Calculation of Stopping-Power Ratio from Multiple CT Numbers Using Photon-Counting CT System: Two- and Three-Parameter-Fitting Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Effective Atomic Number and Electron Density from the Linear Attenuation Coefficient
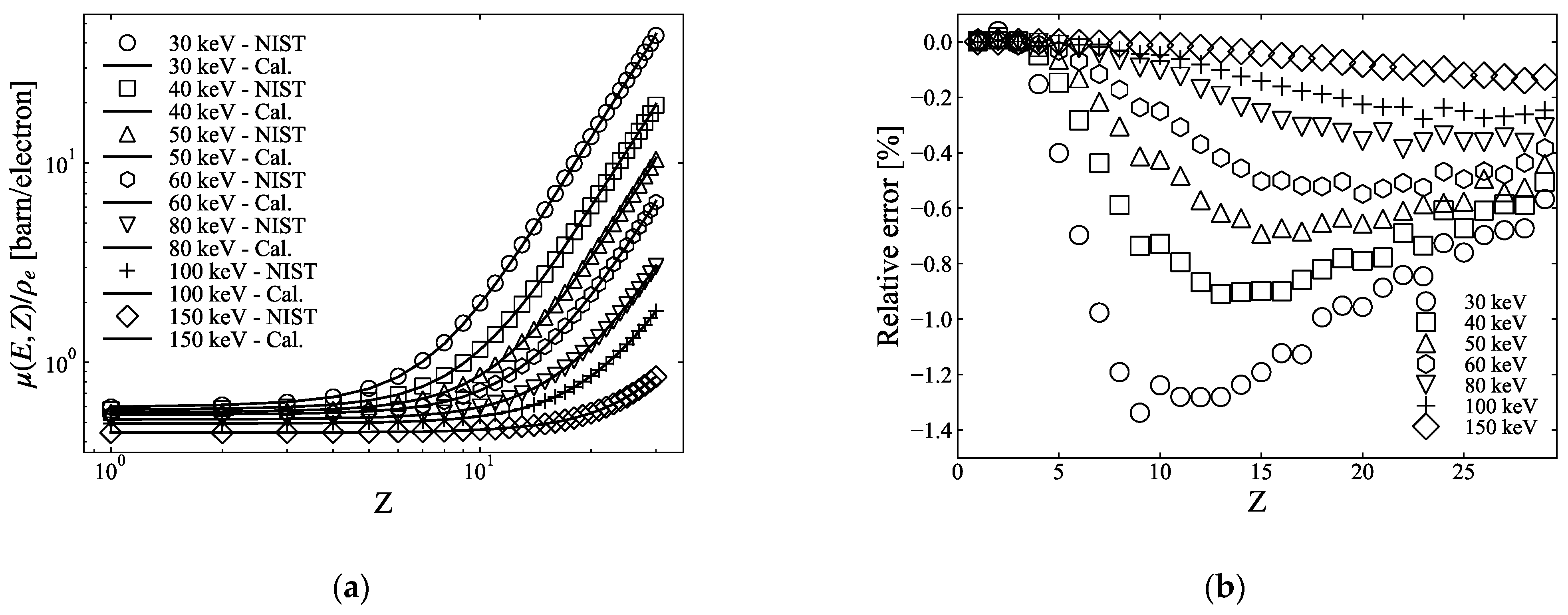
2.1.1. Two-Parameter-Fitting Method
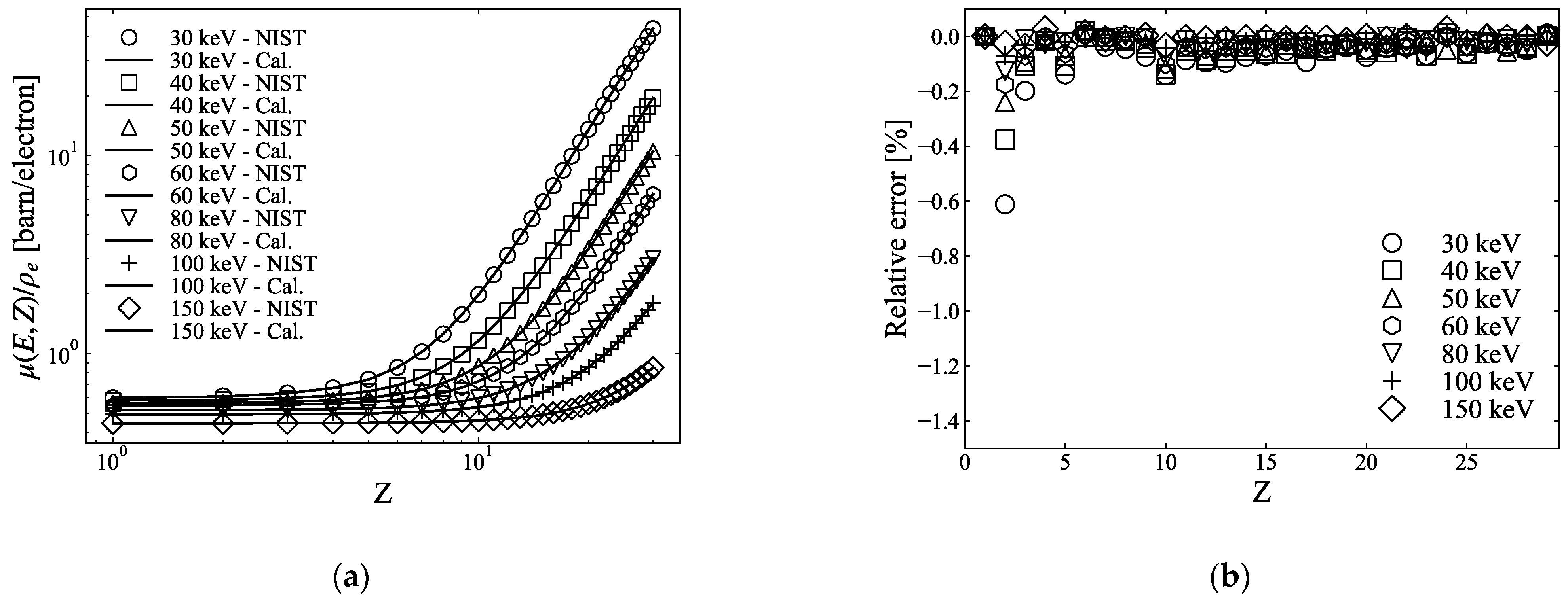
2.1.2. Three-Parameter-Fitting Method
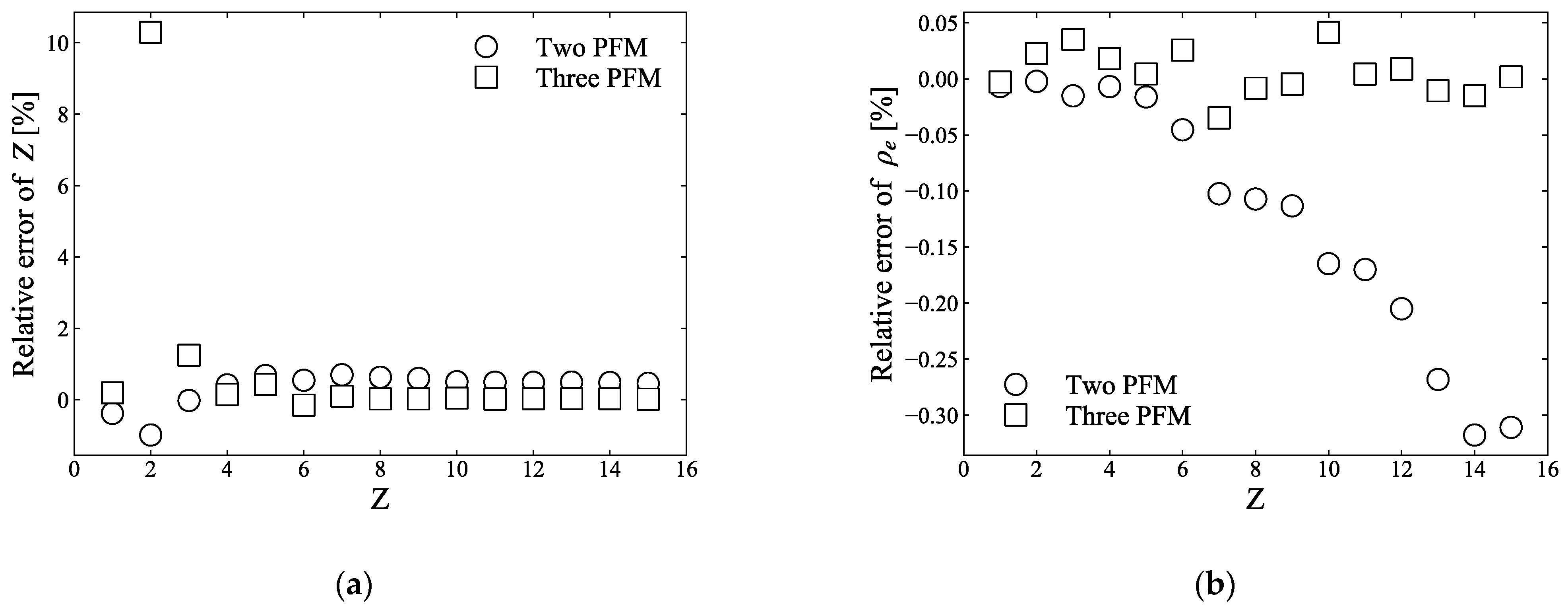
2.1.3. Errors of the Two- and Three-PFM
2.2. Stopping-Power Ratio from I-Value Parameterisation
2.3. Photon-Counting CT System
2.4. Experimental Procedures
2.4.1. Photon-Counting CT Measurement
2.4.2. Spectrum Measurement
2.4.3. Semi-Empirical Correction Method for CT Values
2.4.4. SPR Calculation Using Two-PFM and Three-PFM
2.4.5. Theoretical Values of Effective Atomic Number, Electron Density, I-Value, and SPR
2.5. Random Noise Effect for Theoretical Linear Attenuation Coefficient
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Rutherford, R.A.; Pullan, B.R.; Isherwood, I. Measurement of effective atomic number and electron density using an EMI scanner. Neuroradiology 1976, 11, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.F.; Hawkes, D.J. X-ray attenuation coefficients of elements and mixtures. Phys. Rep. 1981, 70, 169–233. [Google Scholar] [CrossRef]
- Yang, M.; Virshup, G.; Clayton, J.; Zhu, R.X.; Mohan, R.; Dong, L. Theoretical variance analysis of single- and double-energy computed tomography methods for measuring proton stopping power ratios of biological tissues. Phys. Med. Biol. 2010, 55, 1343–1362. [Google Scholar] [CrossRef] [PubMed]
- Bourque, A.E.; Carrier, J.; Bouchard, H. A stoichiometric calibration method for dual energy computed tomography. Phys. Med. Biol. 2014, 59, 2059. [Google Scholar] [CrossRef]
- Shikhaliev, P.M. Beam hardening artefacts in computed tomography with photon counting, charge integrating and energy weighting detectors: A simulation study. Phys. Med. Biol. 2005, 50, 5813–5827. [Google Scholar] [CrossRef]
