Deskilled and Rapid Drug-Resistant Gene Detection by Centrifugal Force-Assisted Thermal Convection PCR Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Chip Fabrication
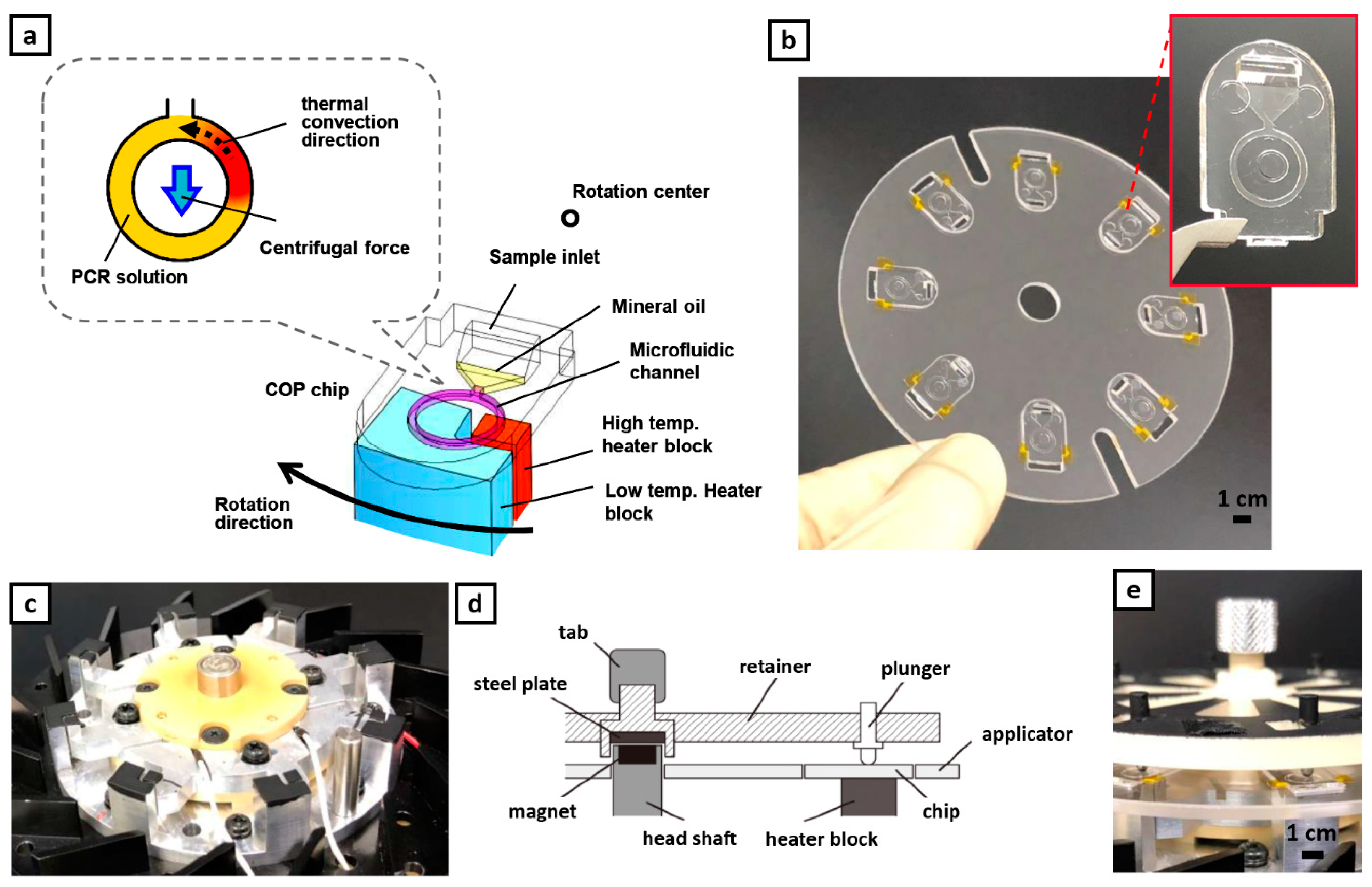
2.2. Device Design and Plain Chip
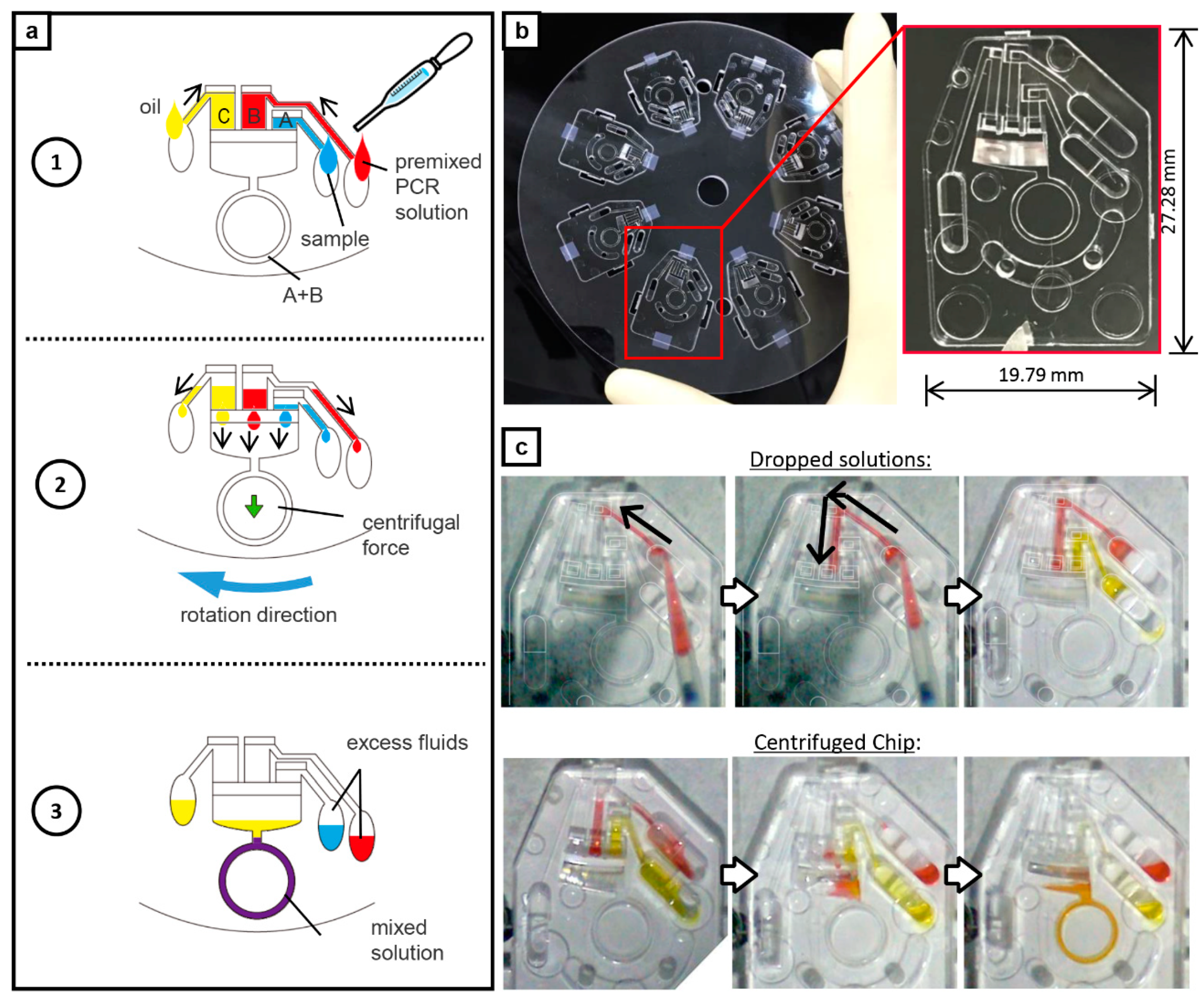
2.3. POCT-Oriented Chip
2.4. Sample Preparation
2.4.1. Isolates and DNA Extraction
2.4.2. Clinical Specimens
2.4.3. DNA Extraction from Clinical Fecal Samples
2.5. On-Chip PCR Assay
2.6. On-Chip PCR Assay with Liquid Delivery Function
2.7. Measurement of Fluorescence Intensity on the PCR Chip
2.8. Gel Shift Assay
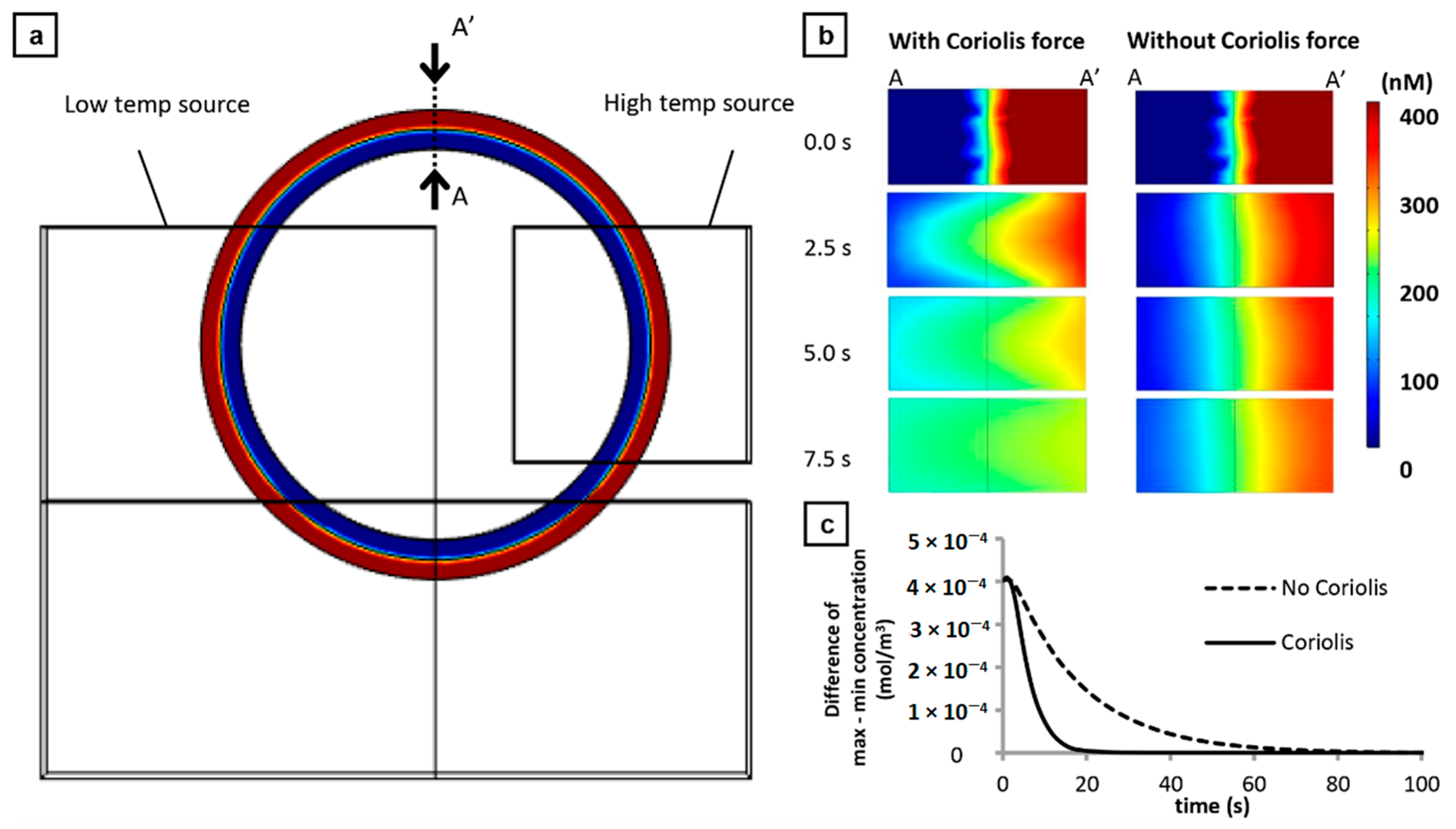
2.9. Simulation Analysis of Centrifugal Thermal Convection
3. Results and Discussion
3.1. Contribution of Coriolis Force to Improved Mixing Efficiency
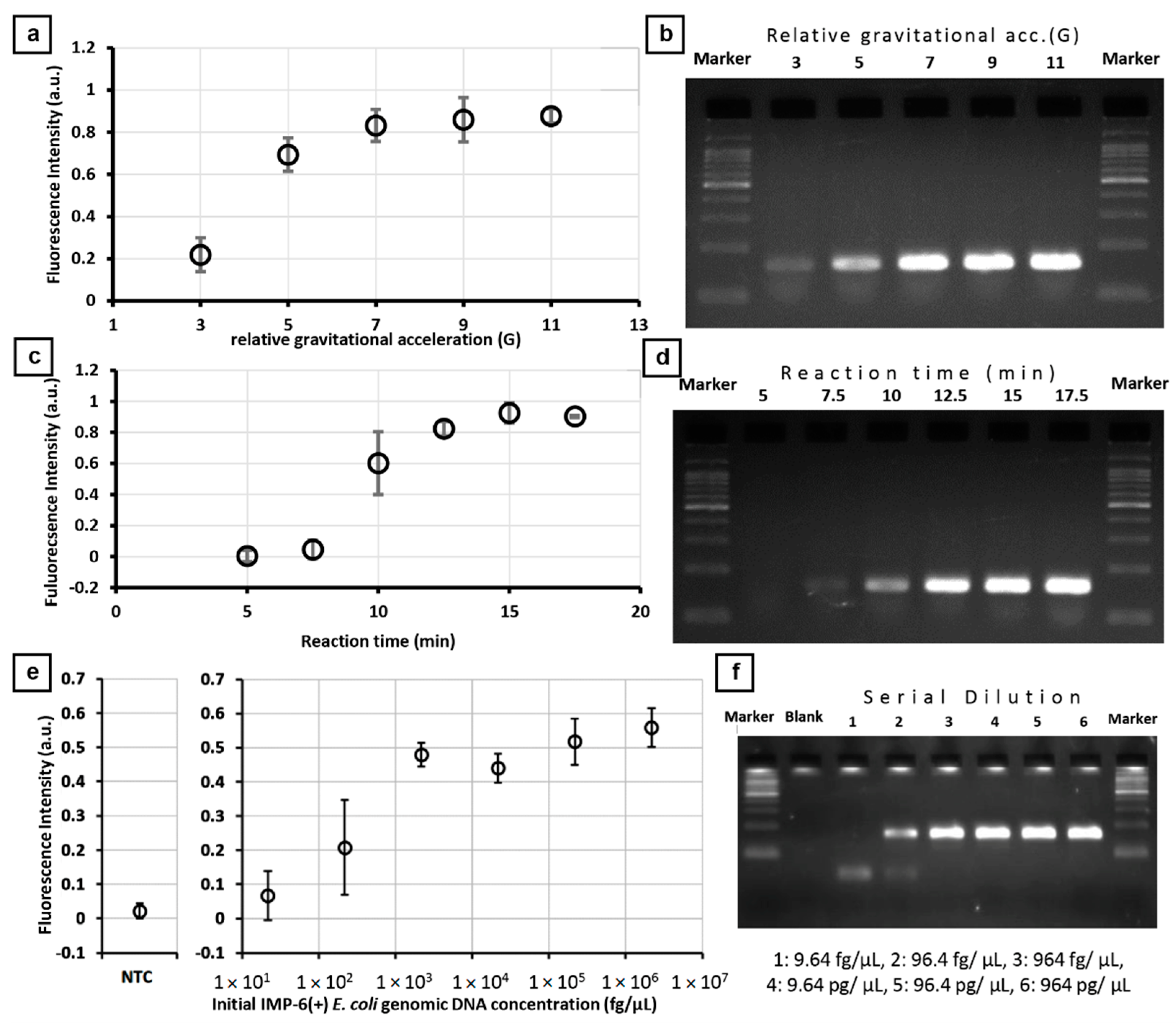
3.2. On-Chip PCR for blaIMP-6 Gene
3.3. Agreement of On-Chip PCR Test Results with Conventional Culture Test Results
3.4. Demonstration of POCT Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pehrsson, E.C.; Tsukayama, P.; Patel, S.; Mejía-Bautista, M.; Sosa-Soto, G.; Navarrete, K.M.; Calderon, M.; Cabrera, L.; Hoyos-Arango, W.; Bertoli, M.T.; et al. Interconnected microbiomes and resistomes in low-income human habitats. Nature 2016, 533, 212–216. [Google Scholar] [CrossRef]
