289 ± 0.12 | |
| 1.250 | 1.17 | 0.44 | 93.593 ± 0.45 | ||
| 2.500 | 2.168 | 0.3 | 86.717 ± 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, W.-Y.; Lee, C.-J.; Sut, T.N.; Kim, H.-H.; Choi, Y.-B. Pentacyanoammineferrate-Based Non-Enzymatic Electrochemical Biosensing Platform for Selective Uric Acid Measurement. Sensors 2021, 21, 1574. https://doi.org/10.3390/s21051574
Jeon W-Y, Lee C-J, Sut TN, Kim H-H, Choi Y-B. Pentacyanoammineferrate-Based Non-Enzymatic Electrochemical Biosensing Platform for Selective Uric Acid Measurement. Sensors. 2021; 21(5):1574. https://doi.org/10.3390/s21051574
Chicago/Turabian StyleJeon, Won-Yong, Chang-Jun Lee, Tun Naw Sut, Hyug-Han Kim, and Young-Bong Choi. 2021. "Pentacyanoammineferrate-Based Non-Enzymatic Electrochemical Biosensing Platform for Selective Uric Acid Measurement" Sensors 21, no. 5: 1574. https://doi.org/10.3390/s21051574
APA StyleJeon, W.-Y., Lee, C.-J., Sut, T. N., Kim, H.-H., & Choi, Y.-B. (2021). Pentacyanoammineferrate-Based Non-Enzymatic Electrochemical Biosensing Platform for Selective Uric Acid Measurement. Sensors, 21(5), 1574. https://doi.org/10.3390/s21051574








