Techniques for Temperature Monitoring of Myocardial Tissue Undergoing Radiofrequency Ablation Treatments: An Overview
Abstract
1. Introduction
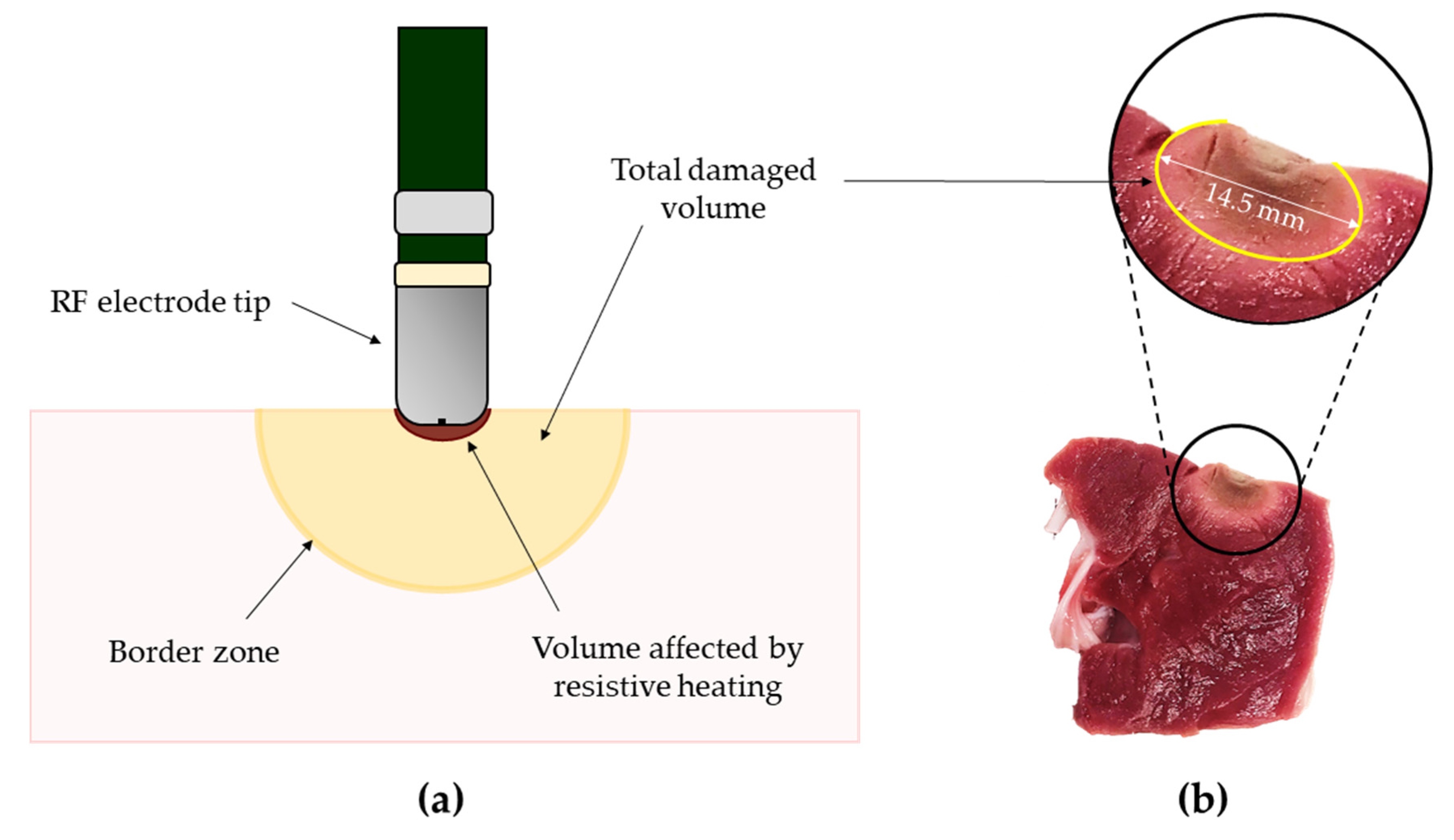
2. General Principles of Radiofrequency Ablation
3. Invasive Solutions for Myocardial Temperature Evaluation during RFA
3.1. Thermocouples: Working Principle and Application in Myocardial RFA
3.1.1. Working Principle
3.1.2. Applications of Thermocouples in Myocardial RFA
3.2. Thermistors: Working Principle and Application in Myocardial RFA
3.2.1. Working Principle
3.2.2. Applications of Thermistors in Myocardial RFA
3.3. Fluoroptic Sensors: Working Principle and Application in Myocardial RFA
3.3.1. Working Principle
3.3.2. Applications of Fluoroptic Sensors in Myocardial RFA
3.4. Fiber Bragg Gratings: Working Principle and Application in Myocardial RFA
3.4.1. Working Principle
3.4.2. Applications of Fiber Bragg Gratings in Myocardial RFA
3.5. Short Summary on Invasive Solutions for Myocardial Temperature Monitoring during RFA
4. Non-Invasive Solutions for Myocardial Temperature Evaluation during RFA
4.1. Magnetic Resonance Imaging: Working Principle and Application in Myocardial RFA
4.1.1. Working Principle
4.1.2. Applications of Magnetic Resonance Imaging in Myocardial RFA
4.2. Ultrasound Imaging: Working Principle and Application in Myocardial RFA
4.2.1. Working Principle
4.2.2. Applications of Ultrasound Imaging in Myocardial RFA
4.3. Infrared Imaging: Working Principle and Application in Myocardial RFA
4.3.1. Working Principle
4.3.2. Applications of Infrared Imaging in Myocardial RFA
4.4. Short Summary on Non-Invasive Solutions for Myocardial Temperature Monitoring during RFA
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Huang, S.K.; Bharati, S.; Graham, A.R.; Lev, M.; Marcus, F.I.; Odell, R.C. Closed chest catheter desiccation of the atrioventricular junction using radiofrequency energy—A new method of catheter ablation. J. Am. Coll. Cardiol. 1987, 9, 349–358. [Google Scholar] [CrossRef]
- Budde, T.; Breithardt, G.; Borggrefe, M.; Podczeck, A.; Langwasser, J. Initial experiences with high-frequency electric ablation of the AV conduction system in the human. Z. Kardiol. 1987, 76, 204–210. [Google Scholar]
- Borggrefe, M.; Budde, T.; Podczeck, A.; Breithardt, G. High frequency alternating current ablation of an accessory pathway in humans. J. Am. Coll. Cardiol. 1987, 10, 576–582. [Google Scholar] [CrossRef]
- Nath, S.; Di Marco, J.P.; Haines, D.E. Basic aspects of radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 1994, 5, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Dinerman, J.L.; Berger, R.D.; Calkins, H. Temperature monitoring during radiofrequency ablation. J. Cardiovasc. Electrophysiol. 1996, 7, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Viles-Gonzalez, J.F.; Berjano, E.; D’Avila, A. Complications of radiofrequency catheter ablation: Can we prevent steam pops? JACC Clin. Electrophysiol. 2018, 4, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, M.; Kojodjojo, P.; Epstein, L.M.; Koplan, B.A.; Michaud, G.F.; Tedrow, U.B.; Stevenson, W.G.; John, R.M. Outcomes of cardiac perforation complicating catheter ablation of ventricular arrhythmias. Circ. Arrhythmia Electrophysiol. 2011, 4, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.E.; Verow, A.F. Observations on electrode-tissue interface temperature and effect on electrical impedance during radiofrequency ablation of ventricular myocardium. Circulation 1990, 82, 1034–1038. [Google Scholar] [CrossRef]
- Morady, F. Radio-frequency ablation as treatment for cardiac arrhythmias. N. Engl. J. Med. 1999, 340, 534–544. [Google Scholar] [CrossRef]
- Stafford, R.J.; Fuentes, D.; Elliott, A.A.; Weinberg, J.S.; Ahrar, K. Laser-induced thermal therapy for tumor ablation. Crit. Rev. Biomed. Eng. 2010, 38, 79–100. [Google Scholar] [CrossRef]
- Hübner, F.; Bazrafshan, B.; Roland, J.; Kickhefel, A.; Vogl, T.J. The influence of Nd: YAG laser irradiation on Fluoroptic® temperature measurement: An experimental evaluation. Lasers Med. Sci. 2013, 28, 487–496. [Google Scholar] [CrossRef]
- Vogl, T.J.; Weinhold, N.; Mack, M.G.; Müller, P.K.; Scholz, W.R.; Straub, R.; Roggan, A.; Felix, R. Verification of MR thermometry by means of an in vivo intralesional, fluoroptic temperature measurement for laser-induced thermotherapy ov liver metastases. RoFo Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nukl. 1998, 169, 182–188. [Google Scholar] [CrossRef]
- Hood, R.L.; Rossmeisl, J.H., Jr.; Andriani, R.T., Jr.; Wilkinson, A.R.; Robertson, J.L.; Rylander, C.G. Intracranial hyperthermia through local photothermal heating with a fiberoptic microneedle device. Lasers Surg. Med. 2013, 45, 167–174. [Google Scholar] [CrossRef]
- Puccini, S.; Bär, N.; Bublat, M.; Kahn, T.; Busse, H. Simulations of thermal tissue coagulation and their value for the planning and monitoring of laser-induced interstitial thermotherapy (LITT). Magn. Reson. Med. An Off. J. Int. Soc. Magn. Reson. Med. 2003, 49, 351–362. [Google Scholar] [CrossRef]
- Reid, A.D.; Gertner, M.R.; Sherar, M.D. Temperature measurement artefacts of thermocouples and fluoroptic probes during laser irradiation at 810 nm. Phys. Med. Biol. 2001, 46, N149. [Google Scholar] [CrossRef]
- Polito, D.; Arturo Caponero, M.; Polimadei, A.; Saccomandi, P.; Massaroni, C.; Silvestri, S.; Schena, E. A needlelike probe for temperature monitoring during laser ablation based on fiber Bragg grating: Manufacturing and characterization. J. Med. Device. 2015, 9. [Google Scholar] [CrossRef]
- Polito, D.; Schena, E.; Saccomandi, P.; Silvestri, S.; Polimadei, A.; Caponero, M.A. Development and characterization of a fibre Bragg grating temperature probe for medical laser ablation therapy. In Proceedings of the SENSORS, 2014 IEEE, Valencia, Spain, 2–5 November 2014; pp. 1134–1137. [Google Scholar]
- Korganbayev, S.; Asadi, S.; Wolf, A.; Dostovalov, A.; Zalteri, M.; Schena, E.; Azhari, H.; Weitz, I.S.; Saccomandi, P. Highly dense FBG arrays for millimeter-scale thermal monitoring during nanocomposite-enhanced laser ablation. In Proceedings of the Optical Sensing and Detection VI, International Society for Optics and Photonics, Strasbourg, France, 29 March–2 April 2020; Volume 11354, p. 113540G. [Google Scholar]
- Di Santo, N.; Cavaiola, C.; Saccomandi, P.; Massaroni, C.; Giurazza, F.; Frauenfelder, G.; Schena, E.; Di Matteo, F.M.; Costamagna, G.; Caponero, M. Feasibility assessment of an FBG-based probe for distributed temperature measurements during laser ablation. In Proceedings of the 2016 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Benevento, Italy, 15–18 May 2016; pp. 1–6. [Google Scholar]
- Pandeya, G.D.; Klaessens, J.; Greuter, M.J.W.; Schmidt, B.; Flohr, T.; Van Hillegersberg, R.; Oudkerk, M. Feasibility of computed tomography based thermometry during interstitial laser heating in bovine liver. Eur. Radiol. 2011, 21, 1733–1738. [Google Scholar] [CrossRef]