- Centers for Disease Contorl and Prevention (CDC). Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Carmeli, Y.; Akova, M.; Cornaglia, G.; Daikos, G.L.; Garau, J.; Harbarth, S.; Rossolini, G.M.; Souli, M.; Giamarellou, H. Controlling the spread of carbapenemase-producing Gram-negatives: Therapeutic approach and infection control. Clin. Microbiol. Infect. 2010, 16, 102–111. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L. Strategies for identification of carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2013, 68, 487–489. [Google Scholar] [CrossRef]
- Vrioni, G.; Daniil, I.; Voulgari, E.; Ranellou, K.; Koumaki, V.; Ghirardi, S.; Kimouli, M.; Zambardi, G.; Tsakris, A. Comparative evaluation of a prototype chromogenic medium (ChromID CARBA) for detecting carbapenemase-producing Enterobacteriaceae in surveillance rectal swabs. J. Clin. Microbiol. 2012, 50, 1841–1846. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kawahara, R.; Akeda, Y.; Shanmugakani, R.K.; Yoshida, H.; Hagiya, H.; Hara, N.; Nishi, I.; Yukawa, S.; Asada, R.; et al. Development of selective medium for IMP-type carbapenemase-producing Enterobacteriaceae in stool specimens. BMC Infect. Dis. 2017, 17, 229. [Google Scholar] [CrossRef]
