The Use of Pulse Oximetry in the Assessment of Acclimatization to High Altitude
Abstract
1. Introduction
2. Methods
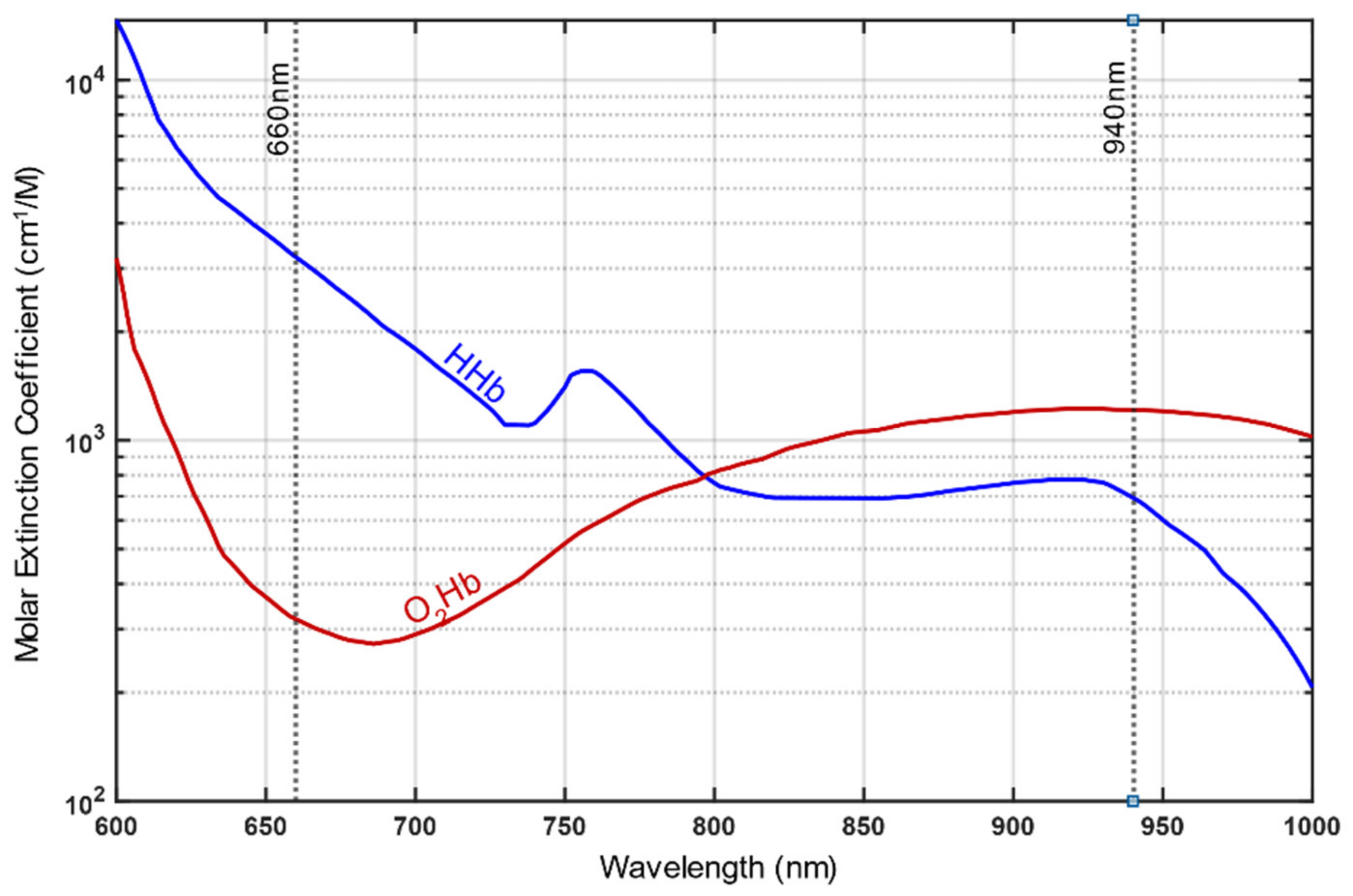
3. Part 1: Basic Principles of Functioning, Most Relevant Pitfalls and Possible Countermeasures for Pulse Oximetry Particularly Concerning Healthy People Going to High Altitudes
4. Part 2: Results from the Literature Review
4.1. Resting SpO2 and HR Changes during Acclimatization to High Altitude
4.2. Exercising SpO2 and HR Changes during Acclimatization to High Altitude
4.3. Changes in AMS Scores during Acclimatization to High Altitude
5. Part 3: Discussion
5.1. Physiologic and Pathophysiologic Mechanisms Explaining Pulse Oximetric Measures When Acutely Exposed to High Altitude and during Acclimatization
5.1.1. Resting SpO2 and HR Changes during Acclimatization to High Altitude
5.1.2. Exercising SpO2 and HR Changes during Acclimatization to High Altitude
5.1.3. The Use of Pulse Oximetry for the Diagnosis of Acute Mountain Sickness (AMS)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, R.T.; Kling, S.R.; Salata, M.J.; Cupp, S.A.; Sheehan, J.; Voos, J.E. Wearable Performance Devices in Sports Medicine. Sports Health Multidiscip. Approach 2016, 8, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Z.; Wong, D.W.; Lam, W.K.; Wan, A.H.; Lee, W.C. Balance Improvement Effects of Biofeedback Systems with State-of-the-Art Wearable Sensors: A Systematic Review. Sensors 2016, 16, 434. [Google Scholar] [CrossRef]
- Altini, M.; Casale, P.; Penders, J.; Ten Velde, G.; Plasqui, G.; Amft, O. Cardiorespiratory fitness estimation using wearable sensors: Laboratory and free-living analysis of context-specific submaximal heart rates. J. Appl. Physiol. 2016, 120, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Welsh, E.J.; Carr, R. Pulse oximeters to self monitor oxygen saturation levels as part of a personalised asthma action plan for people with asthma. Cochrane Database Syst. Rev. 2015, 9, CD011584. [Google Scholar]
- Otani, S.; Miyaoka, Y.; Ikeda, A.; Ohno, G.; Imura, S.; Watanabe, K.; Kurozawa, Y. Evaluating Health Impact at High Altitude in Antarctica and Effectiveness of Monitoring Oxygen Saturation. Yonago Acta Med. 2020, 63, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Tannheimer, M.; Lechner, R. The correct measurement of oxygen saturation at high altitude. Sleep Breath. 2019, 23, 1101–1106. [Google Scholar] [CrossRef]
- Koehle, M.S.; Guenette, J.A.; Warburton, D.E. Oximetry, heart rate variability, and the diagnosis of mild-to-moderate acute mountain sickness. Eur. J. Emerg. Med. 2010, 17, 119–122. [Google Scholar] [CrossRef]
- Burtscher, M.; Flatz, M.; Faulhaber, M. Prediction of susceptibility to acute mountain sickness by SaO2 values during short-term exposure to hypoxia. High Alt. Med. Biol. 2004, 5, 335–340. [Google Scholar] [CrossRef]
- Tannheimer, M.; Thomas, A.; Gerngross, H. Oxygen saturation course and altitude symptomatology during an expedition to broad peak (8047 m). Int. J. Sports Med. 2002, 23, 329–335. [Google Scholar] [CrossRef]
- Reuland, D.S.; Steinhoff, M.C.; Gilman, R.H.; Bara, M.; Olivares, E.G.; Jabra, A.; Finkelstein, D. Prevalence and prediction of hypoxemia in children with respiratory infections in the Peruvian Andes. J. Pediatr. 1991, 119, 900–906. [Google Scholar] [CrossRef]
- Ottestad, W.; Kåsin, J.I.; Høiseth, L.Ø. Arterial Oxygen Saturation, Pulse Oximetry, and Cerebral and Tissue Oximetry in Hypobaric Hypoxia. Aerosp. Med. Hum. Perform. 2018, 89, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Luks, A.M.; Swenson, E.R. Pulse oximetry at high altitude. High Alt. Med. Biol. 2011, 12, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.D.; Chan, M.M.; Chan, M.M. Pulse oximetry: Understanding its basic principles facilitates appreciation of its limitations. Respir. Med. 2013, 107, 789–799. [Google Scholar] [CrossRef]
- Jubran, A. Pulse oximetry. Crit. Care 2015, 19, 272. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T. Current progress of photoplethysmography and SPO2 for health monitoring. Biomed. Eng. Lett. 2019, 9, 21–36. [Google Scholar] [CrossRef]
- Lipnick, M.S.; Feiner, J.R.; Au, P.; Bernstein, M.; Bickler, P.E. The Accuracy of 6 Inexpensive Pulse Oximeters Not Cleared by the Food and Drug Administration: The Possible Global Public Health Implications. Anesth. Analg. 2016, 123, 338–345. [Google Scholar] [CrossRef]
- Petterson, M.T.; Begnoche, V.L.; Graybeal, J.M. The effect of motion on pulse oximetry and its clinical significance. Anesth. Analg. 2007, 105, S78–S84. [Google Scholar] [CrossRef]
- Zaouter, C.; Zavorsky, G.S. The measurement of carboxyhemoglobin and methemoglobin using a non-invasive pulse CO-oximeter. Respir. Physiol. Neurobiol. 2012, 182, 88–92. [Google Scholar] [CrossRef]
- Feiner, J.R.; Rollins, M.D.; Sall, J.W.; Eilers, H.; Au, P.; Bickler, P.E. Accuracy of carboxyhemoglobin detection by pulse CO-oximetry during hypoxemia. Anesth. Analg. 2013, 117, 847–858. [Google Scholar] [CrossRef]
- Prahl, S. Tabulated Molar Extinction Coefficient for Hemoglobin in Water; Oregon Medical Laser Center: Portland, OR, USA, 1998. [Google Scholar]
- Nitzan, M.; Romem, A.; Koppel, R. Pulse oximetry: Fundamentals and technology update. Med. Devices 2014, 7, 231–239. [Google Scholar] [CrossRef]
- Center for Devices and Radiological Health. Pulse Oximeters—Premarket Notification Submissions [510 (k)s] Guidance for Industry and Food and Drug Administration Staff; U.S. Department of Health and Human Services: Washington, DC, USA, 2013.