- Boone, J.M.; Seibert, J.A. An accurate method for computer-generating tungsten anode x-ray spectra from 30 to 140 kV. Med. Phys. 1997, 24, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Hunter, N.; Swain, M.V. Application of polychromatic microCT for mineral density determination. J. Dent. Res. 2011, 90, 18–30. [Google Scholar] [CrossRef]
- Schaffner, B.; Pedroni, E. The precision of proton range calculations in proton radiotherapy treatment planning: Experimental verification of the relation between CT-HU and proton stopping power. Phys. Med. Biol. 1998, 43, 1579–1592. [Google Scholar] [CrossRef]
- Bazalova, M.; Carrier, J.-F.; Beaulieu, L.; Verhaegen, F. Dual-energy CT-based material extraction for tissue segmentation in Monte Carlo dose calculations. Phys. Med. Biol. 2008, 53, 2439–2456. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Ueguchi, T.; Tomiyama, N.; Yoshioka, Y.; Ogawa, K.; Koizumi, M.; Ogata, T.; Yamada, S.; Takahashi, Y.; Sumida, I.; et al. Gemstone spectral imaging: Determination of CT to ED conversion curves for radiotherapy treatment planning. J. Appl. Clin. Med. Phys. 2013, 14, 173–186. [Google Scholar] [CrossRef]
- Wang, X.; Meier, D.; Mikkelsen, S.; Maehlum, G.E.; Wagenaar, D.J.; Tsui, B.M.W.; Patt, B.E.; Frey, E.C. MicroCT with energy-resolved photon-counting detectors. Phys. Med. Biol. 2011, 56, 2791–2816. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.G. Optimal “image-based” weighting for energy-resolved CT. Med. Phys. 2009, 36, 3018–3027. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Frey, E.C.; Wang, X.; Iwanczyk, J.S.; Barber, W.C. An analytical model of the effects of pulse pileup on the energy spectrum recorded by energy resolved photon counting X-ray detectors. Med. Phys. 2010, 37, 3957–3969. [Google Scholar] [CrossRef]
- Shikhaliev, P.M.; Fritz, S.G. Photon counting spectral CT versus conventional CT: Comparative evaluation for breast imaging application. Phys. Med. Biol. 2011, 56, 1905–1930. [Google Scholar] [CrossRef] [PubMed]
