1. Introduction
Physically active individuals have a longer life expectancy and increased health span [
1,
2,
3]. Because physical activity (PA) levels are modifiable for most adults, PA is an attractive target for interventions aimed at improving the quality of life in older adults. In addition, features of PA are known to be highly correlated with prevalence of various health conditions [
4] and risk of mortality [
5,
6,
7,
8,
9]. Taken together, these observations suggest the power of monitoring PA in a free-living environment to both inform the epidemiology of healthy aging and facilitate safe, independent, home living for aging individuals if incorporated into, for example, a clinical monitoring program through individuals’ primary care provider. Historically, PA has been most often measured using self-report questionnaires, which are prone to substantial biases [
10]. Wearable accelerometers provide a convenient, non-invasive, objective alternative for measuring PA, and have become widely adopted in health studies such as the Baltimore Longitudinal Study on Aging (BLSA) [
11], the National Health and Nutrition Survey (NHANES) 2003–2006 and 2011–2014 [
12], and the UK Biobank [
13]. Moreover, in the context of aging, the ability to collect objective measures of physical activity are crucial due to the increasing prevalence of cognitive deficiencies which can further bias self-reported levels of PA.
The increased use of accelerometers in observational studies, clinical trials, large biobanks, and for recreational purposes has provided a wealth of data that can be used to obtain objective measurements of physical activity in free-living environments. Accelerometers typically measure acceleration in three orthogonal axes at the sub-second level, capturing high resolution information on horizontal, lateral, and vertical movement. Typically, these sub-second level data are aggregated at lower resolution time intervals called epochs, most commonly 1 min epochs; this produces multiple days of 24 h minute-by-minute activity trajectories for each subject. When combined across days to create a single 24 h trajectory, these are referred to as daily acceleration or activity profiles. Most often, analysis of data generated by accelerometers focuses on creating one number summaries of the daily profiles for each subject, typically measuring volume of PA (e.g., step count, sedentary time, active time, etc.), circadian rhythm (e.g., relative amplitude), or features of sleep (e.g., sleep efficiency, sleep duration, number of wakes). Summarizing multiple days of data using this approach ignores the highly correlated nature of these features and fails to efficiently exploit information contained in the timing of PA. To overcome this limitation, recent work by Di et al. [
14] used the joint and individual variation explained approach [