- Schena, E.; Saccomandi, P.; Giurazza, F.; Caponero, M.A.; Mortato, L.; Di Matteo, F.M.; Panzera, F.; Del Vescovo, R.; Zobel, B.B.; Silvestri, S. Experimental assessment of CT-based thermometry during laser ablation of porcine pancreas. Phys. Med. Biol. 2013, 58, 5705. [Google Scholar] [CrossRef]
- Bruners, P.; Levit, E.; Penzkofer, T.; Isfort, P.; Ocklenburg, C.; Schmidt, B.; Schmitz-Rode, T.; Günther, R.W.; Mahnken, A.H. Multi-slice computed tomography: A tool for non-invasive temperature measurement? Int. J. Hyperth. 2010, 26, 359–365. [Google Scholar] [CrossRef]
- Bazrafshan, B.; Hübner, F.; Farshid, P.; Hammerstingl, R.; Paul, J.; Vogel, V.; Mäntele, W.; Vogl, T.J. Temperature imaging of laser-induced thermotherapy (LITT) by MRI: Evaluation of different sequences in phantom. Lasers Med. Sci. 2014, 29, 173–183. [Google Scholar] [CrossRef]
- Vogl, T.H.J.; Straub, R.; Zangos, S.; Mack, M.G.; Eichler, K. MR-guided laser-induced thermotherapy (LITT) of liver tumours: Experimental and clinical data. Int. J. Hyperth. 2004, 20, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Allegretti, G.; Saccomandi, P.; Giurazza, F.; Caponero, M.A.; Frauenfelder, G.; Di Matteo, F.M.; Zobel, B.B.; Silvestri, S.; Schena, E. Magnetic resonance-based thermometry during laser ablation on ex-vivo swine pancreas and liver. Med. Eng. Phys. 2015, 37, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.J.; Polhamus, G.D. Measurement and prediction of thermal injury in the retina of the rhesus monkey. IEEE Trans. Biomed. Eng. 1984, 633–644. [Google Scholar] [CrossRef]
- Verdaasdonk, R.M.; Holstege, F.C.; Jansen, E.D.; Borst, C. Temperature along the surface of modified fiber tips for Nd: YAG laser angioplasty. Lasers Surg. Med. 1991, 11, 213–222. [Google Scholar] [CrossRef]
- Germer, C.T.; Albrecht, D.; Roggan, A.; Isbert, C.; Buhr, H.J. Experimental study of laparoscopic laser-induced thermotherapy for liver tumours. Br. J. Surg. 1997, 84, 317–320. [Google Scholar]
- Muschter, R.; Whitfield, H. Interstitial laser therapy of benign prostatic hyperplasia. Eur. Urol. 1999, 35, 147–154. [Google Scholar] [CrossRef]
- Saccomandi, P.; Schena, E.; Caponero, M.A.; Di Matteo, F.M.; Martino, M.; Pandolfi, M.; Silvestri, S. Theoretical analysis and experimental evaluation of laser-induced interstitial thermotherapy in ex vivo porcine pancreas. IEEE Trans. Biomed. Eng. 2012, 59, 2958–2964. [Google Scholar] [CrossRef]
- Sturesson, C.; Ivarsson, K.; Andersson-Engels, S.; Tranberg, K.-G. Changes in local hepatic blood perfusion during interstitial laser-induced thermotherapy of normal rat liver measured by interstitial laser Doppler flowmetry. Lasers Med. Sci. 1999, 14, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.M.K.; Sturesson, C.; Liu, D.L.; Andersson-Engels, S. Changes in spectral shape of tissue optical properties in conjunction with laser-induced thermotherapy. Appl. Opt. 1998, 37, 1256–1267. [Google Scholar] [CrossRef]
- Saccomandi, P.; Schena, E.; Giurazza, F.; Del Vescovo, R.; Caponero, M.A.; Mortato, L.; Panzera, F.; Cazzato, R.L.; Grasso, F.R.; Di Matteo, F.M. Temperature monitoring and lesion volume estimation during double-applicator laser-induced thermotherapy in ex vivo swine pancreas: A preliminary study. Lasers Med. Sci. 2014, 29, 607–614. [Google Scholar] [CrossRef]
- Saccomandi, P.; Schena, E.; Silvestri, S. Techniques for temperature monitoring during laser-induced thermotherapy: An overview. Int. J. Hyperth. 2013, 29, 609–619. [Google Scholar] [CrossRef]
- Lewis, M.A.; Staruch, R.M.; Chopra, R. Thermometry and ablation monitoring with ultrasound. Int. J. Hyperth. 2015, 31, 163–181. [Google Scholar] [CrossRef]
- Yukhnev, A.; Tarkhov, D.; Gataulin, Y.; Ivanova, Y.; Berkovich, A. Neural Network Methods of HIFU-Therapy Control by Infrared Thermography and Ultrasound Thermometry. In Proceedings of the International Symposium on Neural Networks, Moscow, Russia, 10–12 July 2019; Springer: Cham, Switzerland, 2019; pp. 595–602. [Google Scholar]
- Kim, J.; Choi, W.; Park, E.-Y.; Kang, Y.; Lee, K.J.; Kim, H.H.; Kim, W.J.; Kim, C. Real-time photoacoustic thermometry combined with clinical ultrasound imaging and high-intensity focused ultrasound. IEEE Trans. Biomed. Eng. 2019, 66, 3330–3338. [Google Scholar] [CrossRef]
- Van Dongen, K.W.A.; Verweij, M.D. A feasibility study for non-invasive thermometry using non-linear ultrasound. Int. J. Hyperth. 2011, 27, 612–624. [Google Scholar] [CrossRef]
- Holbrook, A.B.; Santos, J.M.; Kaye, E.; Rieke, V.; Pauly, K.B. Real-time MR thermometry for monitoring HIFU ablations of the liver. Magn. Reson. Med. An Off. J. Int. Soc. Magn. Reson. Med. 2010, 63, 365–373. [Google Scholar] [CrossRef]
- Köhler, M.O.; Mougenot, C.; Quesson, B.; Enholm, J.; Le Bail, B.; Laurent, C.; Moonen, C.T.W.; Ehnholm, G.J. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med. Phys. 2009, 36, 3521–3535. [Google Scholar] [CrossRef]
- Lam, M.K.; Huisman, M.; Nijenhuis, R.J.; van den Bosch, M.A.A.J.; Viergever, M.A.; Moonen, C.T.W.; Bartels, L.W. Quality of MR thermometry during palliative MR-guided high-intensity focused ultrasound (MR-HIFU) treatment of bone metastases. J. Ther. Ultrasound 2015, 3, 5. [Google Scholar] [CrossRef]
- Patel, P.R.; Luk, A.; Durrani, A.; Dromi, S.; Cuesta, J.; Angstadt, M.; Dreher, M.R.; Wood, B.J.; Frenkel, V. In vitro and in vivo evaluations of increased effective beam width for heat deposition using a split focus high intensity ultrasound (HIFU) transducer. Int. J. Hyperth. 2008, 24, 537–549. [Google Scholar] [CrossRef]
- Lam, M.K.; Oerlemans, C.; Froeling, M.; Deckers, R.; Barten-Van Rijbroek, A.D.; Viergever, M.A.; Moonen, C.T.W.; Bos, C.; Bartels, L.W. DCE-MRI and IVIM-MRI of rabbit Vx2 tumors treated with MR-HIFU-induced mild hyperthermia. J. Ther. Ultrasound 2016, 4, 9. [Google Scholar] [CrossRef]
- Dasgupta, S.; Banerjee, R.K.; Hariharan, P.; Myers, M.R. Beam localization in HIFU temperature measurements using thermocouples, with application to cooling by large blood vessels. Ultrasonics 2011, 51, 171–180. [Google Scholar] [CrossRef]
- Karaböce, B.; Çetin, E.; Durmuş, H.O.; Öztürk, H.; Mahmat, K.; Güler, M.A.; Korkmaz, H. Variation of Temperature Responses Resulting from HIFU Application According to Acoustic Parameters. In Proceedings of the 2018 Medical Technologies National Congress (TIPTEKNO), Magusa, Cyprus, 8–10 November 2018; pp. 1–4. [Google Scholar]
- Karaböce, B.; Çetin, E.; Durmuş, H.O. Investigation of temperature rise in tissue—Mimicking material induced by a HIFU transducer. In Proceedings of the 2016 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Benevento, Italy, 15–18 May 2016; pp. 1–6. [Google Scholar]