- Pretto, J.J.; Roebuck, T.; Beckert, L.; Hamilton, G. Clinical use of pulse oximetry: Official guidelines from the Thoracic Society of Australia and New Zealand. Respirology 2014, 19, 38–46. [Google Scholar] [CrossRef]
- Hudson, A.J.; Benjamin, J.; Jardeleza, T.; Bergstrom, C.; Cronin, W.; Mendoza, M.; Schultheis, L. Clinical Interpretation of Peripheral Pulse Oximeters Labeled “Not for Medical Use”. Ann. Fam. Med. 2018, 16, 552–554. [Google Scholar] [CrossRef]
- Luks, A.M.; Swenson, E.R. Pulse Oximetry for Monitoring Patients with COVID-19 at Home. Potential Pitfalls and Practical Guidance. Ann. Am. Thorac. Soc. 2020, 17, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.N.; Hofmeyr, R. Perioperative comparison of the agreement between a portable fingertip pulse oximeter v. a conventional bedside pulse oximeter in adult patients (COMFORT trial). S. Afr. Med. J. 2019, 109, 154–158. [Google Scholar] [CrossRef]
- Ross, E.M.; Matteucci, M.J.; Shepherd, M.; Barker, M.; Orr, L. Measuring arterial oxygenation in a high altitude field environment: Comparing portable pulse oximetry with blood gas analysis. Wilderness Environ. Med. 2013, 24, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Bradke, B.; Everman, B. Investigation of Photoplethysmography Behind the Ear for Pulse Oximetry in Hypoxic Conditions with a Novel Device (SPYDR). Biosensors 2020, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Longmore, S.K.; Lui, G.Y.; Naik, G.; Breen, P.P.; Jalaludin, B.; Gargiulo, G.D. A Comparison of Reflective Photoplethysmography for Detection of Heart Rate, Blood Oxygen Saturation, and Respiration Rate at Various Anatomical Locations. Sensors 2019, 19, 1874. [Google Scholar] [CrossRef]
- Yamaya, Y.; Bogaard, H.J.; Wagner, P.D.; Niizeki, K.; Hopkins, S.R. Validity of pulse oximetry during maximal exercise in normoxia, hypoxia, and hyperoxia. J. Appl. Physiol. 2002, 92, 162–168. [Google Scholar] [CrossRef]
- Lorente-Aznar, T.; Perez-Aguilar, G.; García-Espot, A.; Benabarre-Ciria, S.; Mendia-Gorostidi, J.L.; Dols-Alonso, D.; Blasco-Romero, J. Estimation of arterial oxygen saturation in relation to altitude. Med. Clin. 2016, 147, 435–440. [Google Scholar] [CrossRef]
- Barker, S.J. “Motion-resistant” pulse oximetry: A comparison of new and old models. Anesth. Analg. 2002, 95, 967–972. [Google Scholar] [CrossRef]
- Clarke, G.W.J.; Chan, A.D.C.; Adler, A. Effects of motion artifact on the blood oxygen saturation estimate in pulse oximetry. In Proceedings of the 2014 IEEE International Symposium on Medical Measurements and Applications, Lisabon, Portugal, 11–12 June 2014; pp. 1–4. [Google Scholar]
- Giuliano, K.K.; Higgins, T.L. New-generation pulse oximetry in the care of critically ill patients. Am. J. Crit. Care 2005, 14, 26–37. [Google Scholar] [CrossRef]
- Louie, A.; Feiner, J.R.; Bickler, P.E.; Rhodes, L.; Bernstein, M.; Lucero, J. Four Types of Pulse Oximeters Accurately Detect Hypoxia during Low Perfusion and Motion. Anesthesiology 2018, 128, 520–530. [Google Scholar] [CrossRef]
- Cannesson, M.; Talke, P. Recent advances in pulse oximetry. F1000 Med. Rep. 2009, 1, 66. [Google Scholar] [CrossRef]
- Fluck, R.R.; Schroeder, C.; Frani, G.; Kropf, B.; Engbretson, B. Does ambient light affect the accuracy of pulse oximetry? Respir. Care 2003, 48, 677–680. [Google Scholar]
- World Health Organization. Pulse Oximetry Training Manual; WHO Press: Geneva, Switzerland, 2011. [Google Scholar]
- Hafen, B.B.; Sharma, S. Oxygen Saturation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Peacock, A.J. ABC of oxygen: Oxygen at high altitude. BMJ 1998, 317, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. High-altitude medicine. Am. J. Respir. Crit. Care Med. 2012, 186, 1229–1237. [Google Scholar] [CrossRef]
- Severinghaus, J.W.; Naifeh, K.H.; Koh, S.O. Errors in 14 pulse oximeters during profound hypoxia. J. Clin. Monit. 1989, 5, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.C.; Yoon, H.; Kang, H.; Yeom, H. Effects of skin surface temperature on photoplethysmograph. J. Healthc. Eng. 2014, 5, 429–438. [Google Scholar] [CrossRef]
- Khan, M.; Pretty, C.G.; Amies, A.C.; Elliott, R.; Shaw, G.M.; Chase, J.G. Investigating the Effects of Temperature on Photoplethysmography. IFAC PapersOnLine 2015, 48, 360–365. [Google Scholar] [CrossRef]
- Khan, M.; Pretty, C.G.; Amies, A.C.; Elliott, R.; Chiew, Y.S.; Shaw, G.M.; Chase, J.G. Analysing the effects of cold, normal, and warm digits on transmittance pulse oximetry. Biomed. Signal Process. Control 2016, 26, 34–41. [Google Scholar] [CrossRef]
- Feiner, J.R.; Severinghaus, J.W.; Bickler, P.E. Dark skin decreases the accuracy of pulse oximeters at low oxygen saturation: The effects of oximeter probe type and gender. Anesth. Analg. 2007, 105, S18–S23. [Google Scholar] [CrossRef]
- Bickler, P.E.; Feiner, J.R.; Severinghaus, J.W. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology 2005, 102, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Ralston, A.C.; Webb, R.K.; Runciman, W.B. Potential errors in pulse oximetry. III: Effects of interferences, dyes, dyshaemoglobins and other pigments. Anaesthesia 1991, 46, 291–295. [Google Scholar] [CrossRef]
- Sjoding, M.W.; Dickson, R.P.; Iwashyna, T.J.; Gay, S.E.; Valley, T.S. Racial Bias in Pulse Oximetry Measurement. N. Engl. J. Med. 2020, 383, 2477–2478. [Google Scholar] [CrossRef] [PubMed]
- Tannheimer, M. The Use of Pulse Oximetry at High Altitude. Res. Investig. Sports Med. 2020, 6, 10–13. [Google Scholar]
- Rodden, A.M.; Spicer, L.; Diaz, V.A.; Steyer, T.E. Does fingernail polish affect pulse oximeter readings? Intensive Crit. Care Nurs. 2007, 23, 51–55. [Google Scholar] [CrossRef]
- Chan, M.M.; Chan, M.M.; Chan, E.D. What is the effect of fingernail polish on pulse oximetry? Chest 2003, 123, 2163–2164. [Google Scholar] [CrossRef]
- Yeganehkhah, M.; Dadkhahtehrani, T.; Bagheri, A.; Kachoie, A. Effect of Glittered Nail Polish on Pulse Oximetry Measurements in Healthy Subjects. Iran. J. Nurs. Midwifery Res. 2019, 24, 25–29. [Google Scholar]