15] to characterize patterns of PA, circadian rhythms, and sleep using a set of scalar features from each domain. An alternative approach involves analyzing the entire 24 h acceleration profiles in conjunction with health outcomes. Despite evidence that specific patterns of timing and magnitudes of physical activity over the 24 h day are associated with aging [
11] and mortality [
7,
16], analytic approaches that use the full acceleration profiles have been underutilized in the literature, perhaps because of the computational and methodological challenges of working with high dimensional, correlated, structured data.
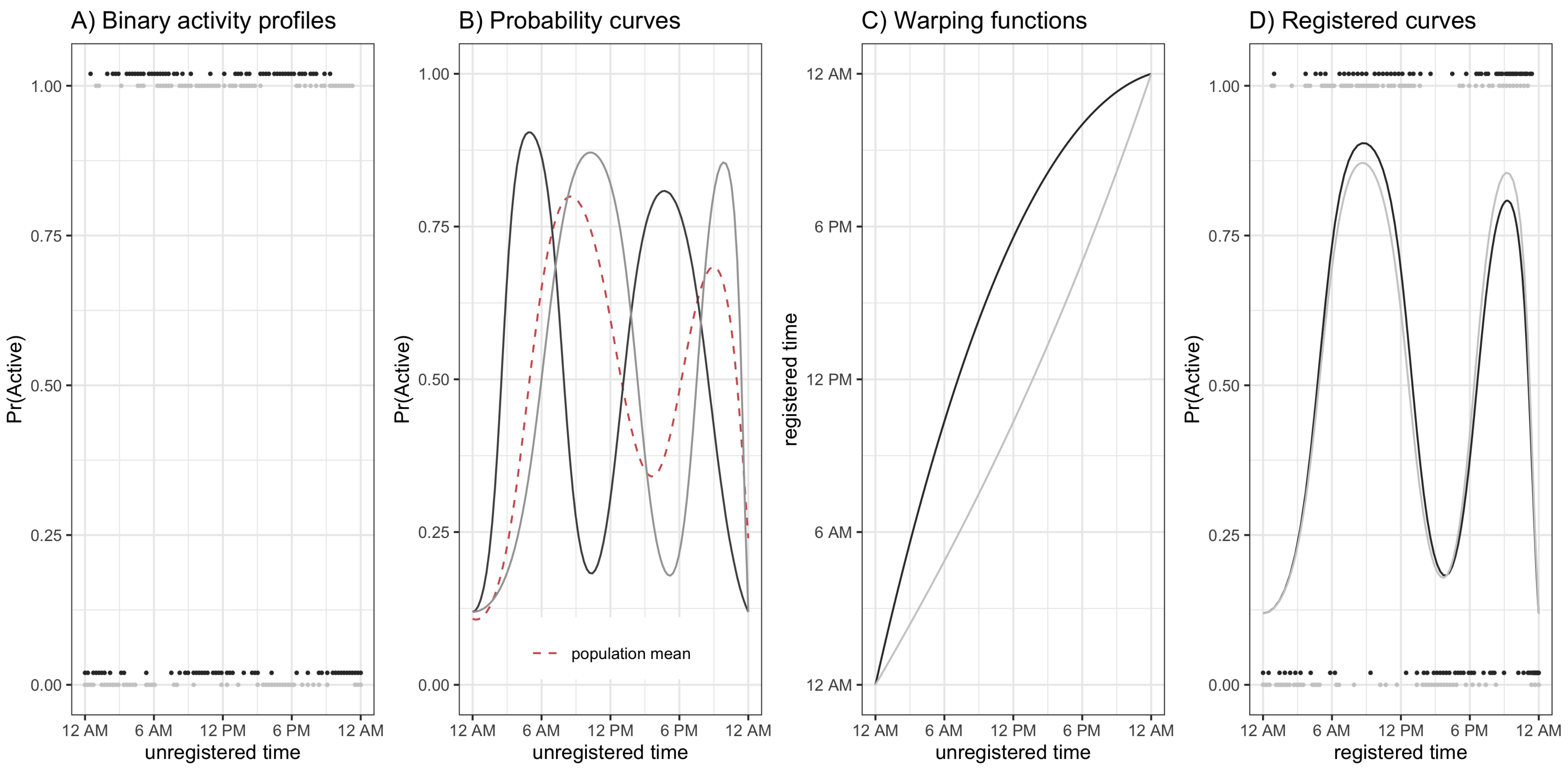
Fortunately, statistical methods developed in the field of functional data analysis [
17] provide a natural framework for analyzing acceleration profiles. From the functional data perspective, each 24 h acceleration profile is a statistical unit of observation that can be analyzed using methods specifically developed to extract patterns of variation and perform inference on noisy, dense, correlated data [
18,
19]. Here we focus on analyzing diurnal patterns of physical activity using two methods from functional data analysis. Specifically, we use function-on-scalar regression (FoSR) [
20], a method which treats an entire “function” (acceleration profile) as an outcome and associates the entire function with scalar features (e.g., age), and curve registration [
21] separates 24 h activity profiles into components of “horizontal” variability and “vertical” variability which correspond to timing and magnitude of PA, respectively. Function-on-scalar regression has been used to analyze age-associated trends in diurnal patterns of physical activity in the BLSA [
22], though that analysis may not scale up to massively large datasets such as the UK Biobank. In addition, the study using BLSA data employed a single axis accelerometer, worn at the chest, and the unit of measurement analyzed was created using a proprietary algorithm which is not transferable across devices. Our analysis is based on open-source and published algorithms using a tri-axial wrist worn accelerometer, which should allow for more general use in other studies. As a result, it is unclear whether and how those analyses would be replicated using a tri-axial accelerometer placed on a different location on the body, and using a different unit of measurement. Though registration of acceleration profiles was recently used to uncover sub-types of circadian rhythms [
23], to our knowledge registration has never been used to study how trends in circadian rhythms change with age, nor has it been paired with functional regression methods. We focus here on functional regression for continuous and binary data, and combine FoSR with curve registration for binary data to simultaneously analyze different aspects of sex-specific age trends in diurnal patterns of PA, leveraging the information contained in the complex high dimensional acceleration profiles to draw novel insights about diurnal patterns in PA across ages.