- Feuerlein, S.; Roessl, E.; Proksa, R.; Martens, G.; Klass, O.; Jeltsch, M.; Rasche, V.; Brambs, H.-J.; Hoffmann, M.H.K.; Schlomka, J.-P. Multienergy Photon-counting K-edge Imaging: Potential for Improved Luminal Depiction in Vascular Imaging. Radiology 2008, 249, 1010–1016. [Google Scholar] [CrossRef]
- McCollough, C.; Leng, S.; Yu, L.; Fletcher, J.G. Dual-and multi-energy computed tomography: Principles, technical approaches, and clinical applications. Radiology 2015, 276, 637. [Google Scholar] [CrossRef]
- Simard, M.; Panta, R.K.; Bell, S.T.; Butler, A.P.; Bouchard, H. Quantitative imaging performance of MARS spectral photon-counting CT for radiotherapy. Med. Phys. 2020, 47, 3423–3434. [Google Scholar] [CrossRef]
- Kanematsu, N.; Matsufuji, N.; Kohno, R.; Minohara, S.; Kanai, T. A CT calibration method based on the polybinary tissue model for radiotherapy treatment planning. Phys. Med. Biol. 2003, 48, 1053–1064. [Google Scholar] [CrossRef]
- Torikoshi, M.; Tsunoo, T.; Sasaki, M.; Endo, M.; Noda, Y.; Ohno, Y.; Kohno, T.; Hyodo, K.; Uesugi, K.; Yagi, N. Electron density measurement with dual-energy x-ray CT using synchrotron radiation. Phys. Med. Biol. 2003, 48, 673–685. [Google Scholar] [CrossRef]
- Ohno, Y.; Torikoshi, M.; Tsunoo, T.; Hyodo, K. Dual-energy X-ray CT with CdTe array and its extension. Nucl. Instrum. Methods Phys. Res. Sect. A 2005, 548, 72–77. [Google Scholar] [CrossRef]
- Berger, M.J.; Hubbell, J.H.; Seltzer, S.M.; Chang, J.; Coursey, J.S.; Sukumar, R.; Zucker, D.S. XCOM: Photon cross Section Database. NBSIR 2005. [Google Scholar] [CrossRef]
- Johns, H.E.; Cunningham, J.R. The Physics of Radiology, 4th ed.; Charles C Thomas: Springfield, IL, USA, 1983; pp. 241–243. [Google Scholar]