- Chen, J.C.; Moriarty, J.A.; Derbyshire, J.A.; Peters, R.D.; Trachtenberg, J.; Bell, S.D.; Doyle, J.; Arrelano, R.; Wright, G.A.; Henkelman, R.M. Prostate cancer: MR imaging and thermometry during microwave thermal ablation-initial experience. Radiology 2000, 214, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Demura, K.; Morikawa, S.; Murakami, K.; Sato, K.; Shiomi, H.; Naka, S.; Kurumi, Y.; Inubushi, T.; Tani, T. An easy-to-use microwave hyperthermia system combined with spatially resolved MR temperature maps: Phantom and animal studies. J. Surg. Res. 2006, 135, 179–186. [Google Scholar] [CrossRef]
- De Senneville, B.D.; Mougenot, C.; Quesson, B.; Dragonu, I.; Grenier, N.; Moonen, C.T.W. MR thermometry for monitoring tumor ablation. Eur. Radiol. 2007, 17, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Gorny, K.R.; Favazza, C.P.; Lu, A.; Felmlee, J.P.; Hangiandreou, N.J.; Browne, J.E.; Stenzel, W.S.; Muggli, J.L.; Anderson, A.G.; Thompson, S.M. Practical implementation of robust MR-thermometry during clinical MR-guided microwave ablations in the liver at 1.5 T. Phys. Med. 2019, 67, 91–99. [Google Scholar] [CrossRef]
- Schena, E.; Giurazza, F.; Massaroni, C.; Fong, Y.; Park, J.J.; Saccomandi, P. Thermometry based on computed tomography images during microwave ablation: Trials on ex vivo porcine liver. In Proceedings of the 2017 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Turin, Italy, 22–25 May 2017; pp. 1–6. [Google Scholar]
- Saccomandi, P.; De Landro, M.; Massaroni, C.; Fong, Y.; Park, J.; Park, J.; Schena, E. Temperature map of kidneys undergoing microwave ablation using computed tomography-thermometry: Ex-vivo experiments and numerical simulations. In Proceedings of the 2019 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Istanbul, Turkey, 26–28 June 2019; pp. 1–6. [Google Scholar]
- Pohlan, J.; Kress, W.; Hermann, K.-G.; Mews, J.; Kroes, M.; Hamm, B.; Diekhoff, T. Computed Tomography Thermography for Ablation Zone Prediction in Microwave Ablation and Cryoablation: Advantages and Challenges in an Ex Vivo Porcine Liver Model. J. Comput. Assist. Tomogr. 2020, 44, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Kastler, A.; Alnassan, H.; Aubry, S.; Kastler, B. Microwave thermal ablation of spinal metastatic bone tumors. J. Vasc. Interv. Radiol. 2014, 25, 1470–1475. [Google Scholar] [CrossRef]
- Kastler, A.; Krainik, A.; Sakhri, L.; Mousseau, M.; Kastler, B. Feasibility of real-time intraprocedural temperature control during bone metastasis thermal microwave ablation: A bicentric retrospective study. J. Vasc. Interv. Radiol. 2017, 28, 366–371. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Nan, Q.; Xiao, J.; Li, L. Phantom experimental study on microwave ablation with a water-cooled antenna. Int. J. Hyperth. 2007, 23, 381–386. [Google Scholar] [CrossRef]
- Fan, W.; Li, X.; Zhang, L.; Jiang, H.; Zhang, J. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. Am. J. Roentgenol. 2012, 198, W46–W50. [Google Scholar] [CrossRef]
- Kuang, M.; Lu, M.D.; Xie, X.Y.; Xu, H.X.; Mo, L.Q.; Liu, G.J.; Xu, Z.F.; Zheng, Y.L.; Liang, J.Y. Liver cancer: Increased microwave delivery to ablation zone with cooled-shaft antenna—experimental and clinical studies. Radiology 2007, 242, 914–924. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Fan, W.; Zhao, M.; Wang, L.; Tang, T.; Jiang, H.; Zhang, J.; Liu, Y. Comparison of microwave ablation and multipolar radiofrequency ablation, both using a pair of internally cooled interstitial applicators: Results in ex vivo porcine livers. Int. J. Hyperth. 2011, 27, 240–248. [Google Scholar] [CrossRef]
- De Vita, E.; Zaltieri, M.; De Tommasi, F.; Massaroni, C.; Faiella, E.; Zobel, B.B.; Iadicicco, A.; Schena, E.; Grasso, R.F.; Campopiano, S. Multipoint Temperature Monitoring of Microwave Thermal Ablation in Bones through Fiber Bragg Grating Sensor Arrays. Sensors 2020, 20, 3200. [Google Scholar] [CrossRef]
- Zaltieri, M.; De Vita, E.; De Tommasi, F.; Massaroni, C.; Faiella, E.; Zobel, B.B.; Iadicicco, A.; Schena, E.; Grasso, R.F.; Campopiano, S. Evaluation of the Thermal Response of Liver Tissue Undergoing Microwave Treatment by Means of Fiber Bragg Grating Sensors. In Proceedings of the 2020 IEEE Sensors, Rotterdam, The Netherlands, 25–28 October 2020; pp. 1–4. [Google Scholar]
- De Tommasi, F.; Zaltieri, M.; Schena, E.; Massaroni, C.; Faiella, E.; Grasso, R.F.; Zobel, B.B.; De Vita, E.; Iadicicco, A.; Campopiano, S. Temperature Monitoring During Microwave Thermal Ablation of Ex Vivo Bovine Bone: A Pilot Test. In Proceedings of the 2020 IEEE International Workshop on Metrology for Industry 4.0 & IoT, Roma, Italy, 3–5 June 2020; pp. 255–259. [Google Scholar]
- Lepetit-Coiffé, M.; Quesson, B.; Seror, O.; Dumont, E.; Le Bail, B.; Moonen, C.T.W.; Trillaud, H. Real-time monitoring of radiofrequency ablation of rabbit liver by respiratory-gated quantitative temperature MRI. J. Magn. Reson. Imaging An Off. J. Int. Soc. Magn. Reson. Med. 2006, 24, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Terraz, S.; Cernicanu, A.; Lepetit-Coiffé, M.; Viallon, M.; Salomir, R.; Mentha, G.; Becker, C.D. Radiofrequency ablation of small liver malignancies under magnetic resonance guidance: Progress in targeting and preliminary observations with temperature monitoring. Eur. Radiol. 2010, 20, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Gellermann, J.; Wlodarczyk, W.; Feussner, A.; Fähling, H.; Nadobny, J.; Hildebrandt, B.; Felix, R.; Wust, P. Methods and potentials of magnetic resonance imaging for monitoring radiofrequency hyperthermia in a hybrid system. Int. J. Hyperth. 2005, 21, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Primavesi, F.; Swierczynski, S.; Klieser, E.; Kiesslich, T.; Jäger, T.; Urbas, R.; Hutter, J.; Neureiter, D.; Öfner, D.; Stättner, S. Thermographic real-time-monitoring of surgical radiofrequency and microwave ablation in a perfused porcine liver model. Oncol. Lett. 2018, 15, 2913–2920. [Google Scholar] [CrossRef]
- Geng, X.; Zhou, Z.; Li, Q.; Wu, S.; Wang, C.-Y.; Liu, H.-L.; Chuang, C.-C.; Tsui, P.-H. Comparison of ultrasound temperature imaging with infrared thermometry during radio frequency ablation. Jpn. J. Appl. Phys. 2014, 53, 47001. [Google Scholar] [CrossRef]
- Liu, Y.-D.; Li, Q.; Zhou, Z.; Yeah, Y.-W.; Chang, C.-C.; Lee, C.-Y.; Tsui, P.-H. Adaptive ultrasound temperature imaging for monitoring radiofrequency ablation. PLoS ONE 2017, 12, e0182457. [Google Scholar] [CrossRef]
- Daniels, M.J.; Varghese, T. Dynamic frame selection for in vivo ultrasound temperature estimation during radiofrequency ablation. Phys. Med. Biol. 2010, 55, 4735. [Google Scholar] [CrossRef][Green Version]
- Varghese, T.; Zagzebski, J.A.; Chen, Q.; Techavipoo, U.; Frank, G.; Johnson, C.; Wright, A.; Lee, F.T., Jr. Ultrasound monitoring of temperature change during radiofrequency ablation: Preliminary in-vivo results. Ultrasound Med. Biol. 2002, 28, 321–329. [Google Scholar] [CrossRef]