- Ballesteros-Pena, S.; Fernandez-Aedo, I.; Picon, A.; Lorrio-Palomino, S. Influence of nail polish on pulse oximeter readings of oxygen saturation: A systematic review. Emergencias 2015, 27, 325–331. [Google Scholar]
- Foutch, R.G.; Henrichs, W. Carbon monoxide poisoning at high altitudes. Am. J. Emerg. Med. 1988, 6, 596–598. [Google Scholar] [CrossRef]
- Buchheit, M.; Simpson, B.M.; Garvican-Lewis, L.A.; Hammond, K.; Kley, M.; Schmidt, W.F.; Aughey, R.J.; Soria, R.; Sargent, C.; Roach, G.D.; et al. Wellness, fatigue and physical performance acclimatisation to a 2-week soccer camp at 3600 m (ISA3600). Br. J. Sports Med. 2013, 47, i100–i106. [Google Scholar] [CrossRef]
- Beidleman, B.A.; Fulco, C.S.; Muza, S.R.; Rock, P.B.; Staab, J.E.; Forte, V.A.; Brothers, M.D.; Cymerman, A. Effect of six days of staging on physiologic adjustments and acute mountain sickness during ascent to 4300 meters. High Alt. Med. Biol. 2009, 10, 253–260. [Google Scholar] [CrossRef]
- Burtscher, M.; Bachmann, O.; Hatzl, T.; Hotter, B.; Likar, R.; Philadelphy, M.; Nachbauer, W. Cardiopulmonary and metabolic responses in healthy elderly humans during a 1-week hiking programme at high altitude. Eur. J. Appl. Physiol. 2001, 84, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, A.; Pooja; Sharma, M.; Singh, K.; Patyal, A.; Bhaumik, G.; Bhargava, K.; Sethy, N.K. Intermittent normobaric hypoxia facilitates high altitude acclimatization by curtailing hypoxia-induced inflammation and dyslipidemia. Eur. J. Physiol. 2019, 471, 949–959. [Google Scholar] [CrossRef]
- Gibson, O.R.; Richardson, A.J.; Hayes, M.; Duncan, B.; Maxwell, N.S. Prediction of physiological responses and performance at altitude using the 6-minute walk test in normoxia and hypoxia. Wilderness Environ. Med. 2015, 26, 205–210. [Google Scholar] [CrossRef]
- Strapazzon, G.; Vezzaro, R.; Hofer, G.; Dal Cappello, T.; Procter, E.; Balkenhol, K.; Platzgummer, S.; Brugger, H. Factors associated with B-lines after exposure to hypobaric hypoxia. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1241–1246. [Google Scholar] [CrossRef][Green Version]
- Bhaumik, G.; Dass, D.; Lama, H.; Chauhan, S.K. Maximum exercise responses of men and women mountaineering trainees on induction to high altitude (4350 m) by trekking. Wilderness Environ. Med. 2008, 19, 151–156. [Google Scholar] [CrossRef]
- Fulco, C.S.; Muza, S.R.; Beidleman, B.A.; Demes, R.; Staab, J.E.; Jones, J.E.; Cymerman, A. Effect of repeated normobaric hypoxia exposures during sleep on acute mountain sickness, exercise performance, and sleep during exposure to terrestrial altitude. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R428–R436. [Google Scholar] [CrossRef] [PubMed]
- Scrase, E.; Laverty, A.; Gavlak, J.C.; Sonnappa, S.; Levett, D.Z.; Martin, D.; Grocott, M.P.; Stocks, J. The Young Everest Study: Effects of hypoxia at high altitude on cardiorespiratory function and general well-being in healthy children. Arch. Dis. Child. 2009, 94, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Severinghaus, J.W.; Bickler, P. Time course of augmentation and depression of hypoxic ventilatory responses at altitude. J. Appl. Physiol. 1994, 77, 313–316. [Google Scholar] [CrossRef]
- Savourey, G.; Garcia, N.; Besnard, Y.; Hanniquet, A.M.; Fine, M.O.; Bittel, J. Physiological changes induced by pre-adaptation to high altitude. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 221–227. [Google Scholar] [CrossRef]
- Reeves, J.T.; McCullough, R.E.; Moore, L.G.; Cymerman, A.; Weil, J.V. Sea-level PCO2 relates to ventilatory acclimatization at 4300 m. J. Appl. Physiol. 1993, 75, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Bender, P.R.; McCullough, R.E.; McCullough, R.G.; Huang, S.Y.; Wagner, P.D.; Cymerman, A.; Hamilton, A.J.; Reeves, J.T. Increased exercise SaO2 independent of ventilatory acclimatization at 4300 m. J. Appl. Physiol. 1989, 66, 2733–2738. [Google Scholar] [CrossRef] [PubMed]
- Voutselas, S.; Stavrou, V.; Zouridis, S.; Vavougios, G.; Gourgroulianis, K.I.; Voutselas, V. The effect of sleep quality in Sherpani Col High Camp Everest. Respir. Physiol. Neurobiol. 2019, 269, 103261. [Google Scholar] [CrossRef] [PubMed]
- Hoiland, R.L.; Foster, G.E.; Donnelly, J.; Stembridge, M.; Willie, C.K.; Smith, K.J.; Lewis, N.C.; Lucas, S.J.E.; Cotter, J.D.; Yeoman, D.J.; et al. Chemoreceptor Responsiveness at Sea Level Does Not Predict the Pulmonary Pressure Response to High Altitude. Chest 2015, 148, 219–225. [Google Scholar] [CrossRef]
- Willie, C.K.; Smith, K.J.; Day, T.A.; Ray, L.A.; Lewis, N.C.; Bakker, A.; Macleod, D.B.; Ainslie, P.N. Regional cerebral blood flow in humans at high altitude: Gradual ascent and 2 wk at 5050 m. J. Appl. Physiol. 2014, 116, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Modesti, P.A.; Rapi, S.; Paniccia, R.; Bilo, G.; Revera, M.; Agostoni, P.; Piperno, A.; Cambi, G.E.; Rogolino, A.; Biggeri, A.; et al. Index measured at an intermediate altitude to predict impending acute mountain sickness. Med. Sci. Sports Exerc. 2011, 43, 1811–1818. [Google Scholar] [CrossRef]
- Agostoni, P.; Swenson, E.R.; Bussotti, M.; Revera, M.; Meriggi, P.; Faini, A.; Lombardi, C.; Bilo, G.; Giuliano, A.; Bonacina, D.; et al. High-altitude exposure of three weeks duration increases lung diffusing capacity in humans. J. Appl. Physiol. 2011, 110, 1564–1571. [Google Scholar] [CrossRef]
- Baillie, J.K.; Thompson, A.A.; Irving, J.B.; Bates, M.G.; Sutherland, A.I.; Macnee, W.; Maxwell, S.R.; Webb, D.J. Oral antioxidant supplementation does not prevent acute mountain sickness: Double blind, randomized placebo-controlled trial. QJM 2009, 102, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Likar, R.; Nachbauer, W.; Philadelphy, M.; Pühringer, R.; Lämmle, T. Effects of aspirin during exercise on the incidence of high-altitude headache: A randomized, double-blind, placebo-controlled trial. Headache 2001, 41, 542–545. [Google Scholar] [CrossRef]