Studying PA across a wide span of age ranges, especially at older ages, typically requires a large number of participants. Opportunely, the UK Biobank is a large prospective cohort study that enrolled more than 500,000 adults. In the UK Biobank accelerometer sub-study, over 100,000 adults wore a wrist-worn accelerometer (Axtivity AX3, Newcastle upon Tyne, UK) for 7 days [
24] between the years of 2013 and 2015. Approximately 88% of those enrolled report British ancestry. The UK Biobank collected vast amounts of information regarding participants’ socio-demographic, lifestyle, environment, accelerometry, imaging and genetics [
25,
26]. In addition, participants’ data can be linked to incident morbidity and mortality through hospital and death records. With data of this size, we can potentially uncover previously undetectable patterns in PA across ages and assess whether diurnal aging trends observed in US based cohorts are replicated in a large UK cohort study.
This work aims to extend the existing literature on objectively measured physical activity using wearable devices in older adults in several ways. First, we use an open source, reproducible summary of raw sub-second level acceleration data aggregated at the minute level to assess the replicability of the general sex-specific trends and differences in the timing and volume of PA observed in US based populations in a large UK based prospective cohort study. Specifically, we wish to validate sex-specific differences in the time-of-day trends these trends using milli-gravity units values as opposed to previously-published proprietary activity counts. Second, we illustrate different patterns of activity of ages if using the values of activity versus an indicator of active vs. inactive using thresholded data. Third, we introduce the concept of curve registration to the intersecting field of physical activity and aging as a tool for analyzing epidemiological trends. Fourth, we show that complex functional regression methods are computationally feasible on very large physical activity datasets using high quality open source software. Finally, to further the goal of the dissemination of our functional data methods applied to physical activity data we provide an accompanying vignette that fully reproduces our analysis in the NHANES 2003–2006 accelerometry data. To avoid the potential complications associated with differential weekend versus weekday patterns of activity, we focus here only on weekdays (Monday–Friday).
3. Results
Our analysis examines weekday (Monday–Friday) activity profiles and binary active versus inactive profiles across ages for 88,797 men and women in the UK Biobank accelerometer study. In the first part of our analysis we apply functional regression to the activity profiles, providing insight into how patterns in magnitude of acceleration vary across ages and gender. These results are shown in
Figure 2 and
Figure 3. The second part of this analysis applies both functional regression and curve registration to the binary activity profiles, providing information on age and gender-related differences in the probability of being active at each time of day as well as age and gender-related shifts in average timing of physical activity. These results are shown in
Figure 4 and
Figure 5.