- Hünemohr, N.; Krauss, B.; Tremmel, C.; Ackermann, B.; Jäkel, O.; Greilich, S. Experimental verification of ion stopping power prediction from dual energy CT data in tissue surrogates. Phys. Med. Biol. 2014, 59, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Landry, G.; Seco, J.; Gaudreault, M.; Verhaegen, F. Deriving effective atomic numbers from DECT based on a parameterization of the ratio of high and low linear attenuation coefficients. Phys. Med. Biol. 2013, 58, 6851–6866. [Google Scholar] [CrossRef] [PubMed]
- Bichsel, H. Passage of Charged Particles through Matter. In American Institute of Physics Handbook, 3rd ed.; Gray, D.E., Ed.; McGraw-Hill: New York, NY, USA, 1972; p. 142. [Google Scholar]
- Schneider, U.; Pedroni, E.; Lomax, A. The calibration of CT Hounsfield units for radiotherapy treatment planning. Phys. Med. Biol. 1996, 41, 111–124. [Google Scholar] [CrossRef]
- Bragg, W.; Kleeman, R. On the α particles of radium, and their loss of range in passing through various atoms and molecules. Philos. Mag. J. Sci. 1905, 10, 318–340. [Google Scholar] [CrossRef]
- Patrignani, C.; Agashe, K.; Aielli, G.; Amsler, C.; Antonelli, M.; Asner, D.M.; Baer, H.; Banerjee, S.; Barnett, R.M.; Basaglia, T.; et al. Review of Particle Physics. Chin. Phys. C 2016, 40, 100001. [Google Scholar] [CrossRef]
- ICRU 1984 Stopping Powers for Electrons and Positrons; ICRU Report No. 37; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1984.
- Cherry, S.R.; Sorenson, J.A.; Phelps, M.E. Physics in Nuclear Medicine, 4th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2012; Chapter 14. [Google Scholar]
- Lee, S.H.; Sunaguchi, N.; Hirano, Y.; Kano, Y.; Liu, C.; Torikoshi, M.; Ohno, T.; Nakano, T.; Kanai, T. A carbon CT system: How to obtain accurate stopping power ratio using a Bragg peak reduction technique. Phys. Med. Biol. 2018, 63, 035025. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Shirayanagi, Y.; Matsui, S.; Aoki, T.; Hatanaka, Y. X-ray color scanner with multiple energy discrimination capability. Med Imaging 2004, 5922, 59220A. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical cone-beam algorithm. J. Opt. Soc. Am. A 1984, 1, 612–619. [Google Scholar] [CrossRef]