- Burtscher, M.; Gatterer, H.; Faulhaber, M.; Burtscher, J. Acetazolamide pre-treatment before ascending to high altitudes: When to start? Int. J. Clin. Exp. Med. 2014, 7, 4378–4383. [Google Scholar]
- Burtscher, M.; Faulhaber, M.; Flatz, M.; Likar, R.; Nachbauer, W. Effects of short-term acclimatization to altitude (3200 m) on aerobic and anaerobic exercise performance. Int. J. Sports Med. 2006, 27, 629–635. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Berryman, C.E.; Wilson, M.A.; Luippold, A.J.; Kenefick, R.W.; Young, A.J.; Pasiakos, S.M. Effects of carbohydrate supplementation on aerobic exercise performance during acute high altitude exposure and after 22 days of acclimatization and energy deficit. J. Int. Soc. Sports Nutr. 2020, 17, 4. [Google Scholar] [CrossRef]
- Vizcardo-Galindo, G.; León-Velarde, F.; Villafuerte, F.C. High-Altitude Hypoxia Decreases Plasma Erythropoietin Soluble Receptor Concentration in Lowlanders. High Alt. Med. Biol. 2020, 21, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Gekeler, K.; Schatz, A.; Fischer, M.D.; Schommer, K.; Boden, K.; Bartz-Schmidt, K.U.; Gekeler, F.; Willmann, G. Decreased contrast sensitivity at high altitude. Br. J. Ophthalmol. 2019, 103, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Aeberli, I.; Erb, A.; Spliethoff, K.; Meier, D.; Götze, O.; Frühauf, H.; Fox, M.; Finlayson, G.S.; Gassmann, M.; Berneis, K.; et al. Disturbed eating at high altitude: Influence of food preferences, acute mountain sickness and satiation hormones. Eur. J. Nutr. 2013, 52, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Nussbaumer-Ochsner, Y.; Ursprung, J.; Siebenmann, C.; Maggiorini, M.; Bloch, K.E. Effect of short-term acclimatization to high altitude on sleep and nocturnal breathing. Sleep 2012, 35, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Willmann, G.; Fischer, M.D.; Schatz, A.; Schommer, K.; Messias, A.; Zrenner, E.; Bartz-Schmidt, K.U.; Gekeler, F. Quantification of optic disc edema during exposure to high altitude shows no correlation to acute mountain sickness. PLoS ONE 2011, 6, e27022. [Google Scholar] [CrossRef] [PubMed]
- Lundeberg, J.; Feiner, J.R.; Schober, A.; Sall, J.W.; Eilers, H.; Bickler, P.E. Increased Cytokines at High Altitude: Lack of Effect of Ibuprofen on Acute Mountain Sickness, Physiological Variables, or Cytokine Levels. High Alt. Med. Biol. 2018, 19, 249–258. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lin, F.C.; Shiao, G.M.; Chang, S.C. Effect of rapid ascent to high altitude on autonomic cardiovascular modulation. Am. J. Med. Sci. 2008, 336, 248–253. [Google Scholar] [CrossRef]
- Staab, J.E.; Beidleman, B.A.; Muza, S.R.; Fulco, C.S.; Rock, P.B.; Cymerman, A. Efficacy of residence at moderate versus low altitude on reducing acute mountain sickness in men following rapid ascent to 4300 m. High Alt. Med. Biol. 2013, 14, 13–18. [Google Scholar] [CrossRef]
- Sareban, M.; Schiefer, L.M.; Macholz, F.; Schäfer, L.; Zangl, Q.; Inama, F.; Reich, B.; Mayr, B.; Schmidt, P.; Hartl, A.; et al. Endurance Athletes Are at Increased Risk for Early Acute Mountain Sickness at 3450 m. Med. Sci. Sports Exerc. 2020, 52, 1109–1115. [Google Scholar] [CrossRef]
- Lenfant, C.; Sullivan, K. Adaptation to high altitude. N. Engl. J. Med. 1971, 284, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Hackett, P.H.; Roach, R.C. High-altitude illness. N. Engl. J. Med. 2001, 345, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Netzer, N.; Strohl, K.; Faulhaber, M.; Gatterer, H.; Burtscher, M. Hypoxia-related altitude illnesses. J. Travel Med. 2013, 20, 247–255. [Google Scholar] [CrossRef]
- Rahn, H.; Otis, A.B. Man’s respiratory response during and after acclimatization to high altitude. Am. J. Physiol. 1949, 157, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Beidleman, B.A.; Muza, S.R.; Rock, P.B.; Fulco, C.S.; Lyons, T.P.; Hoyt, R.W.; Cymerman, A. Exercise responses after altitude acclimatization are retained during reintroduction to altitude. Med. Sci. Sports Exerc. 1997, 29, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Muza, S.R.; Beidleman, B.A.; Fulco, C.S. Altitude preexposure recommendations for inducing acclimatization. High Alt. Med. Biol. 2010, 11, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Wille, M.; Menz, V.; Faulhaber, M.; Gatterer, H. Symptom progression in acute mountain sickness during a 12-hour exposure to normobaric hypoxia equivalent to 4500 m. High Alt. Med. Biol. 2014, 15, 446–451. [Google Scholar] [CrossRef]
- Gaillard, S.; Dellasanta, P.; Loutan, L.; Kayser, B. Awareness, prevalence, medication use, and risk factors of acute mountain sickness in tourists trekking around the Annapurnas in Nepal: A 12-year follow-up. High Alt. Med. Biol. 2004, 5, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Philadelphy, M.; Gatterer, H.; Burtscher, J.; Likar, R. Submaximal exercise testing at low altitude for prediction of exercise tolerance at high altitude. J. Travel Med. 2018, 25, tay011. [Google Scholar] [CrossRef] [PubMed]
- Valli, G.; Internullo, M.; Ferrazza, A.M.; Onorati, P.; Cogo, A.; Palange, P. Minute ventilation and heart rate relationship for estimation of the ventilatory compensation point at high altitude: A pilot study. Extreme Physiol. Med. 2013, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.E.; Gore, C.J.; Ebert, T.R.; Martin, D.T.; Hahn, A.G.; Chow, C.M. Ventilatory acclimatisation is beneficial for high-intensity exercise at altitude in elite cyclists. Eur. J. Sport Sci. 2016, 16, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Horstman, D.; Weiskopf, R.; Jackson, R.E. Work capacity during 3-wk sojourn at 4300 m: Effects of relative polycythemia. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 49, 311–318. [Google Scholar] [PubMed]
- Maher, J.T.; Jones, L.G.; Hartley, L.H. Effects of high-altitude exposure on submaximal endurance capacity of men. J. Appl. Physiol. 1974, 37, 895–898. [Google Scholar] [CrossRef]