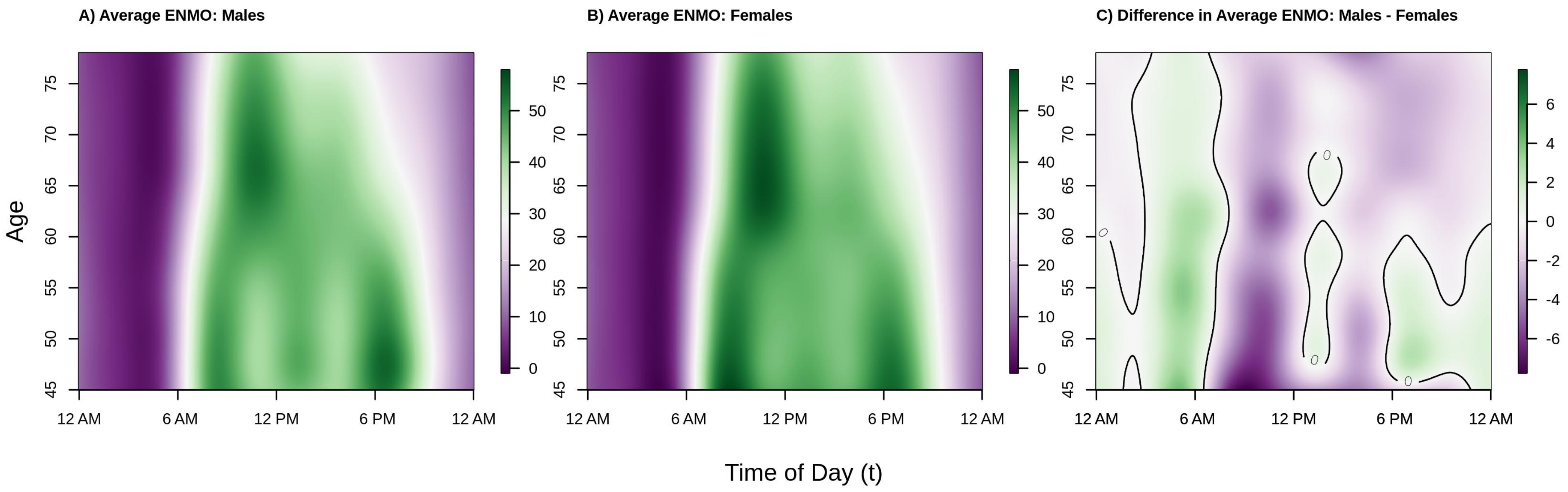
Figure 2 shows the population estimated average acceleration by time of day over the age range in our analytic sample separately for males (panel A), females (panel B), and the difference between males and females (panel C). The color in the panels A/B of
Figure 2, corresponding to average activity patterns in males and females, respectively, denotes lower (dark/light purple) versus higher (light/dark green) acceleration (volume of PA). Color in panel C of
Figure 2 indicates periods of time where men are more (green) or less (purple) active than women and a solid black line demarcates areas where the estimated difference crosses 0 (i.e., no difference in activity levels between men and women, white color). We observe that among both males and females, younger participants tended to start activity earlier in the day (06:00 a.m. for 45–55 year olds vs. 07:00 a.m. for 65+ year olds) and maintain higher levels of activity later in the day, on average. In addition, with the exception of the youngest males in the sample, the peak average activity in this population occurs in the morning.
We also see a clear shift in activity patterns beginning around age 60 that manifest similarly in males and females; specifically, these individuals start their activity later in the morning, and tend to wind down earlier in the afternoon, consistent with previous findings in US populations, specifically NHANES 2003–2006 [
38] and BLSA [
22]. In addition, while both males and females under the age of 60 have two clear peaks in activity (around 8–9 a.m. and 6–7 p.m.), there is only one clear peak (around 10–11 a.m.) in older men and women (age ≥ 60). This change from two peaks to one peak after age 60 can also be seen in the BLSA study from
Figure 3 of [
22]. This clear shift in activity patterns around the age of 60, seen in both US studies and now the UK Biobank study, may be a result of individuals beginning to exit the work force via retirement, leading to a change in the structure of individuals’ weekday schedules.
In the period roughly between 10 a.m.–12 p.m. and 2 p.m.–4 p.m. in males, and, to a lesser extent females, average activity dips in the younger individuals (ages 45–60). The 2 p.m.–4 p.m. dip in PA has been previously observed in the BLSA [
22], but the 10 a.m.–12 p.m. dip has not been previously reported. It may be that the larger sample size of the UK Biobank enables detection of more nuanced patterns than in smaller studies, or it may be specific to the UK population. Alternatively, the observed shift may be due to differences in the ENMO summary measure as compared to proprietary activity counts used by Xiao et al. [
22] generated by similar activities, the different location of the device (wrist versus hip/chest), or some combination of the two. Because this pattern may be a result of structured lunch breaks for employed individuals, we term it the “lunch effect”.