- Crowley, J.D.; Shelton, J.; Iverson, A.J.; Burton, M.P.; Dalrymple, N.C.; Bishoff, J.T. Laparoscopic and computed tomography-guided percutaneous radiofrequency ablation of renal tissue: Acute and chronic effects in an animal model. Urology 2001, 57, 976–980. [Google Scholar] [CrossRef]
- Buy, X.; Tok, C.-H.; Szwarc, D.; Bierry, G.; Gangi, A. Thermal protection during percutaneous thermal ablation procedures: Interest of carbon dioxide dissection and temperature monitoring. Cardiovasc. Intervent. Radiol. 2009, 32, 529–534. [Google Scholar] [CrossRef]
- Diehn, F.E.; Neeman, Z.; Hvizda, J.L.; Wood, B.J. Remote thermometry to avoid complications in radiofrequency ablation. J. Vasc. Interv. Radiol. 2003, 14, 1569–1576. [Google Scholar] [CrossRef]
- Patterson, E.J.; Scudamore, C.H.; Owen, D.A.; Nagy, A.G.; Buczkowski, A.K. Radiofrequency ablation of porcine liver in vivo: Effects of blood flow and treatment time on lesion size. Ann. Surg. 1998, 227, 559. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, D.E.; Hong, R.; Oliver, B.; Goldberg, S.N. Radiofrequency ablation of spinal tumors: Temperature distribution in the spinal canal. Am. J. Roentgenol. 2000, 175, 1263–1266. [Google Scholar] [CrossRef]
- Tosi, D.; Macchi, E.G.; Gallati, M.; Braschi, G.; Cigada, A.; Rossi, S.; Leen, G.; Lewis, E. Fiber-optic chirped FBG for distributed thermal monitoring of ex-vivo radiofrequency ablation of liver. Biomed. Opt. Express 2014, 5, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Saccomandi, P.; Schena, E.; Diana, M.; Di Matteo, F.M.; Costamagna, G.; Marescaux, J. Multipoint temperature monitoring in liver undergoing computed tomography-guided radiofrequency ablation with fiber Bragg grating probes. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5174–5179. [Google Scholar]
- Tosi, D.; Macchi, E.G.; Cigada, A. Fiber-optic temperature and pressure sensors applied to radiofrequency thermal ablation in liver phantom: Methodology and experimental measurements. J. Sens. 2015, 2015. [Google Scholar] [CrossRef]
- Macchi, E.G.; Tosi, D.; Braschi, G.; Gallati, M.; Cigada, A.; Lewis, E. Optical fiber sensors-based temperature distribution measurement in ex vivo radiofrequency ablation with submillimeter resolution. J. Biomed. Opt. 2014, 19, 117004. [Google Scholar] [CrossRef]
- Cao, H.; Vorperian, V.R.; Tsai, J.-Z.; Tungjitkusolmun, S.; Woo, E.J.; Webster, J.G. Temperature measurement within myocardium during in vitro RF catheter ablation. IEEE Trans. Biomed. Eng. 2000, 47, 1518–1524. [Google Scholar]
- Kovoor, P.; Daly, M.P.J.; Pouliopoulos, J.; Byth, K.; Dewsnap, B.I.; Eipper, V.E.; Yung, T.; Uther, J.F.B.; Ross, D.L. Comparison of radiofrequency ablation in normal versus scarred myocardium. J. Cardiovasc. Electrophysiol. 2006, 17, 80–86. [Google Scholar] [CrossRef]
- Wood, M.A.; Shaffer, K.M.; Ellenbogen, A.L.; Ownby, E.D. Microbubbles during radiofrequency catheter ablation: Composition and formation. Hear. Rhythm 2005, 2, 397–403. [Google Scholar] [CrossRef]
- Zaltieri, M.; Allegretti, G.; Massaroni, C.; Schena, E.; Cauti, F.M. Fiber Bragg Grating Sensors for Millimetric-Scale Temperature Monitoring of Cardiac Tissue Undergoing Radiofrequency Ablation: A Feasibility Assessment. Sensors 2020, 20, 6490. [Google Scholar] [CrossRef] [PubMed]
- Schena, E.; Tosi, D.; Saccomandi, P.; Lewis, E.; Kim, T. Fiber optic sensors for temperature monitoring during thermal treatments: An overview. Sensors 2016, 16, 1144. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.H.; Stephens, D.N.; Cannata, J.; Dentinger, A.; Lin, F.; Park, S.; Wildes, D.; Thomenius, K.E.; Chen, P.; Nguyen, T. The feasibility of using thermal strain imaging to regulate energy delivery during intracardiac radio-frequency ablation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 1406–1417. [Google Scholar] [PubMed]
- Wood, M.; Goldberg, S.; Lau, M.; Goel, A.; Alexander, D.; Han, F.; Feinstein, S. Direct measurement of the lethal isotherm for radiofrequency ablation of myocardial tissue. Circ. Arrhythmia Electrophysiol. 2011, 4, 373–378. [Google Scholar] [CrossRef]
- Kolandaivelu, A.; Zviman, M.M.; Castro, V.; Lardo, A.C.; Berger, R.D.; Halperin, H.R. Noninvasive assessment of tissue heating during cardiac radiofrequency ablation using MRI thermography. Circ. Arrhythmia Electrophysiol. 2010, 3, 521–529. [Google Scholar] [CrossRef]
- Rieke, V.; Butts Pauly, K. MR thermometry. J. Magn. Reson. Imaging An Off. J. Int. Soc. Magn. Reson. Med. 2008, 27, 376–390. [Google Scholar] [CrossRef]
- Rieke, V.; Vigen, K.K.; Sommer, G.; Daniel, B.L.; Pauly, J.M.; Butts, K. Referenceless PRF shift thermometry. Magn. Reson. Med. An Off. J. Int. Soc. Magn. Reson. Med. 2004, 51, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Burton, C. V RF lesion generation. Stereotact. Funct. Neurosurg. 1976, 39, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Organ, L.W. Electrophysiologic principles of radiofrequency lesion making. Stereotact. Funct. Neurosurg. 1976, 39, 69–76. [Google Scholar] [CrossRef]
- Haines, D.E.; Watson, D.D.; Verow, A.F. Electrode radius predicts lesion radius during radiofrequency energy heating. Validation of a proposed thermodynamic model. Circ. Res. 1990, 67, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Ulucakli, M.E. Simulation of Radiofrequency Ablation and Thermal Damage to Tissue. In Proceedings of the IEEE 32nd Annual Northeast Bioengineering Conference, Easton, PA, USA, 1–2 April 2006; pp. 93–94. [Google Scholar]
- Labonte, S. Numerical model for radio-frequency ablation of the endocardium and its experimental validation. IEEE Trans. Biomed. Eng. 1994, 41, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Childs, P.R.N.; Greenwood, J.R.; Long, C.A. Review of temperature measurement. Rev. Sci. Instrum. 2000, 71, 2959–2978. [Google Scholar] [CrossRef]
- Carnochan, P.; Dickinson, R.J.; Joiner, M.C. The practical use of thermocouples for temperature measurement in clinical hyperthermia. Int. J. Hyperth. 1986, 2, 1–19. [Google Scholar] [CrossRef]
- Christensen, D.A. Thermal dosimetry and temperature measurements. Cancer Res. 1979, 39, 2325–2327. [Google Scholar]
- Calkins, H.; Prystowsky, E.; Carlson, M.; Klein, L.S.; Saul, J.P.; Gillette, P. Temperature monitoring during radiofrequency catheter ablation procedures using closed loop control. Atakr Multicenter Investigators Group. Circulation 1994, 90, 1279–1286. [Google Scholar] [CrossRef]
- Anter, E.; Neužil, P.; Rackauskas, G.; Peichl, P.; Aidietis, A.; Kautzner, J.; Nakagawa, H.; Jackman, W.M.; Natale, A.; Reddy, V.Y. A lattice-tip temperature-controlled radiofrequency ablation catheter for wide thermal lesions: First-in-human experience with atrial fibrillation. JACC Clin. Electrophysiol. 2020, 6, 507–519. [Google Scholar] [CrossRef]