- Cuzon, G.; Ouanich, J.; Gondret, R.; Naas, T.; Nordmann, P. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrob. Agents Chemother. 2011, 55, 2420–2423. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Poirel, L.; Carrër, A.; Toleman, M.A.; Walsh, T.R. How to detect NDM-1 producers. J. Clin. Microbiol. 2011, 49, 718–721. [Google Scholar] [CrossRef]
- Yano, H.; Ogawa, M.; Endo, S.; Kakuta, R.; Kanamori, H.; Inomata, S.; Ishibashi, N.; Aoyagi, T.; Hatta, M.; Gu, Y.; et al. High frequency of IMP-6 among clinical isolates of metallo-β-lactamase-producing Escherichia coli in Japan. Antimicrob. Agents Chemother. 2012, 56, 4554–4555. [Google Scholar] [CrossRef]
- Codjoe, F.; Donkor, E. Carbapenem Resistance: A Review. Med. Sci. 2017, 6, 1. [Google Scholar] [CrossRef]
- Goldstein, F.W.; Labigne-Roljssel, A.; Gerbaud, G.; Carlier, C.; Collatz, E.; Courvalin, P. Transferable Plasmid-Mediated Antibiotic Resistance in Acinetobacter. Plasmid 1983, 10, 138–147. [Google Scholar] [CrossRef]
- Poyart-Salmeron, C.; Carlier, C.; Trieu-Cuot, P.; Courvalin, P.; Courtieu, A. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet 1990, 335, 1422–1426. [Google Scholar] [CrossRef]
- Chua, A.C.; Cunningham, J.; Moussy, F.; Perkins, M.D.; Formenty, P. The case for improved diagnostic tools to control ebola virus disease in West Africa and how to get there. PLoS Negl. Trop. Dis. 2015, 9, 4–9. [Google Scholar] [CrossRef]