Moving to the right panel of
Figure 2, we see that across the age range of this sample, women tend to be more active than men during the daytime hours of 6 a.m.–6 p.m. (mostly light/dark purple color during this period) with the exception of 12 p.m.–1 p.m. (white/light green color), and less active during the nighttime hours of 12 a.m.–6 a.m. (mostly light green color). Interestingly, younger women (ages 45–60) tend to be less active during the evening hours of 6 p.m.–12 a.m., but older women (ages 60–80) are more active during this period. The observed higher level of average activity in males as compared to females in the early morning hours could be driven by poorer sleep quality (more movement during sleep), more variable sleep periods, or less stable weekday circadian patterns in men.
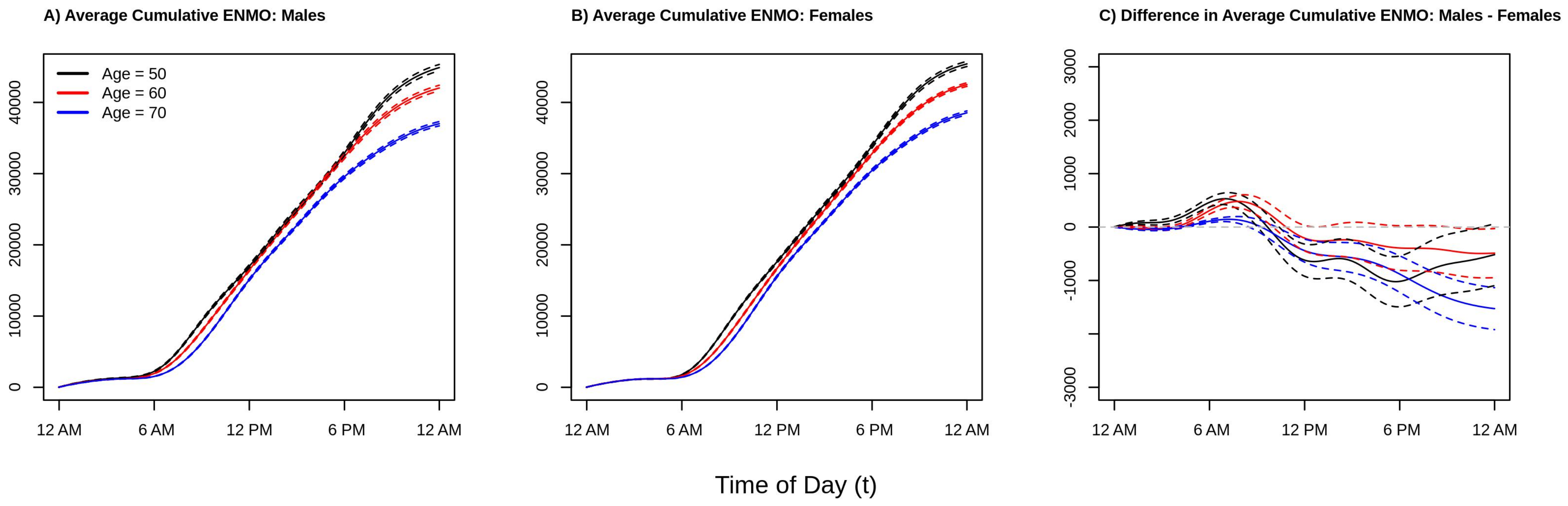
To give perspective on the average total amount of activity accumulated over the 12 a.m.–12 a.m. period,
Figure 3 plots the estimated cumulative average activity at ages 50 (black line), 60 (red line), an 70 (blue line) separately for males (panel A), females (panel B), and the difference between males and female (panel C). Solid lines present point estimates and dashed lines represent 95% confidence intervals obtained via bootstrap. Consistent with the results from
Figure 2, we see that younger individuals accumulate as much or more activity at any point of the day as compared to older individuals (black curve ≥ red curve ≥ blue curve at all times of the day). Comparing the estimated cumulative activity curves for age 50 versus 60 in both males and females, we see that the younger age group accumulate more activity early in the morning (larger difference between the two curves between 6 a.m. and 10 a.m.) which shrinks to near zero difference moving into the early afternoon due to the aforementioned “lunch effect” for younger adults, separating again in the late afternoon/early evening, resulting in more overall activity for the younger group. In addition, from
Figure 3 panel C, we see that the estimated total daily activity for females and males is roughly equal for the younger ages groups as the confidence intervals at 12 a.m. just overlap 0 (95% confidence intervals for ages 50 and 60 contain 0), while older (age 70) females have noticeably higher estimated levels of total activity than older males (point estimate for age 70 at 12 a.m. is negative and the 95% confidence interval does not contain 0). This suggests that the lower levels of activity observed in men ages 50–60 during the day seen in the right panel of
Figure 2 are offset by increased activity during the evening and early morning hours. In contrast, by age 70, the increased activity of males during the early a.m. hours is not enough to “make up” for the higher levels of activity in females during the rest of the day. These results find that in this population women are estimated to be as or more active than men ages 45–80 with women being relatively more active after age 60. This may indicate that activity patterns changed deferentially by gender as individuals begin to exit the workforce as they approach retirement age, though future investigations to validate this hypothesis are required.