- Hindricks, G.; Haverkamp, W.; Gülker, H.; Rissel, U.; Budde, T.; Richter, K.D.; Borggrefe, M.; Breithardt, G. Radiofrequency coagulation of ventricular myocardium: Improved prediction of lesion size by monitoring catheter tip temperature. Eur. Heart J. 1989, 10, 972–984. [Google Scholar] [CrossRef]
- Petersen, H.H.; Chen, X.; Pietersen, A.; Svendsen, J.H.; Haunsø, S. Temperature-controlled radiofrequency ablation of cardiac tissue: An in vitro study of the impact of electrode orientation, electrode tissue contact pressure and external convective cooling. J. Interv. Card. Electrophysiol. 1999, 3, 257–262. [Google Scholar] [CrossRef]
- Petersen, H.H.; Chen, X.; Pietersen, A.; Svendsen, J.H.; Haunsø, S. Lesion dimensions during temperature-controlled radiofrequency catheter ablation of left ventricular porcine myocardium: Impact of ablation site, electrode size, and convective cooling. Circulation 1999, 99, 319–325. [Google Scholar] [CrossRef]
- Jain, M.K.; Wolf, P.D. In Vitro Temperature Map of Cardiac Ablation Demonstrates the Effect of Flow on Lesion Development. Ann. Biomed. Eng. 2000, 28, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Pollock, D.D. Thermocouples: Theory and Properties; CRC Press: Boca Raton, FL, USA, 1991; ISBN 0849342430. [Google Scholar]
- Kinzie, P.A.; Rubin, L.G. Thermocouple temperature measurement. PhT 1973, 26, 52. [Google Scholar] [CrossRef]
- Cao, H.; Vorperian, V.R.; Tungjitkusolmun, S.; Tsai, J.-Z.; Haemmerich, D.; Choy, Y.B.; Webster, J.G. Flow effect on lesion formation in RF cardiac catheter ablation. IEEE Trans. Biomed. Eng. 2001, 48, 425–433. [Google Scholar]
- Eick, O.J.; Bierbaum, D. Tissue Temperature-Controlled Radiofrequency Ablation. Pacing Clin. Electrophysiol. 2003, 26, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Halm, U.; Gaspar, T.; Zachäus, M.; Sack, S.; Arya, A.; Piorkowski, C.; Knigge, I.; Hindricks, G.; Husser, D. Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am. J. Gastroenterol. 2010, 105, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Halbfass, P.; Müller, P.; Nentwich, K.; Krug, J.; Roos, M.; Hamm, K.; Barth, S.; Szöllösi, A.; Mügge, A.; Schieffer, B. Incidence of asymptomatic oesophageal lesions after atrial fibrillation ablation using an oesophageal temperature probe with insulated thermocouples: A comparative controlled study. Ep Eur. 2017, 19, 385–391. [Google Scholar] [CrossRef]
- Chakraborty, D.; Brezovich, I. Error sources affecting thermocouple thermometry in RF electromagnetic fields. J. Microw. Power 1982, 17, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.P.; Brezovich, I.A. A source of thermocouple error in radiofrequency electric fields. Electron. Lett. 1980, 16, 853–854. [Google Scholar] [CrossRef]
- Blouin, L.T.; Marcus, F.I.; Lampe, L. Assessment of effects of a radiofrequency energy field and thermistor location in an electrode catheter on the accuracy of temperature measurement. Pacing Clin. Electrophysiol. 1991, 14, 807–813. [Google Scholar] [CrossRef]
- Pires, L.A.; Huang, S.K.S.; Wagshal, A.B.; Mittleman, R.S.; Rittman, W.J. Temperature-guided radiofrequency catheter ablation of closed-chest ventricular myocardium with a novel thermistor-tipped catheter. Am. Heart J. 1994, 127, 1614–1618. [Google Scholar] [CrossRef]
- McRury, I.A.N.D.; Whayne, J.G.; Haines, D.E. Temperature measurement as a determinant of tissue heating during radiofrequency catheter ablation: An examination of electrode thermistor positioning for measurement accuracy. J. Cardiovasc. Electrophysiol. 1995, 6, 268–278. [Google Scholar] [CrossRef]
- Langberg, J.J.; Calkins, H.; El-Atassi, R.; Borganelli, M.; Leon, A.; Kalbfleisch, S.J.; Morady, F. Temperature monitoring during radiofrequency catheter ablation of accessory pathways. Circulation 1992, 86, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Willems, S.; Chen, X.; Kottkamp, H.; Hindricks, G.; Haverkamp, W.; Rotman, B.; Shenasa, M.; Breithardt, G.; Borggrefe, M. Temperature-controlled radiofrequency catheter ablation of manifest accessory pathways. Eur. Heart J. 1996, 17, 445–452. [Google Scholar] [CrossRef]
- Thiagalingam, A.; D’Avila, A.; McPherson, C.; Malchano, Z.; Ruskin, J.; Reddy, V.Y. Impedance and temperature monitoring improve the safety of closed-loop irrigated-tip radiofrequency ablation. J. Cardiovasc. Electrophysiol. 2007, 18, 318–325. [Google Scholar] [CrossRef]
- Wood, S.D.; Mangum, B.W.; Filliben, J.J.; Tillett, S.B. An investigation of the stability of thermistors. J. Res. Natl. Bur. Stand 1978, 83, 247–263. [Google Scholar] [CrossRef]
- Redfearn, D.P.; Trim, G.M.; Skanes, A.C.; Petrellis, B.; Krahn, A.D.; Yee, R.; Klein, G.J. Esophageal temperature monitoring during radiofrequency ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2005, 16, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Lequerica, J.L.; Berjano, E.J.; Herrero, M.; Hornero, F. Esophageal temperature monitoring during radiofrequency catheter ablation: Experimental study based on an agar phantom model. Physiol. Meas. 2007. [Google Scholar] [CrossRef] [PubMed]
- Wickersheim, K.A.; Sun, M.H. Fiberoptic thermometry and its applications. J. Microw. Power Electromagn. Energy 1987, 22, 85–94. [Google Scholar] [CrossRef]
- Gottueb, C.F.; Hagmann, M.J.; Babü, T.M.; Abitbol, A.A.; Lewin, A.A.; Houdek, P.V.; Schwade, J.G. Interstitial microwave hyperthermia applicators having submillimetre diameters. Int. J. Hyperth. 1990, 6, 707–714. [Google Scholar] [CrossRef]
- Whayne, J.G.; Nath, S.; Haines, D.E. Microwave catheter ablation of myocardium in vitro. Assessment of the characteristics of tissue heating and injury. Circulation 1994, 89, 2390–2395. [Google Scholar] [CrossRef]
- Lopresto, V.; Pinto, R.; Cavagnaro, M. Experimental characterisation of the thermal lesion induced by microwave ablation. Int. J. Hyperth. 2014, 30, 110–118. [Google Scholar] [CrossRef]
- Mattei, E.; Triventi, M.; Calcagnini, G.; Censi, F.; Kainz, W.; Bassen, H.I.; Bartolini, P. Temperature and SAR measurement errors in the evaluation of metallic linear structures heating during MRI using fluoroptic® probes. Phys. Med. Biol. 2007, 52, 1633. [Google Scholar] [CrossRef] [PubMed]
- Viallon, M.; Terraz, S.; Roland, J.; Dumont, E.; Becker, C.D.; Salomir, R. Observation and correction of transient cavitation-induced PRFS thermometry artifacts during radiofrequency ablation, using simultaneous Ultrasound/MR imaging. Med. Phys. 2010, 37, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Thiagalingam, A.; Pouliopoulos, J.; Barry, M.A.; Salisbury, E.; Pathmanathan, N.; Boyd, A.; Ross, D.L.; Kovoor, P. A thermochromic dispersive electrode can measure the underlying skin temperature and prevent burns during radiofrequency ablation. J. Cardiovasc. Electrophysiol. 2005, 16, 781–788. [Google Scholar] [CrossRef]