- Ward, K.; Fan, Z.H. Mixing in microfluidic devices and enhancement methods. J. Micromech. Microeng. 2015, 25, 094001. [Google Scholar] [CrossRef]
- Steigert, J.; Grumann, M.; Brenner, T.; Riegger, L.; Harter, J.; Zengerle, R.; Ducrée, J. Fully integrated whole blood testing by real-time absorption measurement on a centrifugal platform. Lab Chip 2006, 6, 1040–1044. [Google Scholar] [CrossRef]
- Sudarsan, A.P.; Ugaz, V.M. Multivortex micromixing. Proc. Natl. Acad. Sci. USA 2006, 103, 7228–7233. [Google Scholar] [CrossRef]
- Lee, N.Y. A review on microscale polymerase chain reaction based methods in molecular diagnosis, and future prospects for the fabrication of fully integrated portable biomedical devices. Microchim. Acta 2018, 185, 285. [Google Scholar] [CrossRef]
- Espulgar, W.; Tadokoro, T.; Tamiya, E.; Saito, M. Utility of Centrifugation-Controlled Convective (C3) Flow for Rapid On-chip ELISA. Sci. Rep. 2019, 9, 20150. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Takahashi, K.; Kiriyama, Y.; Espulgar, W.V.; Aso, H.; Sekiya, T.; Tanaka, Y.; Sawazumi, T.; Furui, S.; Tamiya, E. Centrifugation-Controlled Thermal Convection and Its Application to Rapid Microfluidic Polymerase Chain Reaction Devices. Anal. Chem. 2017, 89, 12797–12804. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.L.; McCuiston, A.; Mittendorf, I.; Ottway, R.; Johnson, R.D. Behavior of capillary valves in centrifugal microfluidic devices prepared by three-dimensional printing. Microfluid. Nanofluidics 2011, 10, 877–888. [Google Scholar] [CrossRef]