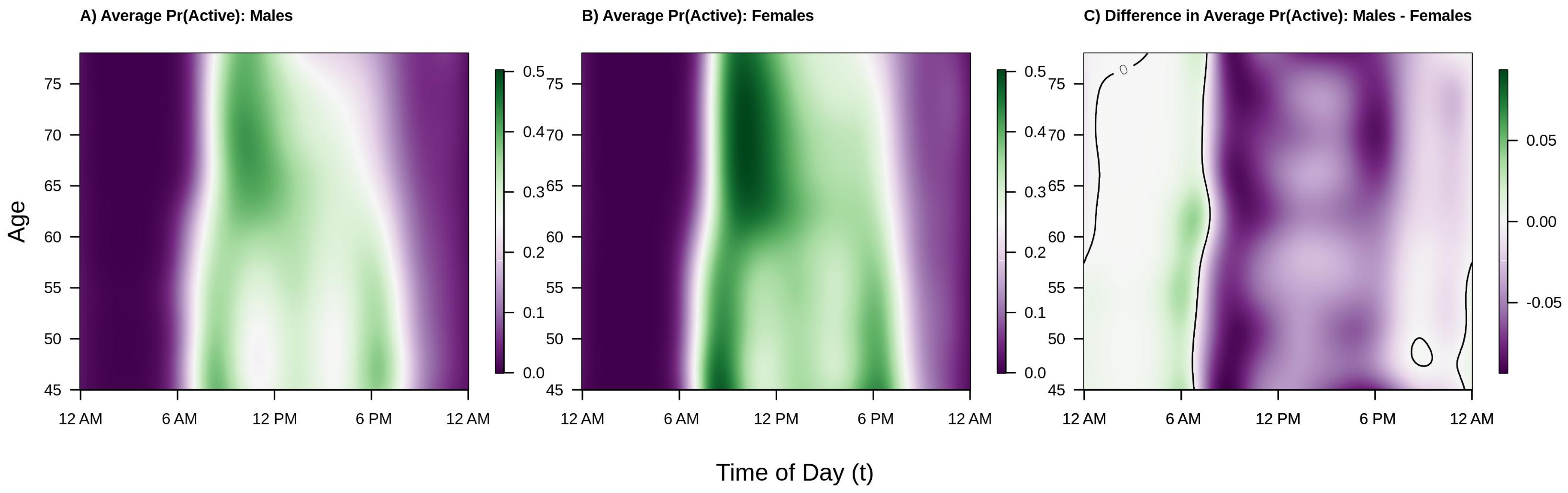
Shifting focus to population level diurnal probabilities of being active versus inactive, consider
Figure 4. The interpretation and layout of the figure is the same as
Figure 2 except that color intensity now denotes probability of being active (or, in the right panel, difference in probability of being active). We find that the overall trends are largely the same as those seen in
Figure 2 and
Figure 3 with a few key differences. Regarding similarities, from the left two panels of
Figure 4 we see that the estimated probability of being active tends to be highest in the morning shortly after waking, that this peak occurs later in older individuals, that there are morning and early evening peaks in younger males and females, and that there is a “lunch effect” that is present in both men and women. Analyzing the binary activity profiles provides additional information that was not revealed by the analysis of participants’ continuous activity profiles. Specifically, from the panel C of
Figure 4, we see that females tend to have a higher probability of being active during the daytime hours (6 a.m.–6 p.m.) across all age groups and a lower probability of being active during the early a.m. hours (12 a.m.–6 a.m.). Interestingly, although males ages 45–60 were generally found to have higher levels of average activity 12 p.m.–2 p.m. and 6 p.m.–12 a.m., women have nearly the same or higher estimated probability of being active. This suggests that women are, on average, engaging in more low-light levels of activity and less moderate-vigorous intensity activity during this time period. Alternatively, the average volume of PA may be pulled upward by a few individuals engaging in very vigorous levels of activity, though future work is needed to confirm this theory.