- Matsudaira, K.; Nakagawa, H.; Wittkampf, F.H.M.; Yamanashi, W.S.; Imai, S.; Pitha, J.V.; Lazzara, R.; Jackman, W.M. High incidence of thrombus formation without impedance rise during radiofrequency ablation using electrode temperature control. Pacing Clin. Electrophysiol. 2003, 26, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Grattan, K.T.V.; Palmer, A.W. Fiber-optic high-temperature sensor based on the fluorescence lifetime of alexandrite. Rev. Sci. Instrum. 1992, 63, 3869–3873. [Google Scholar] [CrossRef]
- Lou, J.; Finegan, T.Μ.; Mohsen, P.; Hatton, T.A.; Laibinis, P.E. Fluorescence-based thermometry: Principles and applications. Rev. Anal. Chem. 1999, 18, 235–284. [Google Scholar] [CrossRef]
- Thyer, I.A.; Kovoor, P.; Barry, M.A.; Pouliopoulos, J.I.M.; Ross, D.L.; Thiagalingam, A. Protection of the coronary arteries during epicardial radiofrequency ablation with intracoronary chilled saline irrigation: Assessment in an in vitro model. J. Cardiovasc. Electrophysiol. 2006, 17, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, I.; Nuo, M.; Okumura, Y.; Ohkubo, K.; Ashino, S.; Kofune, M.; Kofune, T.; Nakai, T.; Kasamaki, Y.; Hirayama, A. Temperature-controlled cooled-tip radiofrequency ablation in left ventricular myocardium. Int. Heart J. 2010, 51, 193–198. [Google Scholar] [CrossRef][Green Version]
- Tosi, D.; Schena, E.; Molardi, C.; Korganbayev, S. Fiber optic sensors for sub-centimeter spatially resolved measurements: Review and biomedical applications. Opt. Fiber Technol. 2018, 43, 6–19. [Google Scholar] [CrossRef]
- Johnson, D. Novel Optical Fibers-Draw-tower process creates high-quality FBG arrays. Laser Focus World 2012, 48, 53. [Google Scholar]
- Shamir, A.; Ishaaya, A.A. Effect of femtosecond photo-treatment on inscription of fiber Bragg gratings. Opt. Lett. 2016, 41, 765–768. [Google Scholar] [CrossRef]
- Jelbuldina, M.; Korganbayev, S.; Seidagaliyeva, Z.; Sovetov, S.; Tuganbekov, T.; Tosi, D. Fiber Bragg Grating Sensor for Temperature Monitoring During HIFU Ablation of Ex Vivo Breast Fibroadenoma. IEEE Sens. Lett. 2019, 3, 1–4. [Google Scholar] [CrossRef]
- Erdogan, T. Fiber grating spectra. J. Light. Technol. 1997, 15, 1277–1294. [Google Scholar] [CrossRef]
- Fani, F.; Schena, E.; Saccomandi, P.; Silvestri, S. CT-based thermometry: An overview. Int. J. Hyperth. 2014, 30, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Calderon, A.; Watanabe, H.; Okamoto, K.; Suzuki, Y.; Kuroda, K.; Suzuki, Y. A precise and fast temperature mapping using water proton chemical shift. Magn. Reson. Med. 1995, 34, 814–823. [Google Scholar] [CrossRef]
- Poorter, J.D.; Wagter, C.D.; Deene, Y.D.; Thomsen, C.; Ståhlberg, F.; Achten, E. Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: In vivo results in human muscle. Magn. Reson. Med. 1995, 33, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Vigen, K.K.; Daniel, B.L.; Pauly, J.M.; Butts, K. Triggered, navigated, multi-baseline method for proton resonance frequency temperature mapping with respiratory motion. Magn. Reson. Med. An Off. J. Int. Soc. Magn. Reson. Med. 2003, 50, 1003–1010. [Google Scholar] [CrossRef]
- Gellermann, J.; Wlodarczyk, W.; Hildebrandt, B.; Ganter, H.; Nicolau, A.; Rau, B.; Tilly, W.; Fähling, H.; Nadobny, J.; Felix, R. Noninvasive magnetic resonance thermography of recurrent rectal carcinoma in a 1.5 Tesla hybrid system. Cancer Res. 2005, 65, 5872–5880. [Google Scholar] [CrossRef] [PubMed]
- Gellermann, J.; Hildebrandt, B.; Issels, R.; Ganter, H.; Wlodarczyk, W.; Budach, V.; Felix, R.; Tunn, P.; Reichardt, P.; Wust, P. Noninvasive magnetic resonance thermography of soft tissue sarcomas during regional hyperthermia: Correlation with response and direct thermometry. Cancer 2006, 107, 1373–1382. [Google Scholar] [CrossRef]
- Tempany, C.M.C.; Stewart, E.A.; McDannold, N.; Quade, B.J.; Jolesz, F.A.; Hynynen, K. MR imaging–guided focused ultrasound surgery of uterine leiomyomas: A feasibility study. Radiology 2003, 226, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Baik, J.-H.; Pham, L.D.; Jacobs, M.A. MR-guided high-intensity focused ultrasound treatment for symptomatic uterine leiomyomata: Long-term outcomes. Acad. Radiol. 2011, 18, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Odéen, H.; Parker, D.L. Magnetic resonance thermometry and its biological applications–Physical principles and practical considerations. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 110, 34–61. [Google Scholar] [CrossRef]
- Winter, L.; Oberacker, E.; Paul, K.; Ji, Y.; Oezerdem, C.; Ghadjar, P.; Thieme, A.; Budach, V.; Wust, P.; Niendorf, T. Magnetic resonance thermometry: Methodology, pitfalls and practical solutions. Int. J. Hyperth. 2016, 32, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Stollberger, R.; Ascher, P.W.; Huber, D.; Renhart, W.; Radner, H.; Ebner, F. Temperature monitoring of interstitial thermal tissue coagulation using MR phase images. J. Magn. Reson. imaging 1998, 8, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Depoorter, J.; Dewagter, C.; Dedeene, Y.; Thomsen, C.; Stahlberg, F.; Achten, E. The proton-resonance-frequency-shift method compared with molecular diffusion for quantitative measurement of two-dimensional time-dependent temperature distribution in a phantom. J. Magn. Reson. Ser. B 1994, 103, 234–241. [Google Scholar] [CrossRef]
- Hindman, J.C. Proton resonance shift of water in the gas and liquid states. J. Chem. Phys. 1966, 44, 4582–4592. [Google Scholar] [CrossRef]
- Hore, P.J. Nuclear Magnetic Resonance; Oxford University Press: New York, NY, USA, 2015; ISBN 0198703414. [Google Scholar]
- Kuroda, K.; Oshio, K.; Chung, A.H.; Hynynen, K.; Jolesz, F.A. Temperature mapping using the water proton chemical shift: A chemical shift selective phase mapping method. Magn. Reson. Med. 1997, 38, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Hey, S.; Cernicanu, A.; De Senneville, B.D.; Roujol, S.; Ries, M.; Jaïs, P.; Moonen, C.T.W.; Quesson, B. Towards optimized MR thermometry of the human heart at 3T. NMR Biomed. 2012, 25, 35–43. [Google Scholar] [CrossRef]
- De Senneville, B.D.; Roujol, S.; Jaïs, P.; Moonen, C.T.W.; Herigault, G.; Quesson, B. Feasibility of fast MR-thermometry during cardiac radiofrequency ablation. NMR Biomed. 2012, 25, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Toupin, S.; Bour, P.; Lepetit-Coiffé, M.; Ozenne, V.; De Senneville, B.D.; Schneider, R.; Vaussy, A.; Chaumeil, A.; Cochet, H.; Sacher, F. Feasibility of real-time MR thermal dose mapping for predicting radiofrequency ablation outcome in the myocardium in vivo. J. Cardiovasc. Magn. Reson. 2017, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ozenne, V.; Toupin, S.; Bour, P.; De Senneville, B.D.; Lepetit-Coiffé, M.; Boissenin, M.; Benois-Pineau, J.; Hansen, M.S.; Inati, S.J.; Govari, A. Improved cardiac magnetic resonance thermometry and dosimetry for monitoring lesion formation during catheter ablation. Magn. Reson. Med. 2017, 77, 673–683. [Google Scholar] [CrossRef]