- Buskirk, E.R.; Kollias, J.; Akers, R.F.; Prokop, E.K.; Reategui, E.P. Maximal performance at altitude and on return from altitude in conditioned runners. J. Appl. Physiol. 1967, 23, 259–266. [Google Scholar] [CrossRef]
- Burtscher, M.; Niedermeier, M.; Burtscher, J.; Pesta, D.; Suchy, J.; Strasser, B. Preparation for Endurance Competitions at Altitude: Physiological, Psychological, Dietary and Coaching Aspects. A Narrative Review. Front. Physiol. 2018, 9, 1504. [Google Scholar] [CrossRef]
- O’Connor, T.; Dubowitz, G.; Bickler, P.E. Pulse oximetry in the diagnosis of acute mountain sickness. High Alt. Med. Biol. 2004, 5, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Lin, W.L.; Wu, J.Y.; Wang, S.H.; Chiu, T.F.; Weng, Y.M.; Hsu, T.Y.; Wu, M.H. Change in oxygen saturation does not predict acute mountain sickness on Jade Mountain. Wilderness Environ. Med. 2012, 23, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Leichtfried, V.; Basic, D.; Burtscher, M.; Gothe, R.M.; Siebert, U.; Schobersberger, W. Diagnosis and prediction of the occurrence of acute mountain sickness measuring oxygen saturation--independent of absolute altitude? Sleep Breath. 2016, 20, 435–442. [Google Scholar] [CrossRef]
- Karinen, H.M.; Peltonen, J.E.; Kähönen, M.; Tikkanen, H.O. Prediction of acute mountain sickness by monitoring arterial oxygen saturation during ascent. High Alt. Med. Biol. 2010, 11, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Roach, R.C.; Greene, E.R.; Schoene, R.B.; Hackett, P.H. Arterial oxygen saturation for prediction of acute mountain sickness. Aviat. Space Environ. Med. 1998, 69, 1182–1185. [Google Scholar] [PubMed]
- Mandolesi, G.; Avancini, G.; Bartesaghi, M.; Bernardi, E.; Pomidori, L.; Cogo, A. Long-term monitoring of oxygen saturation at altitude can be useful in predicting the subsequent development of moderate-to-severe acute mountain sickness. Wilderness Environ. Med. 2014, 25, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, M.; Likar, R.; Nachbauer, W.; Philadelphy, M. Aspirin for prophylaxis against headache at high altitudes: Randomised, double blind, placebo controlled trial. BMJ 1998, 316, 1057–1058. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Causes of Unreliable SpO2 Readings | Effects on The Measurement Result | Special Influence Conditioned by The High Altitude | Feasible Countermeasures |
|---|---|---|---|
| Excessive movement | Motion artefacts may cause a decrease of measured SpO2 [13,32,33], however modern devices implement advanced algorithm to reduce motion artefacts [14,15,17,33,34]. These devices may be identified by indications such as “motion tolerant” or “motion resistant” [34,35,36]. | With increasing altitude, the temperature drops. This may result in cold extremities and an increased shivering and affects the sensor position and the sensor signal. | During measurement, keep the measuring position steady and avoid too much trembling. |
| Poor probe positioning | The red or infrared light of the sensor may bypass the tissue or too high levels of ambient light hit the light-detector of the sensor [13,15]. This results in a wrong SpO2 reading. | None | An imperfectly fitting of the sensor should be avoided, and the sensor should always be used in the appropriate position. If these countermeasures do not achieve the desired results, measurement at a different site (e.g., earlobe or forehead) may be considered. |
| Excess ambient light | Excessive ambient light can lead to erroneous SpO2 readings. However, modern devices are capable of handling strong ambient light more effectively [13,37]. | Especially snow-covered areas with high solar radiation can lead to increased ambient light intensity at the sensor. | Protect the sensor from sunlight (e.g., by covering the measuring site). |
| Decreased arterial pressures of oxygen (PaO2) | A decrease in PaO2 (<60 mmHg) results in a significant change in oxygen saturation where small variations of the pressure have a strong effect on the saturation [12,38,39]. | Increasing altitude results in a decreasing PaO2. Especially at altitudes above 3000 m a PaO2 below 60% can be expected [12,40,41]. | To reduce fluctuations in PaO2, SpO2 measurements should be conducted after the person keeps silent and gentle breathing for several minutes. The measurement duration should be extended over a few minutes and the most frequent occurring value should be used [27]. |
| SpO2 saturation below 70% | Devices complying with the international standard ISO 80601-2-61 (medical electrical equipment. Part 2-61: Specific requirements for basic safety and essential performance of pulse oximeter equipment) must measure accurate oxygen saturations (Arms ≤ 4%) between 70–100%. Below 70%, they are less reliable [11,12,16,23,25,42]. | the oxygen saturation is estimated based on human calibration data measured from 100% to 70%. Saturation values below 70% are only based on an extrapolation of this determined curve [13,25]. At high altitudes, however, the occurrence of low saturation values is not abnormal. | The possibility of a slight deviation of the measured value should be considered if oxygen saturation is below 70%. Especially when comparing data with devices of several manufacturers. |
| Cold-induced vasoconstriction (poor perfusion) | Cold skin temperatures reduce SpO2 reading accuracy [43,44,45]. This effect is based on a reduced blood flow due to cold-induced vasoconstriction [12,31]. However, modern devices can handle this condition and/or report it to the user. These devices may be identified by indications such as “oximeter with perfusion index” or “sensitivity to low perfusion signals”. | With increasing altitude, the temperature drops. | Warming the measuring site before and preferably during the measurement (e.g., using heating pads). |