- Sijbers, J.; Postnov, A. Reduction of ring artefacts in high resolution micro-CT reconstructions. Phys. Med. Biol. 2004, 49, N247–N253. [Google Scholar] [CrossRef]
- Prell, D.; Kyriakou, Y.; Beister, M.; Kalender, W.A. A novel forward projection-based metal artifact reduction method for flat-detector computed tomography. Phys. Med. Biol. 2009, 54, 6575–6591. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Mori, H.; Neo, Y.; Mimura, H.; Aoki, T. Application of CdTe photon-counting X-ray imager to material discriminated X-ray CT. Proc. SPIE 2007, 6706, 67060C. [Google Scholar] [CrossRef]
- Phantoms for Medical Imaging; Kyoto Kagaku Co., Ltd.: Kyoto, Japan, 2018. (In Japanese)
- Chen, G.T.Y.; Singh, R.P.; Castro, J.R.; Lyman, J.T.; Quivey, J.M. Treatment planning for heavy ion radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 1809–1819. [Google Scholar] [CrossRef]
- Jäkel, O.; Jacob, C.; Schardt, D.; Karger, C.P.; Hartmann, G.H. Relation between carbon ion ranges and x-ray CT numbers. Med. Phys. 2001, 28, 701–703. [Google Scholar] [CrossRef]
- LaLonde, A.; Bär, E.; Bouchard, H. A Bayesian approach to solve proton stopping powers from noisy multi-energy CT data. Med. Phys. 2017, 44, 5293–5302. [Google Scholar] [CrossRef] [PubMed]
- Taasti, V.T.; Hansen, D.C.; Michalak, G.J.; Deisher, A.J.; Kruse, J.J.; Muren, L.P.; Petersen, J.B.; McCollough, C.H. Theoretical and experimental analysis of photon counting detector CT for proton stopping power prediction. Med. Phys. 2018, 45, 5186–5196. [Google Scholar] [CrossRef]
- Matsumoto, G.; Imura, Y.; Morii, H.; Miyake, A.; Aoki, T. Analysis of artifact with X-ray CT using energy band by photon counting CdTe detector. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2010, 621, 292–294. [Google Scholar] [CrossRef][Green Version]
- Miyajima, S. Thin CdTe detector in diagnostic x-ray spectroscopy. Med. Phys. 2003, 30, 771–777. [Google Scholar] [CrossRef]