- Toupin, S.; De Senneville, B.D.; Ozenne, V.; Bour, P.; Lepetit-Coiffe, M.; Boissenin, M.; Jais, P.; Quesson, B. Combination of principal component analysis and optical-flow motion compensation for improved cardiac MR thermometry. Phys. Med. Biol. 2017, 62, 1208. [Google Scholar] [CrossRef]
- Grissom, W.A.; Rieke, V.; Holbrook, A.B.; Medan, Y.; Lustig, M.; Santos, J.; McConnell, M.V.; Pauly, K.B. Hybrid referenceless and multibaseline subtraction MR thermometry for monitoring thermal therapies in moving organs. Med. Phys. 2010, 37, 5014–5026. [Google Scholar] [CrossRef] [PubMed]
- Nasoni, R.L.; Bowen, T.; Connor, W.G.; Sholes, R.R. In vivo temperature dependence of ultrasound speed in tissue and its application to noninvasive temperature monitoring. Ultrason. Imaging 1979, 1, 34–43. [Google Scholar] [CrossRef]
- Bowen, T.; Connor, W.G.; Nasoni, R.L.; Pifer, A.E.; Sholes, R.R. Measurement of the temperature dependence of the velocity of ultrasound in soft tissues. Ultrason. Tissue Charact. II 1979, 525, 57–61. [Google Scholar]
- Bamber, J.C.; Hill, C.R. Ultrasonic attenuation and propagation speed in mammalian tissues as a function of temperature. Ultrasound Med. Biol. 1979, 5, 149–157. [Google Scholar] [CrossRef]
- Prakash, O.; Fabbri, M.; Drocourt, M.; Escanye, J.-M.; Marchal, C.; Gaulard, M.-L.; Robert, J. Hyperthermia induction and its measurement using ultrasound. In Proceedings of the 1980 Ultrasonics Symposium, Boston, MA, USA, 5–7 November 1980; pp. 1063–1066. [Google Scholar]
- Simon, C.; VanBaren, P.; Ebbini, E.S. Two-dimensional temperature estimation using diagnostic ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1998, 45, 1088–1099. [Google Scholar] [CrossRef]
- Liu, D.; Ebbini, E.S. Real-time 2-D temperature imaging using ultrasound. IEEE Trans. Biomed. Eng. 2009, 57, 12–16. [Google Scholar] [PubMed]
- Giurazza, F.; Massaroni, C.; Silvestri, S.; Zobel, B.B.; Schena, E. Preliminary analysis of ultrasound elastography imaging-based thermometry on non-perfused ex vivo swine liver. J. Ultrasound 2020, 23, 69–75. [Google Scholar] [CrossRef]
- Lemor, R.M.; Kleffner, B.V.; Tretbar, S.; Schmitt, R.M. Ultrasound temperature and attenuation monitoring for controlling the laser induced thermo therapy. In Acoustical Imaging; Springer: Boston, MA, USA, 2002; pp. 395–400. [Google Scholar]
- Garrean, S.; Hering, J.; Saied, A.; Hoopes, P.J.; Helton, W.S.; Ryan, T.P.; Espat, N.J. Ultrasound monitoring of a novel microwave ablation (MWA) device in porcine liver: Lessons learned and phenomena observed on ablative effects near major intrahepatic vessels. J. Gastrointest. Surg. 2009, 13, 334–340. [Google Scholar] [CrossRef]
- Abu-Zidan, F.M.; Hefny, A.F.; Corr, P. Clinical ultrasound physics. J. Emergencies Trauma Shock 2011, 4, 501. [Google Scholar]
- Pernot, M.; Tanter, M.; Bercoff, J.; Waters, K.R.; Fink, M. Temperature estimation using ultrasonic spatial compound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 606–615. [Google Scholar] [CrossRef]
- Seip, R.; Ebbini, E.S. Noninvasive estimation of tissue temperature response to heating fields using diagnostic ultrasound. IEEE Trans. Biomed. Eng. 1995, 42, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Arthur, R.M.; Straube, W.L.; Trobaugh, J.W.; Moros, E.G. Non-invasive estimation of hyperthermia temperatures with ultrasound. Int. J. Hyperth. 2005, 21, 589–600. [Google Scholar] [CrossRef]
- Ni, Y.; Mulier, S.; Miao, Y.; Michel, L.; Marchal, G. A review of the general aspects of radiofrequency ablation. Abdom. Imaging 2005, 30, 381–400. [Google Scholar] [CrossRef]
- Ueno, S.; Hashimoto, M.; Fukukita, H.; Yano, T. Ultrasound thermometry in hyperthermia. In Proceedings of the IEEE Symposium on Ultrasonics, Honolulu, HI, USA, 4–7 December 1990; pp. 1645–1652. [Google Scholar]
- Arthur, R.M.; Trobaugh, J.W.; Straube, W.L.; Moros, E.G.; Sangkatumvong, S. Temperature dependence of ultrasonic backscattered energy in images compensated for tissue motion. In Proceedings of the IEEE Symposium on Ultrasonics, Honolulu, HI, USA, 5–8 October 2003; pp. 990–993. [Google Scholar]
- Milonov, O.B.; Lebedeva, O.D.; Pomelova, L.A. Use of echography and thermography in parasitic liver diseases. Sov. Med. 1980, 62. [Google Scholar]
- Köşüş, N.; Köşüş, A.; Duran, M.; Simavlı, S.; Turhan, N. Comparison of standard mammography with digital mammography and digital infrared thermal imaging for breast cancer screening. J. Turkish Ger. Gynecol. Assoc. 2010, 11, 152. [Google Scholar] [CrossRef]
- Bagavathiappan, S.; Saravanan, T.; Philip, J.; Jayakumar, T.; Raj, B.; Karunanithi, R.; Panicker, T.M.; Korath, P.; Jagadeesan, K. Investigation of peripheral vascular disorders using thermal imaging. Br. J. Diabetes Vasc. Dis. 2008, 8, 102–104. [Google Scholar] [CrossRef]
- Tan, J.-H.; Ng, E.Y.K.; Acharya, U.R.; Chee, C. Study of normal ocular thermogram using textural parameters. Infrared Phys. Technol. 2010, 53, 120–126. [Google Scholar] [CrossRef]
- Zhou, Y. Noninvasive thermometry in high-intensity focused ultrasound ablation. Ultrasound Q. 2017, 33, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Yu, Y.; Guofeng, S. Theoretical framework for quantitatively estimating harmonic intensity in focused ultrasound field using infrared thermometry. In Proceedings of the 2020 13th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Chengdu, China, 17–19 October 2020; pp. 778–783. [Google Scholar]
- Jones, B.F. A reappraisal of the use of infrared thermal image analysis in medicine. IEEE Trans. Med. Imaging 1998, 17, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Meola, C.; Carlomagno, G.M. Recent advances in the use of infrared thermography. Meas. Sci. Technol. 2004, 15, R27. [Google Scholar] [CrossRef]
- Gaussorgues, G.; Chomet, S. Infrared Thermography; Springer Science & Business Media: London, UK, 1993; Volume 5, ISBN 0412479001. [Google Scholar]
- Watanabe, H.; Ishii, J.; Wakabayashi, H.; Kumano, T.; Hanssen, L. Spectral emissivity measurements. In Experimental Methods in the Physical Sciences; Elsevier: Amsterdam, The Netherlands, 2014; Volume 46, pp. 333–366. ISBN 1079-4042. [Google Scholar]
- Steketee, J. Spectral emissivity of skin and pericardium. Phys. Med. Biol. 1973, 18, 686. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.P.; Burke, M.C.; Hong, T.E.; McAuley, A.; Amundson, D.; Hanlin, J.; Blankenship, L.; Ferguson, T.B., Jr.; Nazarian, S.; Berger, R.D. Direct imaging of transvenous radiofrequency cardiac ablation using a steerable fiberoptic infrared endoscope. Hear. Rhythm 2005, 2, 1116–1121. [Google Scholar] [CrossRef]
- Singh-Moon, R.P.; Marboe, C.C.; Hendon, C.P. Near-infrared spectroscopy integrated catheter for characterization of myocardial tissues: Preliminary demonstrations to radiofrequency ablation therapy for atrial fibrillation. Biomed. Opt. Express 2015, 6, 2494–2511. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.G.; Melton, I.; Roper, G.; Lim, G.; Crozier, I.G. High-resolution infrared thermography of esophageal temperature during radiofrequency ablation of atrial fibrillation. Circ. Arrhythmia Electrophysiol. 2018, 11, e005667. [Google Scholar] [CrossRef]