- Andersson, P.; Jesson, G.; Kylberg, G.; Ekstrand, G.; Thorsén, G. Parallel nanoliter microfluidic analysis system. Anal. Chem. 2007, 79, 4022–4030. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Weber, P.; Lutz, S.; Focke, M.; Zengerle, R.; Von Stetten, F. Aliquoting on the centrifugal microfluidic platform based on centrifugo-pneumatic valves. Microfluid. Nanofluid. 2011, 10, 1279–1288. [Google Scholar] [CrossRef]
- Stumpf, F.; Schwemmer, F.; Hutzenlaub, T.; Baumann, D.; Strohmeier, O.; Dingemanns, G.; Simons, G.; Sager, C.; Plobner, L.; Von Stetten, F.; et al. LabDisk with complete reagent prestorage for sample-to-answer nucleic acid based detection of respiratory pathogens verified with influenza A H3N2 virus. Lab Chip 2016, 16, 199–207. [Google Scholar] [CrossRef]
- Yamamoto, N.; Asada, R.; Kawahara, R.; Hagiya, H.; Akeda, Y.; Shanmugakani, R.K.; Yoshida, H.; Yukawa, S.; Yamamoto, K.; Takayama, Y.; et al. Prevalence of, and risk factors for, carriage of carbapenem-resistant Enterobacteriaceae among hospitalized patients in Japan. J. Hosp. Infect. 2017, 97, 212–217. [Google Scholar] [CrossRef]