- Tsutsui, H.; Ohtsuchi, T.; Ohmori, K.; Baba, S. Measurement of X-ray spectrum using a small size CdTe multichannel detector. IEEE Trans. Nucl. Sci. 1992, 39, 2282–2285. [Google Scholar] [CrossRef]
- Zou, W.; Nakashima, T.; Onishi, Y.; Koike, A.; Shinomiya, B.; Morii, H.; Neo, Y.; Mimura, H.; Aoki, T. Atomic Number and Electron Density Measurement Using a Conventional X-ray Tube and a CdTe Detector. Jpn. J. Appl. Phys. 2008, 47, 7317–7323. [Google Scholar] [CrossRef]
- Hiraoka, T.; Kawashima, K.; Hoshino, K.; Fukumura, A.; Bichsel, H. Energy loss of 70 MeV protons in tissue substitute materials. Phys. Med. Biol. 1994, 39, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, P.E.; Hasegawa, B.H.; Beyer, T. X-ray-based attenuation correction for positron emission tomography/computed tomography scanners. Semin. Nucl. Med. 2003, 33, 166–179. [Google Scholar] [CrossRef] [PubMed]











| Elemental Composition (Percentage by Mass) | Mass Density (g cm−3) | |||||||
|---|---|---|---|---|---|---|---|---|
| H | C | N | O | P | Cl | Ca | ||
| BE-T-10 | 3.69 | 29.22 | 1.19 | 32.66 | 10.24 | 0.06 | 22.92 | 1.730 |
| BE-H-10 | 5.11 | 42.45 | 1.73 | 28.13 | 7.00 | 0.09 | 15.49 | 1.500 |
| BE-N-10 | 6.97 | 60.03 | 2.45 | 21.79 | 2.30 | 0.13 | 6.33 | 1.240 |
| WD-3010 | 8.63 | 68.89 | 2.18 | 17.88 | 0.15 | 2.27 | 1.018 | |
| Effective Atomic Number | Electron Density | I-Value | SPR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Theory | Two PFM | Three PFM | Theory (1e23) | Two PFM | Three PFM | Theory | Two PFM | Three PFM | Theory | Two PFM | Three PFM |
| BE-H-10 | 12.4 | 11.3 (−8.3) | 11.2 (−9.6) | 4.73 | 4.42 (−6.5) | 4.79 (1.2) | 87.5 | 87.8 (0.3) | 86.8 (−0.8) | 1.39 | 1.47 (5.5) | 1.42 (1.7) |
| BE-N-10 | 9.8 | 9.1 (−7.3) | 9.0 (−8.4) | 3.98 | 3.68 (−7.6) | 4.07 (2.0) | 74.0 | 78.2 (5.7) | 77.9 (5.3) | 1.23 | 1.24 (1.2) | 1.22 (−0.6) |
| BE-T-10 | 13.8 | 12.7 (−7.8) | 12.5 (−9.4) | 5.38 | 4.98 (−7.4) | 5.35 (−0.4) | 100.0 | 99.4 (−0.6) | 96.9 (−3.0) | 1.58 | 1.63 (3.5) | 1.56 (−0.7) |