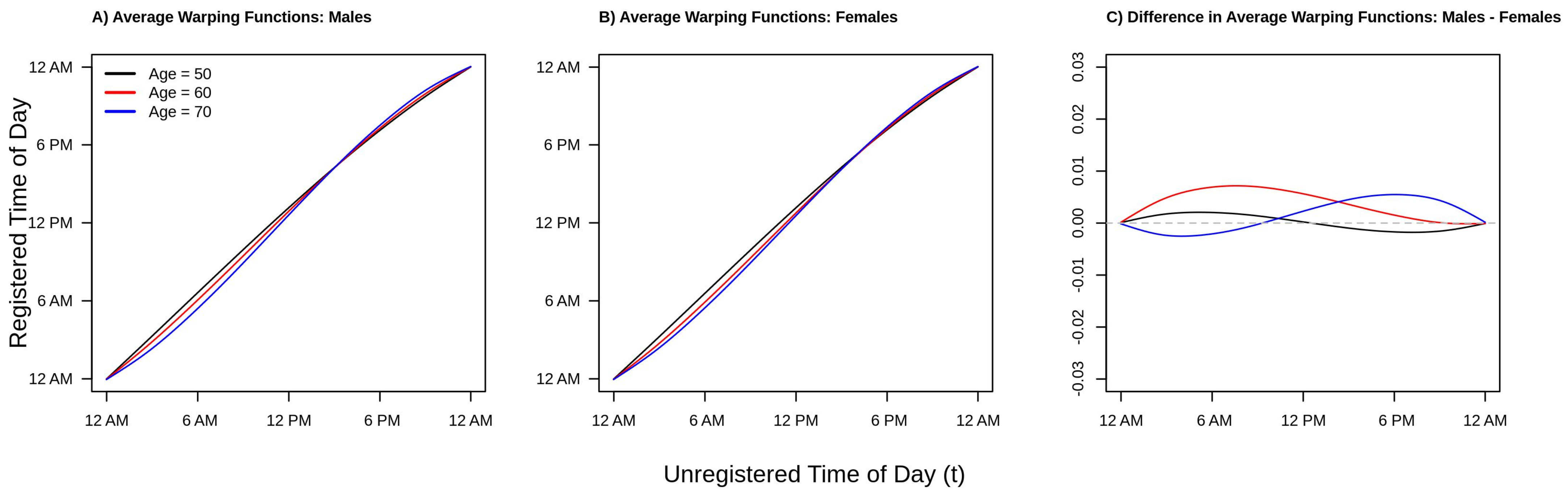
Finally, consider
Figure 5, which plots the estimated average warping functions for males (panel A), females (panel B), and the difference between males and females (panel C) for ages 50 (black line), 60 (red line), and 70 (blue line) years old. From panels A/B of
Figure 5 we see that in the morning period, the ordering of the lines is black (age 50) greater than red (age 60) greater than blue (age 70). This pattern reverses after around 10 a.m. in both men and women. This ordering of the curves is consistent with a compressed chronological “active” time during the daytime hours and an expanded “inactive” time during the nighttime/early morning hours, with active time being more compressed with increasing age. We also see this phenomena in
Figure 2 and
Figure 4. However, the observed differences in warping functions are visually quite small. Looking at panel C, we see that for the youngest age group shown (red line, age 50), the estimated difference is very close to zero at all times of day, indicating similar active/inactive timing between men and women age 50. In contrast, for the next youngest age group (red line, age 60), the estimated warping function is estimated to be positive for the entirety of the day, suggesting that men’s active periods are uniformly shifted to earlier in the day relative to women age 60. Finally, for the older age group (blue line, age 70), the estimated warping function starts negative and then becomes positive after around 8 a.m., suggesting men have a compressed active period relative to women age 70. These observations are all consistent with the patterns observed in
Figure 2 and
Figure 4. However, once again, the estimated differences in average warping functions do not appear large in absolute value.