| Skin pigmentation | Pulse oximeters are possibly less accurate during hypoxia in dark-skinned individuals at lower saturation (<80%) resulting in overestimations [46,47]. Feiner et al. mentioned [48]: “further study is needed to confirm these observations in the relevant populations.” However, an actual study is consistent with Bickler et al. and Feiner et al. [49]. | At high altitude, the occurrence of saturation values below 80% is not abnormal [12,50]. | Until the scientific data is more definite, the possibility of a slight overestimation (about +2% [51]) of the measured value should be kept in mind when interpreting the data for a person with oxygen saturation below approx. 90% combined with a dark pigmentation of the skin. |
| Nail polish | Some fingernail polish can lower the SpO2 readings [48]. Previous studies, however, have shown that the variance is not clinically relevant using actual devices [51,52,53,54]. | None | Especially with older devices, the nail polish should be removed to avoid variations in the measurement accuracy. However, the deviation in the SpO2 readings is less than 2% [53,54]. |
| Limited knowledge of technology (devices) and data interpretation | A lack of knowledge regarding device application and interpretation of the measurement data can lead to incorrect conclusions [14,23]. | Conditions at high altitudes complicate the use of the device and the accurate interpretation of the data [12,27,50]. | As Tannheimer et al. [50] concluded it “requires an experienced examiner who can include altitude anamnesis, clinical examination and mountaineering aspects in the overall assessment” to avoid possible pitfalls during SpO2 measurement and interpretation on high altitudes. |
| Dyshemoglobins (carboxyhemoglobin and methemoglobin) | Based on an absorption of the red and infrared light, methemoglobin (MetHb) and carboxyhemoglobin (HbCO) cause SpO2 overestimation and mask serious hypoxia [14,23,48]. However, as already mentioned, certain multiple-wavelengths devices are capable of detecting dyshemoglobins. | Unlikely at high altitude, however, it can be a danger using a cooking stove in small, enclosed areas like tents. In the worst case, this can lead to carbon monoxide poisoning [12,55]. | When using devices that are not capable of analyzing dyshemoglobins, possible carbon monoxide poisoning should be considered if the person has remained in a small, enclosed space for an extended period while a combustion process (e.g., a stove) has taken place. Symptoms of carbon monoxide poisoning may include headache, nausea and drowsiness. However, these symptoms are similar to those associated to altitude sickness [56]. |
| Authors | Participants M/F; Age (Means ± SD or Median (Range)) | Altitude | Exposure Time at Target Altitude (days) | Information about SpO2 Measurements | Pre-Acclimatization/Prolonged Ascent Phase | Type of Exposure [Stay, Ascents] | Change in Resting SpO2 (%) (Means ± SD or Median (Range)) d (Days), Bl (Baseline) | Change in Resting HR (bpm) (Means ± SD or Median (Range)) |
|---|---|---|---|---|---|---|---|---|
| Gangwar et al. (2019) [59] | 20 M; 22–25 years # | 3520 m | 7 | n.a. | - | stay | Bl: 99.0 * d1: 89.7 * d2: 90.5 * d3: 91.2 * d4: 92.0 * d5: 93.0 * d6: 94.2 * d7: 95.2 * | |
| Voutselas et al. (2019) [69] | 8 M; 48.0 ± 9.2 years | 5700 m | 7 | environment temperature: 0.6–5 °C | - | stay | d1: 83.0 ± 3.7 d2: 82.9 ± 4.3 d3: 84.3 ± 3.3 d4: 86.9 ± 1.6 d5: 87.1 ± 5.5 d6: 84.6 ± 4.4 d7: 89.4 ± 1.8 | d1: 87.9 ± 9.7 d2: 87.1 ± 8.5 d3: 86.6 ± 11.9 d4: 84.8 ± 10.5 d5: 95.1 ± 13.8 d6: 81.4 ± 9.3 d7: 78.8 ± 9.1 |
| Gibson et al. (2015) [60] | 29 (15 M, 14 F); 22.2 ± 5.4 years | 3400 m | 9 | temperature (°C) (mean (95%CI)): Bl: 14.7 (14.1–15.4) d2: 24.2 (24.2–24.2) d6: 27.0 (27.0–27.0) d9: 21.9 (21.9–21.9) | - | stay/outdoor tests (6MWT) at 3 times at 42, 138 and 210 h | Bl: 97.7 (96.6–98.7) d2: 92.5 (91.6–93.3) d6: 91.6 (90.8–92.4) d9: 92.2 (91.4–93.0) | Bl: 82.0 (75.7–88.3) d2: 100.5 (95.8–105.2) d6: 85.1 (95.8–105.2) d9: 80.5 (75.4–85.7) |
| Hoiland et al. (2015) [70] | 20 (15 M, 5 W); 34 ± 7 years | 5050 m | 14 (max 21) | n.a. | - | stay | Bl: 98.6 ± 1.1 d2: 79.5 ± 2.9 d5: 83.4 ± 1.9 d14–21: 80.5 ± 1.6 | |
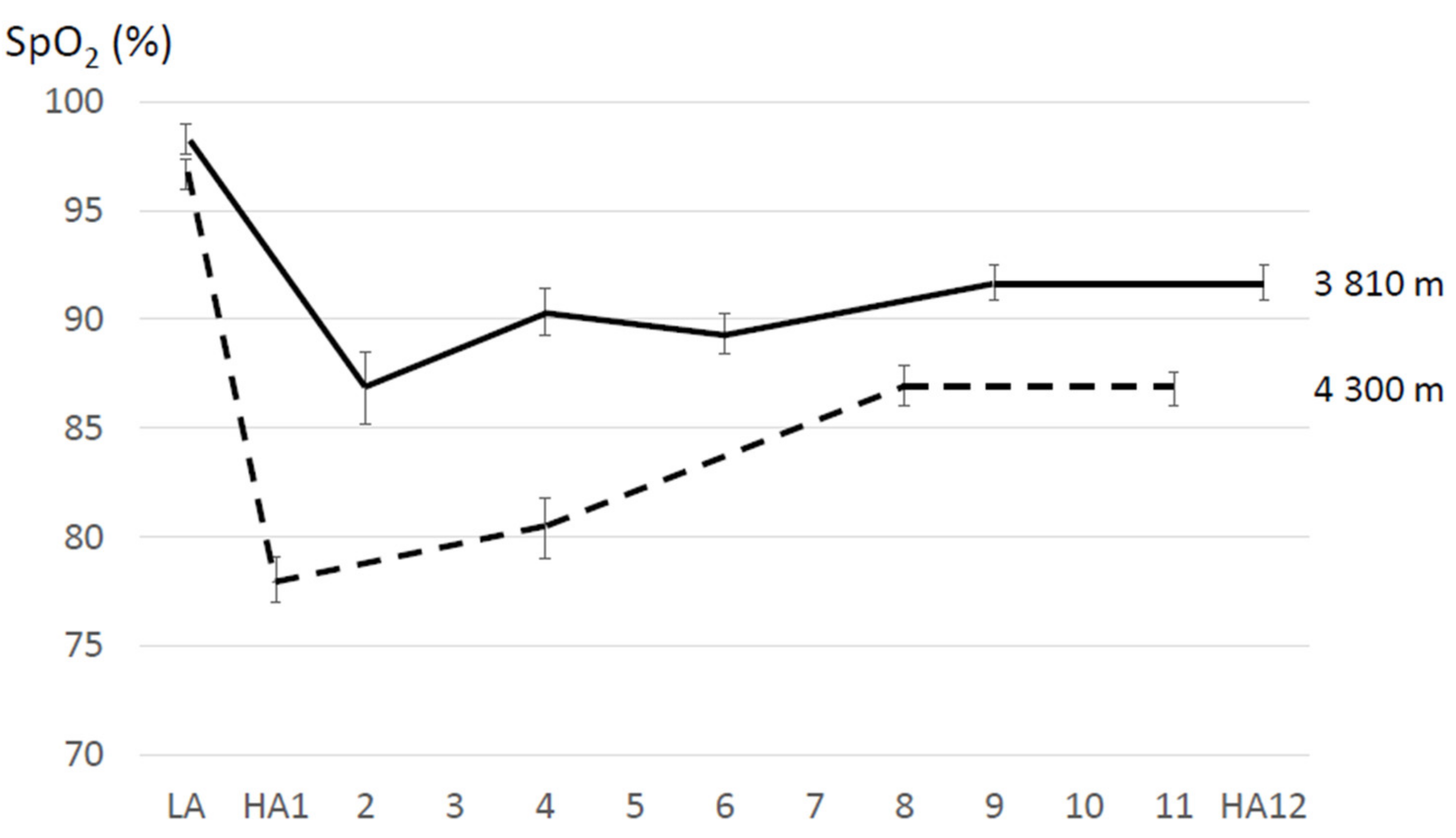
| Strapazzon et al. (2015) [61] | 19 (15 M, 4 F); 39 ± 9 years | 3830 m | 8 | SpO2 measured after signal stabilization; subjects at rest and with warm hands (SpO2: average of three consecutive measurements) | - | stay | Bl: 98.6 ± 1.4 9 h: 86.2 ± 5.8 d1: 87.1 ± 4.6 d2: 89.2 ± 3.9 d3: 91.5 ± 2.2 d8: 91.5 ± 3.1 | Bl: 62.1 ± 8.0 9 h: 86.9 ± 18.4 d1: 82.2 ± 11.1 d2: 79.3 ± 14.7 d3: 76.3 ± 14.7 d8: 79.0 ± 10.8 |
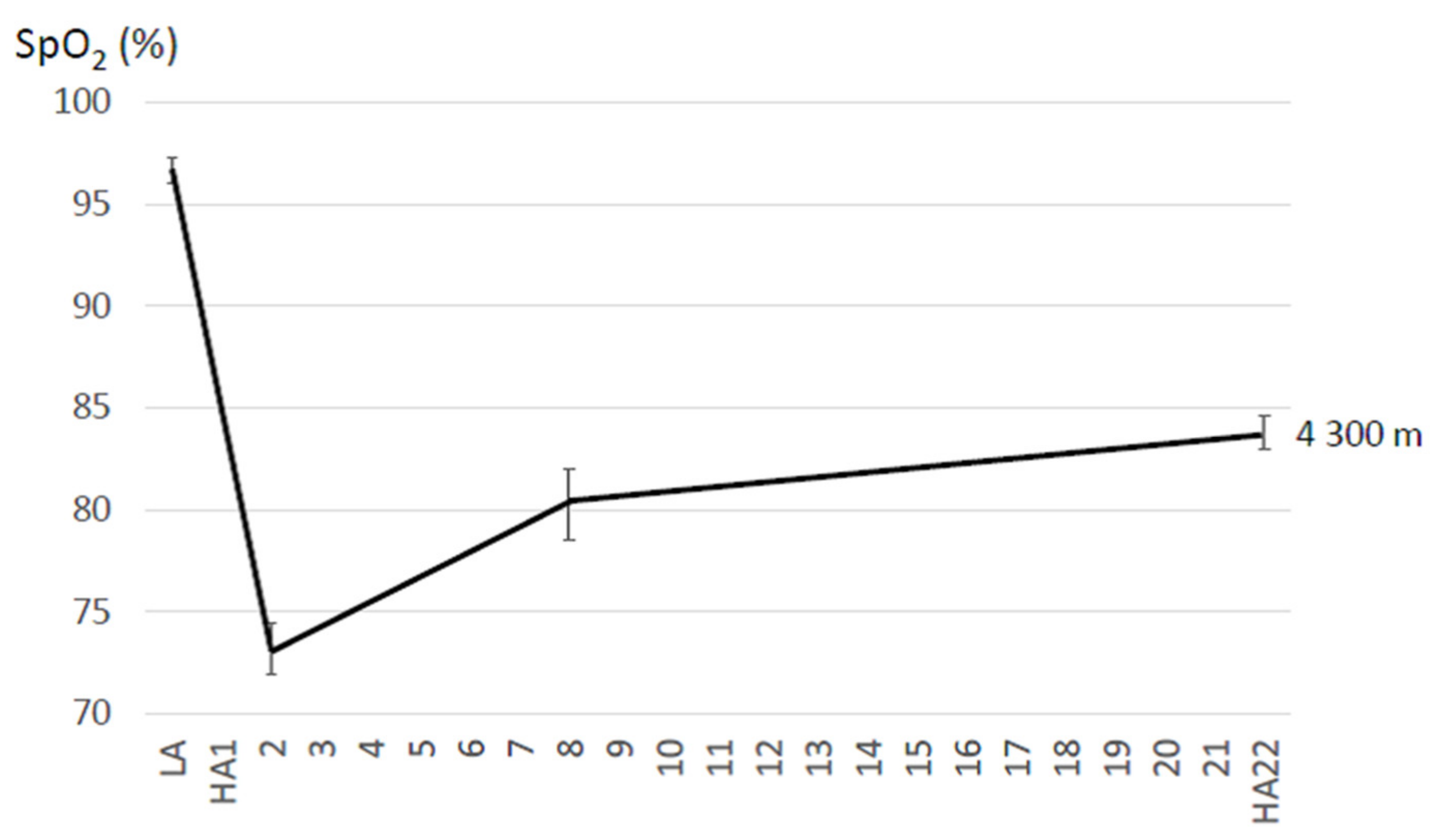
| Willie et al. (2014) [71] | 8 (M,F); 28 ± 6 years | 5050 m | 14 | SpO2 measured in triplicate, after 10 min rest in prewarmed sleeping bag (subjects: warm and calm before measurement) | 1week at 1338 m; 6–8 day trek from 2860 m–5050 m (incl. 1day at 3440 m, 1–3day at 4371 m) | stay | Bl: 99.0 ± 0.3 d2: 80.0 ± 0.9 d8: 82.0 ± 0.9 d14: 86.0 ± 0.7 | Bl: 58 ± 3 d2: 76 ± 4 d8: 75 ± 6 d14: 75 ± 5 |
| Bhaumik et al. (2013) [62] | 6 M; 24.8 ± 2.9 years | 3500 m | 5 | subjects rested quietly in supine position; ambient temperature varied between 10–20 °C | - | stay | Bl: 98.3 ± 0.2 d2: 92.8 ± 0.5 d5: 96.5 ± 0.2 | Bl: 67.0 ± 3.8 d2: 81.2 ± 4.1 d5: 75.7 ± 6.9 |
| Agostoni et al. (2011) [73] | 33 (22 M, 11 F); 40.8 ± 10.4 years | 5400 m | 14 | experiments were performed in a heated tent | 9 day ascent | stay | Bl: 97.6 ± 0.6 d1–2: 77.2 ± 6.0 d14–15: 85.3 ± 3.6 | Bl: 73 ± 13 d1–2: 82 ± 19 d14–15: 77 ± 18 |
| Fulco et al. (2011) [63] | 9 (8 M, 1 F); 25 ± 6 years # | 4300 m | 5 | subjects rested in a seating position for 30 min; temperature was maintained at 21 ± 3 °C | - | stay | Bl: 97 ± 1 d1/d2: 82 ± 4 d5: 85 ± 5 | |
| Modesti et al. (2011) [72] | 47 (32 M, 15 F); 40 ± 9 years | 5400 m | 9–11 | tests were carried out in a heated tent | 2 day hike from 3440 m to 4200 m; 1 day stay at 4200 m; 2 day hike to 5400 m | stay | Bl: 98 ± 1 d1: 78 ± 6 d9–11: 86 ± 4 | Bl: 61 ± 12 d1: 84 ± 16 d9–11: 78 ± 15 |