- Hummel, J.P.; Kadado, A.J.; Baker, M.; Gehi, A.K.; Mounsey, J.P.; Sadaf, M.I.; Enriquez, A.D.; Freeman, J.V.; Akar, J.G. Atrial Fibrillation Thermographic and Endoscopic Monitoring of Patients: Safety Algorithm for the Esophagus: AF TEMP-SAFE Study. Circ. Arrhythmia Electrophysiol. 2018, 11, e006814. [Google Scholar] [CrossRef]
- Borne, R.T.; Nguyen, D.T. Red Alert: Infrared Thermography for Esophageal Monitoring. Circ. Arrhythmia Electrophysiol. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef]
| First Author, Year, Ref. | Measurement System | Type of Experiment | Features |
|---|---|---|---|
| Cao et al., 2000−2001 [80,106] | Probe embedding 3 thermocouples | RFA on ex vivo bovine myocardium in saline bath | Time constant = 0.08 s (but approximately 0.2 s when inserted in the proposed probe) |
| Eick et al., 2003 [107] | Probe embedding a single thermocouple | RFA on ex vivo swine myocardium in saline bath | |
| Halm et al., 2010 [108] | Esophageal probe embedding 5 thermocouples | 185 patients undergoing myocardial RFA | |
| Halbfass et al., 2017 [109] | Esophageal probe embedding 12 thermocouples | 80 patients undergoing myocardial RFA | |
| Redfearm et al., 2005 [119] | Esophageal probe embedding a single thermistor | 15 patients undergoing myocardial RFA | |
| Kovoor et al., 2006 [81] | 5 probes embedding a single thermistor | 5 mongrel dogs undergoing myocardial RFA | Time constant = 0.2 s |
| Rodrìguez et al., 2007 [120] | Esophageal probe embedding a single thermistor + single exposed thermistor | Agar phantom | Time constants = 8.0 s for the esophageal probe and 1.5 s for the exposed thermistor |
| Wood et al., 2005 [82] | 4 fluoroptic probes | RFA on ex vivo swine myocardium in saline bath | |
| Thyer et al., 2006 [131] | 2 fluoroptic probes | RFA on ex vivo ovine myocardium in saline bath | |
| Watanabe et al., 2010 [132] | 4 fluoroptic probes | 10 mongrel dogs undergoing myocardial RFA | |
| Zaltieri et al., 2020 [83] | 2 fiber optics embedding 7 FBGs 1 each | RFA on ex vivo swine myocardium in saline bath | Sensing length = 1 mm Thermal sensitivity = 0.01 nm∙°C−1 |
| First Author, Year, Ref. | Type of Sensor | Model (In Vivo, Ex Vivo, In Vitro) | Features |
|---|---|---|---|
| Kolandaivelu et al., 2010 [87] | MRI 1 | 6 mongrel dogs undergoing myocardial RFA | FOV 3 = 220 × 165 mm Resolution = 256 × 192 Slice thickness = 4 mm |
| Hey et al., 2011 [153] | MRI 1 | 8 patients undergoing myocardial RFA | FOV 3 =350 × 350 × 8 mm3 Resolution = 3.5 mm2 Slice thickness = 8 mm |
| De Senneville et al., 2012 [154] | MRI 1 | 2 sheep undergoing myocardial RFA | FOV 3 = 250 × 166 mm Resolution = 2.6 mm2 Slice thickness = 7 mm |
| Toupin et al., 2017 [155] | MRI 1 | 5 patients + 3 sheep undergoing myocardial RFA | FOV 3 = 225 × 225 mm2 Resolution = 1.8 × 1.8 × 4 mm3 Slice thickness = 3 mm |
| Ozenne et al., 2017 [156] | MRI 1 | 10 patients undergoing myocardial RFA | FOV 3 = 180 × 180 mm2 Resolution =1.6 × 1.6 × 4 mm2 Slice thickness = 3 mm |
| Toupin et al., 2017 [157] | MRI 1 | 9 patients + 1 sheep undergoing myocardial RFA + 1 agar phantom | FOV 3 = 180 × 180 mm2 Resolution = 1.6 × 1.6 × 3 mm3 |
| Seo et al., 2001 [85] | Ultrasound Imaging | RFA on ex vivo swine myocardium in saline bath + in vivo on swine myocardium | Imaging depth = 5 mm/10 mm Imaging width = 45° Frame rate = 32 Hz/1 Hz |
| Wood et al., 2011 [86] | IR 2 Imaging | RFA on ex vivo swine myocardium in saline bath | Sensitivity = 0.005 °C Accuracy = ±2% |
| Daly et al., 2018 [188] | IR 2 Imaging | 16 patients undergoing myocardial RFA | Dimension = 3 mm of diameter Resolution = 0.1 °C |
| Hummel et al., 2018 [189] | IR 2 Imaging | 34 patients undergoing myocardial RFA | Dimension = 3.5 mm of diameter |
| Borne et al., 2018 [190] | IR 2 Imaging | 16 patients undergoing myocardial RFA | Dimension = 3 mm/6 mm of diameter |
| Technology | Features |
|---|---|
| Thermocouples | Invasive; single point measurement; accuracy of approximately 1 °C; robust; well-known technology; almost constant sensitivity in a wide range of T; adequate dynamic response considering the application of interest; wide measuring interval; potential presence of measurement artifacts. |
| Thermistors | Invasive; single point measurement; accuracy better than 0.3 °C; robust; well-known technology; high sensitivity but can significantly decrease for high T; adequate dynamic response considering the application of interest; wide measuring interval; potential presence of measurement artifacts. |
| Fluoroptic Sensors | Invasive; single point measurement; accuracy up to 0.2 °C; fragile; adequate dynamic response considering the application of interest; wide measuring interval; immunity to electromagnetic interference. |
| FBGs | Invasive; multi-point measurement with resolution even better than 1 mm; accuracy even higher than 0.1 °C; fragile; adequate dynamic response considering the application of interest; wide measuring interval; constant sensitivity in a wide range of T; immunity to electromagnetic interferences; potential presence of motion artifacts. |
| MRI | Non-invasive; 3D distribution of T; sensitivity up to −0.01 ppm·°C−1; constant sensitivity for T from −15 °C to 100 °C; no tissue dependency in case of PRFST 1; temporal resolution better than 2 s; motion artifacts. |
| Ultrasound Imaging | Non-invasive; 3D distribution of T; easy supply in clinical settings; high T resolution at high computational costs; motion artifacts; decrease in sensitivity for T up to 50 °C. |
| IR Imaging | Non-invasive; 2D color-coded T map; no T information regarding the inner layers; measurement affected by the surrounding environment. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaltieri, M.; Massaroni, C.; Cauti, F.M.; Schena, E. Techniques for Temperature Monitoring of Myocardial Tissue Undergoing Radiofrequency Ablation Treatments: An Overview. Sensors 2021, 21, 1453. https://doi.org/10.3390/s21041453
Zaltieri M, Massaroni C, Cauti FM, Schena E. Techniques for Temperature Monitoring of Myocardial Tissue Undergoing Radiofrequency Ablation Treatments: An Overview. Sensors. 2021; 21(4):1453. https://doi.org/10.3390/s21041453
Chicago/Turabian StyleZaltieri, Martina, Carlo Massaroni, Filippo Maria Cauti, and Emiliano Schena. 2021. "Techniques for Temperature Monitoring of Myocardial Tissue Undergoing Radiofrequency Ablation Treatments: An Overview" Sensors 21, no. 4: 1453. https://doi.org/10.3390/s21041453
APA StyleZaltieri, M., Massaroni, C., Cauti, F. M., & Schena, E. (2021). Techniques for Temperature Monitoring of Myocardial Tissue Undergoing Radiofrequency Ablation Treatments: An Overview. Sensors, 21(4), 1453. https://doi.org/10.3390/s21041453