| Aluminium | 13.0 | 13.2 (1.6) | 13.0(−0.3) | 7.83 | 7.56 (−3.5) | 7.75 (−1.1) | 166.0 | 105.1 (−36.7) [164.4 (−1.0)] | 102.0 (−38.6) [161.2 (−2.9)] | 2.10 | 2.46 (17.1) [2.37 (12.7)] | 2.25 (7.1) [2.16 (2.9)] |
| PMMA | 6.5 | 7.2 (10.8) | 7.2 (9.8) | 3.80 | 3.40 (−10.6) | 3.80 (0.1) | 68.5 | 72.0 (−3.3) | 71.7 (−3.6) | 1.17 | 1.15 (−1.6) | 1.15 (−2.0) |
| WD-3010 | 7.9 | 7.0 (−11.2) | 7.0 (−11.2) | 3.32 | 2.95 (−11.1) | 3.35 (0.9) | 67.2 | 70.9 (5.4) | 71.0 (5.5) | 1.01 | 1.01 (−0.1) | 1.02 (0.8) |
| Graphite | 6.0 | 7.3 (21.1) | 7.2 (20.0) | 6.65 | 5.51 (−17.2) | 5.61 (−15.7) | 78.0 | 72.2 (−7.4) | 72.0 (−7.8) | 1.67 | 1.87 (12.0) | 1.70 (1.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Sunaguchi, N.; Nagao, A.; Hirano, Y.; Sakurai, H.; Kano, Y.; Torikoshi, M.; Kanai, T.; Tashiro, M. Calculation of Stopping-Power Ratio from Multiple CT Numbers Using Photon-Counting CT System: Two- and Three-Parameter-Fitting Method. Sensors 2021, 21, 1215. https://doi.org/10.3390/s21041215
Lee SH, Sunaguchi N, Nagao A, Hirano Y, Sakurai H, Kano Y, Torikoshi M, Kanai T, Tashiro M. Calculation of Stopping-Power Ratio from Multiple CT Numbers Using Photon-Counting CT System: Two- and Three-Parameter-Fitting Method. Sensors. 2021; 21(4):1215. https://doi.org/10.3390/s21041215
Chicago/Turabian StyleLee, Sung Hyun, Naoki Sunaguchi, Akie Nagao, Yoshiyuki Hirano, Hiroshi Sakurai, Yosuke Kano, Masami Torikoshi, Tatsuaki Kanai, and Mutsumi Tashiro. 2021. "Calculation of Stopping-Power Ratio from Multiple CT Numbers Using Photon-Counting CT System: Two- and Three-Parameter-Fitting Method" Sensors 21, no. 4: 1215. https://doi.org/10.3390/s21041215
APA StyleLee, S. H., Sunaguchi, N., Nagao, A., Hirano, Y., Sakurai, H., Kano, Y., Torikoshi, M., Kanai, T., & Tashiro, M. (2021). Calculation of Stopping-Power Ratio from Multiple CT Numbers Using Photon-Counting CT System: Two- and Three-Parameter-Fitting Method. Sensors, 21(4), 1215. https://doi.org/10.3390/s21041215