| Baillie et al. (2009) [74] | 42 (26 M, 16 F); 22.4 ± 6.3 years | 5200 m | 7 | n.a. | 4 day acclimatization at 3800 m | stay | Bl: 98 ± 1.3 d1: 77 ± 8 d3: 75 ± 5 d7: 77 ± 7 | |
| Beidleman et al. (2009) [57] | 11 M; 21 ± 3 years | 2200 m | 6 | testing was performed in a climatically controlled room (temperature: 22 ± 2.8 °C) | - | stay | Bl: 97 ± 2 d1: 94 ± 1 d3: 93 ± 2 d6: 94 ± 2 | Bl: 69 ± 6 d1: 68 ± 10 d2: 67 ± 10 d3: 66 ± 3 |
| Scrase et al. (2009) [64] | 9 (5 M, 4 F); 8 (6–13) years | 3500 m | 9 | n.a. | 4 day ascent 1300 m–3500 m | trekking (up to 3860 m) | Bl: 98.5 ± 0.9 d1: 88.9 ± 2.4 d9: 91.8 ± 1.5 | Bl: 78 ± 13 d1: 99 ± 14 d9: 98 ± 14 |
| Burtscher et al. (2001) [58] | 20 (10 M, 10 F); 63.7 + 7.4 years | 2000 m | 7 | 10 min rest in a sitting position before measurement; HR and SpO2 measured continuously for 3 min and averaged over 15 s intervals (mean of the intervals in the final minute was taken as rest value) | - | daily hiking; 2.5 h (day1)- 5 h (day6); 50% VO2max | Bl: 96 ± 2 d1 (PM): 89.7 * d2 (AM/PM): 91.3/91.5 * d3 (AM/PM): 93.2/91.7 * d4 (AM/PM): 93.6/92.5 * d5 (AM/PM): 93.3/92.5 * d6 (AM/PM): 93.6/92.7 * | Bl: 60 ± 7 d2: 68.5 * d3: 70.5 * d4: 69.8 * d5: 69.9 * d6: 67.8 * |
| Sato et al. (1994) [65] | 6 M | 3810 m | 12 | n.a. | - | stay | Bl: 98.6 ± 0.37 d2: 86.2 ± 2.3 d4: 90.3 ± 1.1 d6: 89.4 ± 0.9 d9: 91.9 ± 0.6 d12: 91.0 ± 0.6 (means ± SEM) | |
| Savourey et al. (1994) [66] | 7 (6 M, 1 W) | 4350 m | 7 | n.a. | - | during stay: 3 ascents to Mont Blanc (4807 m) | Bl: 98 * d1: 85.0 (SEM 0.5) d7: 86.0 (SEM 0.7) | |
| Reeves et al. (1993) [67] | 37 M | 4300 m | 19 | n.a. | - | stay | Bl: 97 * d1: 81.0 ± 0.9 d2: 83 * d3: 85 * d4: 85 * d5: 86 * d7: 87 * d10: 88 * d19: 87.9 ± 0.4 | |
| Bender et al. (1989) [68] | 6 M; 21 ± 1 (mean ± SEM) years | 4300 m | 22 | 4-min measurement period; subjects sat upright after relaxing for at least 20 min | - | stay | 97 * d1: 78.4 ± 1.6 d8: 87.5 ± 1.4 d20: 86.4 ± 0.6 |
| Authors | Participants M/F; Age (Mean ± SD or Median (Range) | Altitude | Exposure Time at Target Altitude (days) | Information about SpO2 Measurements | Type of Exercise Test | Change in Exercise SpO2 (%) (Means ± SD or Median (range)) d (Days), Bl (Baseline) | Change in Exercise HR (bpm) (Means ± SD or Median (Range)) |
|---|---|---|---|---|---|---|---|
| Bradbury et al. (2020) [78] | 6 M; 26.6 ± 8.5 years # | 4300 | 22 | n.a. | 80 min of metabolically-matched treadmill walking (2-mile time trial) | Bl: 95 ± 3 d1: 73 ± 4 d22: 81 ± 4 (data are means during 80 min time trial) | Bl: 175 ± 9 d1: 168 ± 14 d22: 161 ± 18 (data are means during 80 min time trial) |
| Gibson et al. (2015) [60] | 29 (15 M, 14 F); 22.2 ± 5.4 years | 3400 m | 9 | temperature [°C] (mean (95%CI)): Bl: 14.7 (14.1–15.4) d2: 24.2 (24.2–24.2) d6: 27.0 (27.0–27.0) d9: 21.9 (21.9–21.9) | 6MWT | Bl: Pre: 97.7 (96.6–98.7) Post: 98.0 (97.5–98.6) d2: Pre: 92.5 (91.6–93.3) Post: 83.5 (81.8–85.2) d6: Pre: 91.6 (90.8–92.4) Post: 86.7 (85.2–88.2) d9: Pre: 92.2 (91.4–93.0) Post: 85.4 (83.7–87.2) (Pre/post exercise test) | Bl: Pre: 82.0 (75.7–88.3) Post: 116.3 (103.4–129.2) d2: Pre: 100.5 (95.8–105.2) Post: 154.7 (147.2–162.1) d6: Pre: 85.1 (95.8–105.2) Post: 148.1 (138.5–157.7) D9: Pre: 80.5 (75.4–85.7) Post: 149.1 (143.0–155.3) (Pre/post exercise test) |
| Burtscher et al. (2014) [76] | 7 (4 M, 3 F); 44.7 ± 8.6 years # | 3480 m | 3 | SpO2 and HR were continuously monitored | 3 min step test (stepping 90 times up and down; 4 cm step) | Bl: 95.2 ± 1.5 d1: 74.9 ± 5.9 d2: 76.1 ± 4.1 d3: 74.4 ± 3.7 | Bl: 125 ± 12 HAd1: 144± 14 HAd2: 140 ± 12 HAd3: 140 ± 12 |
| Fulco et al. (2011) [63] | 9 (8 M, 1 F); 25 ± 6 years # | 4300 m | 5 | n.a. | 20 min steady -state exercise at 45± 5% of SL VO2peak. (speed: 5.6 m/h) | Bl: 97 ± 1 d1: 75 ± 4 d2: 75 ± 4 d5: 78 ± 4 | Bl: 129 ± 18 d1: 140 ± 15 d2: 138 ± 15 d5: 132 ± 12 |
| Burtscher et al. (2006) [77] | 5 M; 51.4 ± 7.7 years # | 2800 m | 3 | SpO2 was determined 5 times (minute 9, 19, 29, 39, 49); room temperature: ∼24 °C; | 50 min cycle ergometer test at individually chosen power output | Bl: 94.2 ± 0.8 d1: 79.2 ± 3.2 d3: 82.1 ± 2.1 | Bl: 167.6 ± 4.5 d1: 166.2 ± 5.1 d3: 164.2 ± 5.0 |
| Burtscher et al. (2001) [60] | 20 (10 M,10 F); 63.7 + 7.4 years | 2000 m | 7 | SpO2 and HR measured continuously and averaged over 15-sec intervals; means of the intervals of the final minute indicate exercise responses | Step test (step up and down on a 24 cm-high step, 90 times in 3 min) | Bl: 93.2 ± 2.0 d1: 84.9 ± 2.8 d4 (AM): 88.1 ± 2.1 | Bl: 124.3 ± 20.3 d1: 138.6 ± 19.2 d4 (AM): 124.7 ± 6.8 |
| Savourey et al. (1994) [66] | 7 (6 M, 1 F) | 4350 m | 7 | n.a. | moderate cycle ergometer exercise at a constant power (100 W) | Bl: 98 * d1: 79.0 (SEM 1.8) d7: 82.0 (SEM 1.3) | Bl: 115 * d1: 135 * d7: 130 * |
| Bender et al. (1989) [68] | 6 M; 21 ± 1 years | 4300 m | 22 | n.a. | submaximal cycle exercise | d2: 72.7 d8: 78.6 d22: 82.3 (means of measurements at min 5, 15 and 30) | d2: (5 min): 155 ± 3 d2: (15 min): 157± 5 d2: (30 min): 150± 3 d8: (5 min): 162 ± 2 d8: (15 min): 165 ± 4 d8: (30 min): 159 ± 4 d22: (5 min): 168 ± 2, d22: (15 min): 169 ± 4 d22: (30 min): 163 ± 4 |
| Authors | Participants M/F; Age (Men ± SD or Median (Range) | Altitude | Exposure Time at Target Altitude (days) | Information about SpO2 Measurements | Pre-Acclimatization/Prolonged Ascent Phase | Change in Resting SpO2 (%) (Means ± SD or Median (Range)) d (Days), h (Hours), Bl [Baseline] | Change in resting HR (bpm) (Means ± SD or Median (Range)) | AMS (Lake Louise Score) |
|---|---|---|---|---|---|---|---|---|
| Vizcardo-Galindo et al. (2020) [79] | 22 (21 M, 1 F); 32.7 ± 1.9 years | 4340 m | 4 | n.a. | - | Bl: 98 * 12 h: 77 * 24 h: 77 * 36 h: 76 * 48 h: 81 * 72 h: 81 * | Bl: 72 * 12 h:80 * 24 h: 86 * 36 h: 89 * 42 h: 85 * 72 h: 84 * | Bl: 0 * 12 h: 1.2 * 24 h: 2.7 * 36 h: 1.3 * 42 h: 1.3 * 72 h: 0.8 * |