- Swayne, R.; Ellington, M.J.; Curran, M.D.; Woodford, N.; Aliyu, S.H. Utility of a novel multiplex TaqMan PCR assay for metallo-β-lactamase genes plus other TaqMan assays in detecting genes encoding serine carbapenemases and clinically significant extended-spectrum β-lactamases. Int. J. Antimicrob. Agents 2013, 42, 352–356. [Google Scholar] [CrossRef]
- Eswaran, J.; Koronakis, E.; Higgins, M.K.; Hughes, C.; Koronakis, V. Three’s company: Component structures bring a closer view of tripartite drug efflux pumps. Curr. Opin. Struct. Biol. 2004, 14, 741–747. [Google Scholar] [CrossRef]
- Poole, K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005, 56, 20–51. [Google Scholar] [CrossRef]
- Zwama, M.; Yamasaki, S.; Nakashima, R.; Sakurai, K.; Nishino, K.; Yamaguchi, A. Multiple entry pathways within the efflux transporter AcrB contribute to multidrug recognition. Nat. Commun. 2018, 9, 124. [Google Scholar] [CrossRef]
- Nishino, K.; Yamaguchi, A. Analysis of a Complete Library of Putative Drug Transporter Genes in Escherichia coli. J. Bacteriol. 2001, 183, 5803–5812. [Google Scholar] [CrossRef]
- Nordmann, P.; Dortet, L.; Poirel, L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends Mol. Med. 2012, 18, 263–272. [Google Scholar] [CrossRef]