4. Discussion
This work extends the existing literature on physical activity and aging through the (1) validation of aging trends seen in several US populations in a large UK cohort; (2) establishment of functional regression approaches as a set of computationally feasible, open source tools for analyzing physical activity in the largest publicly available accelerometry dataset; (3) combination of functional regression and registration for analyzing the associations among age, gender, and the timing and the volume of physical activity; (4) analysis of multiple types of PA acceleration profiles (activity count versus binary activity profiles) which showed that conclusions based about PA and aging are potentially dependent on profile choice; and dissemination of template code which allows researchers to reproduce our analytic procedure on any accelerometry dataset via an application to the NHANES 2003–2006 accelerometry data. In the future, the tools presented here could be used to create reference quantities for normative patterns of physical activity by jointly considering multiple types of PA profiles. Further, the methods applied in this study could be applied to other bio-signals measured continuously using wearable devices which have circadian patterns; for example, heart rate, continuous glucose monitoring, or skin temperature.
This study has several important limitations. First, we reduced multiple days of subjects’ accelerometry data to one day per subject by collapsing across days. It may be possible to gain additional insights into the epidemiology of the timing and volume of physical activity and aging by analyzing individuals’ day-level data using a multi-day approach to both registration and regression. We also restricted our analysis to weekdays only. It may be that analyzing individuals weekend activity patterns, when many employed people’s activity are not restricted by their occupational requirements, could provide additional insights into individuals’ leisure time activities. Moreover, the registration method we applied here was not designed to handle individuals who tend to sleep during the daytime. That is, registration does not respect the circular nature of clock time.
In addition, the composition of the UK Biobank accelerometry sub-study has been shown to be overall healthier than the larger UK Biobank study, which is itself healthier than the UK population. As a result, the generalizability of our results is unclear. Nevertheless, we were able to replicate key findings regarding the epidemiology of circadian patterns of PA from previous studies in both nationally representative and healthy aging US cohorts, suggesting that the observed aging trends are robust to some sources of sampling bias and are likely to be reproduced in other studies which draw from similar populations. Finally, driving and other systematic behavioral differences that are not physical activity but affect accelerometry readings across age ranges and sexes can have a minor effect on results, so all results must be viewed with this caveat [
39]. Threshold-based methods such as our binary-curve registration are more robust.
Synthesizing the results of our analysis, we find that the diurnal patterns of both volume of physical activity and the probability of being active change with age and that there are sizable gender difference in these trends. In addition, there does appear to be an age trend in the timing of PA as older adults have a more compressed “active” period. However, there does not appear to be a substantial gender difference in the changes of the active period with age. Ultimately, from a scientific perspective, this study validates previous studies’ findings in a new aging cohort (diurnal patterns of volume), presents novel findings regarding the difference in analyzing various summaries of physical activity profiles (probability of being active/inactive, registration and changes in phase in timing of PA). In addition, we introduce new methodologies to the study of PA and aging and, crucially, provide the code for reproducing our methods using publicly available software through the accompanying online supplemental material to be uploaded to Github on publication.