| Sareban et al. (2020) [87] | 38 M; 19 endurance athletes: 31 ± 7 years 19 untrained: 38 ± 9 years | 3450 m | 2 | SpO2 measured after rest in supine position for 10 min (stable SpO2 values were reached) | - | athletes: Bl: 97 ± 1 3 h: 82 ± 6 8 h: 81 ± 6 24 h: 87 ± 3 34 h: 85 ± 4 48 h: 87 ± 4 untrained: Bl: 96 ± 1 3 h: 83 ± 4 8 h: 83 ±4 24 h: 85 ± 5 34 h: 84 ± 4 48 h: 86 ± 3 | athletes: Bl: 52 ± 9 3 h: 59 ± 8 8 h: 59 ± 11 24 h: 64 ± 8 34 h: 58 ± 9 48 h: 60 ± 8 untrained: Bl: 58 ±9 3 h: 72 ± 12 8 h: 68 ± 12 24 h: 76 ± 11 34 h: 69 ± 9 48 h: 73 ± 11 | athletes: Bl: 0.1 * 3 h: 1.7 * 8 h: 2.1 * 24 h: 1.7 * 34 h: 0.7 * 48 h: 0.7 * untrained: Bl: 0.2 * 3 h: 1.2 * 8 h: 0.9 * 24 h: 1.7 * 34 h: 1.0 * 48 h: 1.0 * |
| Gekeler at al. (2019) [80] | 14 (7 M, 7 F); 35 ± 8 years | 4559 m | 4 | SpO2 measured after >5 min at rest after 1 min of steady recording | ascent: 1635 m to 4559 m within 24 h | Bl: 98.6 ± 1.3 d1 PM: 69.4 ± 4.4 d2 AM: 72.1 ± 5.9 d2 PM: 74.4 ± 7.1 d3 AM: 73.9 ± 6.0 d3 PM: 79.9 ± 5.4 d4 AM: 79.4 ± 4.3 | Bl: 57.9 ± 7.0 d1 PM: 88.4 ± 6.0 d2 AM: 83.43 ± 10.1 d2 PM: 82.7 ± 9.5 d3 AM: 77.1 ± 12.2 d3 PM: 75.2 ± 16.6 d4 AM: 73.6 ± 13.4 | Bl: 0 d1 PM: 5.4 ± 2.2 d2 AM: 5.4 ± 2.6 d2 PM: 3.9 ± 2.1 d3 AM: 4.0 ± 3.4 d3 PM: 2.1 ± 1.5 d4 AM: 2.4 ± 2.1 |
| Lundeberg et al. (2018) [84] | 9 (4 M, 5 F); 32.7 ± 11.7 years # | 3800 m | 2.5 | recordings lasted ~300 s; pulse oximeter was always placed on the same finger | - | Bl: 97.5 * h0: 85.5 * h12: 87.5 * h36: 89.5 * h60: 88.0 * | Bl: 70 * h0: 82 * h12: 82 * h36: 85 * h60: 85 * | h0: 2.6 * h12: 5.3 * h36: 3.4 * h60: 3.5 * |
| Gibson et al. (2015) [60] | 29 (15 M, 14 F); 22.2 ± 5.4 years | 3400 m | 9 | temperature [°C] (mean (95%CI)): Bl: 14.7 (14.1–15.4) d2: 24.2 (24.2–24.2) d6: 27.0 (27.0–27.0) d9: 21.9 (21.9–21.9) | - | Bl: 97.7 (96.6–98.7) d2: 92.5 (91.6–93.3) d6: 91.6 (90.8–92.4) d9: 92.2 (91.4–93.0) | Bl: 82.0 (75.7–88.3) d2: 100.5 (95.8–105.2) d6: 85.1 (95.8–105.2) d9: 80.5 (75.4–85.7) | Bl: 0.8 (0.4–1.1) d2: 2.0 (1.1–2.9) d6: 1.0 (0.3–1.6) d9: 1.0 (0.4–1.6) |
| Strapazzon et al. (2015) [61] | 19 (15 M, 4 F); 39 ± 9 years | 3830 m | 8 | SpO2 measured after signal stabilization, subject at rest and with warm hands (SpO2: average of three consecutive measurements) | - | Bl: 98.6 ± 1.4 9 h: 86.2 ± 5.8 d1: 87.1 ± 4.6 d2: 89.2 ± 3.9 d3: 91.5 ± 2.2 d8: 91.5 ± 3.1 | Bl: 62.1 ± 8.0 9 h: 86.9 ± 18.4 d1: 82.2 ± 11.1 d2: 79.3 ± 14.7 d3: 76.3 ± 14.7 d8: 79.0 ± 10.8 | Bl: 0.0 (0.0) 9 h: 0.6 (0.7) 24 h: 1.7 (2.7) 48 h: 0.5 (0.7) 72 h: 0.4 (0.5) d8: 0.1 (0.4) |
| Staab et al. (2013) [86] | 18 M; 25 ± 5 years, | 4300 m | 3 | data collection of at least 10 min; mean over the last 5–8 min of the session was calculated and used in the analyses; room temperature: 21 ± 2 °C | - | Bl: 99 ± 1 24 h: 81± 5 48 h: 83 ± 6 72 h: 83 ± 5 | Bl: 52 ± 7 24 h: 75 ± 9 48 h: 75± 12 72 h: 74 ± 10 | AMS-C: Bl: 0.2 * 24 h: 1.4 * 48 h: 1.4 * 72 h: 0.7* |
| Aeberli et al. (2012) [81] | 25 (15 M, 10 F); 43.8 ± 9.5 years | 4559 m | 4 | n.a. | 1 night at 3650 m | Bl: 97.4 ± 1.5 d2: 78.4 ± 6.0 d4: 81.6 ± 8.6 | Bl: 0.9 ± 1.0 d2: 3.4 ± 1.4 d4: 2.3 ± 1.3 | |
| Nussbaumer-Ochsner et al. (2012) [82] | 16 (13 M, 3 F); 45 (33–50) years | 4559 m | 3 nights (n) | SpO2: mean value during sleep | - | Bl: 96 (95,96) n1: 67 (64,69) n3: 71 (69,78) (medians and quartiles) | Bl: 56 (50,61) n1: 81 (74,92) n3: 84 (75,89) | Bl: 1 (0,1) n1: 6 (3,9) n3: 4 (3,5) |
| Modesti et al. (2011) [72] | 47 (32 M, 15 F) 40 ± 9 years | 5400 m | 9–11 | tests were carried out in a heated tent | 2 day hike from 3440 m to 4200 m; 1 day stay at 4200 m; 2 day hike to 5400 m | Bl: 98 ± 1 d1: 78 ± 6 d9–11: 86 ± 4 | Bl: 61 ± 12 d1: 84 ± 16 d9–11: 78 ± 15 | Bl: 0 d1: 2.7 ± 2.0 d9–11: 0.6 ± 1.0 |
| Willmann et al. (2011) [83] | 18 (11 M, 7 F); 35 ± 8 years | 3647 m | 4 | SpO2 measurement performed after >5 min of rest | ascent within 24 h from 1635 m to 4559 m | Bl: 98.5 ± 1.3 d1 PM: 70.6 ± 5.2 d2 AM: 73.0 ± 6.0 d2 PM: 74.3 ± 6.6 d3 AM: 74.2v5.6 d3 PM: 77.3 ± 3.8 d4 AM: 79.6 ± 5.2 | Bl: 60.3 ± 7.2 d1 PM: 89.4 ± 5.9 d2 AM: 82.7 ± 9.7 d2 PM: 80.1 ± 11.7 d3 AM: 76.8 ± 11.4 d3 PM: 75.3 ± 13.2 d4 AM:72.2 ± 12.7 | Bl: 0 d1 PM: 5.7 ± 3.1 d2 AM: 5.2 ± 2.6 d2 PM: 3.8 ± 2.0 d3 AM: 4.1 ± 3.0 d3 PM: 2.1 ± 1.4 d4 AM: 2.3 ± 1.9 |
| Baillie et al. (2009) [74] | 42 (26 M, 16 F); 22.4 ± 6.3 years | 5200 m | 7 | n.a. | 4 day acclimatization at 3800 m | Bl: 98 ± 1 d1: 77 ± 8 d3: 75 ± 5 d7: 77 ± 7 | d1: 4 (2–5) d2: 4 (2–6) d3: 3 (1–5) d7: 1 (0–2) median (IQR) | |
| Chen et al. (2008) [85] | 27 (11 M, 16 F); 39 ± 12 years | 3180 m | 2 nights (n) | measurements performed with the subjects in a supine position after resting for 10 min | - | AMS+ (N = 13) Bl: 97.7 ± 0.8 4–6 h: 85.5 ± 3.2 n2: 87.3 ± 2.1 Non-AMS: Bl: 97.4 ± 1.1 4–6 h: 85.5 ± 4.3 n2: 87.1 ± 4.4 | AMS +: Bl: 63.5 ± 6.5 4–6 h: 77.7 ± 7.8 n2: 79.1 ± 11.3 Non-AMS: Bl: 64.5 ± 8.2 4–6 h: 75.0 ± 9.1 n2: 80.1 ± 9.3 | AMS +: Bl: 0.0 ± 0.0 4–6 h: 3.9 ± 2.4 n2: 2.9± 2.7 Non-AMS: Bl: 0.0 ± 0.0 4–6 h: 0.6 ± 0.9 n2: 0.0 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dünnwald, T.; Kienast, R.; Niederseer, D.; Burtscher, M. The Use of Pulse Oximetry in the Assessment of Acclimatization to High Altitude. Sensors 2021, 21, 1263. https://doi.org/10.3390/s21041263
Dünnwald T, Kienast R, Niederseer D, Burtscher M. The Use of Pulse Oximetry in the Assessment of Acclimatization to High Altitude. Sensors. 2021; 21(4):1263. https://doi.org/10.3390/s21041263
Chicago/Turabian StyleDünnwald, Tobias, Roland Kienast, David Niederseer, and Martin Burtscher. 2021. "The Use of Pulse Oximetry in the Assessment of Acclimatization to High Altitude" Sensors 21, no. 4: 1263. https://doi.org/10.3390/s21041263
APA StyleDünnwald, T., Kienast, R., Niederseer, D., & Burtscher, M. (2021). The Use of Pulse Oximetry in the Assessment of Acclimatization to High Altitude. Sensors, 21(4), 1263. https://doi.org/10.3390/s21041263