- Warner, D.M.; Yang, Q.; Duval, V.; Chen, M.; Xu, Y.; Levy, S.B. Involvement of MarR and YedS in Carbapenem Resistance in a Clinical Isolate of Escherichia coli from China. Antimicrob. Agents Chemother. 2013, 57, 1935–1937. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espulgar, W.V.; Saito, M.; Takahashi, K.; Ushiro, S.; Yamamoto, N.; Akeda, Y.; Hamaguchi, S.; Tomono, K.; Tamiya, E. Deskilled and Rapid Drug-Resistant Gene Detection by Centrifugal Force-Assisted Thermal Convection PCR Device. Sensors 2021, 21, 1225. https://doi.org/10.3390/s21041225
Espulgar WV, Saito M, Takahashi K, Ushiro S, Yamamoto N, Akeda Y, Hamaguchi S, Tomono K, Tamiya E. Deskilled and Rapid Drug-Resistant Gene Detection by Centrifugal Force-Assisted Thermal Convection PCR Device. Sensors. 2021; 21(4):1225. https://doi.org/10.3390/s21041225
Chicago/Turabian StyleEspulgar, Wilfred Villariza, Masato Saito, Kazuya Takahashi, Sakiko Ushiro, Norihisa Yamamoto, Yukihiro Akeda, Shigeto Hamaguchi, Kazunori Tomono, and Eiichi Tamiya. 2021. "Deskilled and Rapid Drug-Resistant Gene Detection by Centrifugal Force-Assisted Thermal Convection PCR Device" Sensors 21, no. 4: 1225. https://doi.org/10.3390/s21041225
APA StyleEspulgar, W. V., Saito, M., Takahashi, K., Ushiro, S., Yamamoto, N., Akeda, Y., Hamaguchi, S., Tomono, K., & Tamiya, E. (2021). Deskilled and Rapid Drug-Resistant Gene Detection by Centrifugal Force-Assisted Thermal Convection PCR Device. Sensors, 21(4), 1225. https://doi.org/10.3390/s21041225




