Micro-Lensed Negative-Curvature Fibre Probe for Raman Spectroscopy
Abstract
:1. Introduction
2. Experimental
2.1. NCF and Probe Fabrication
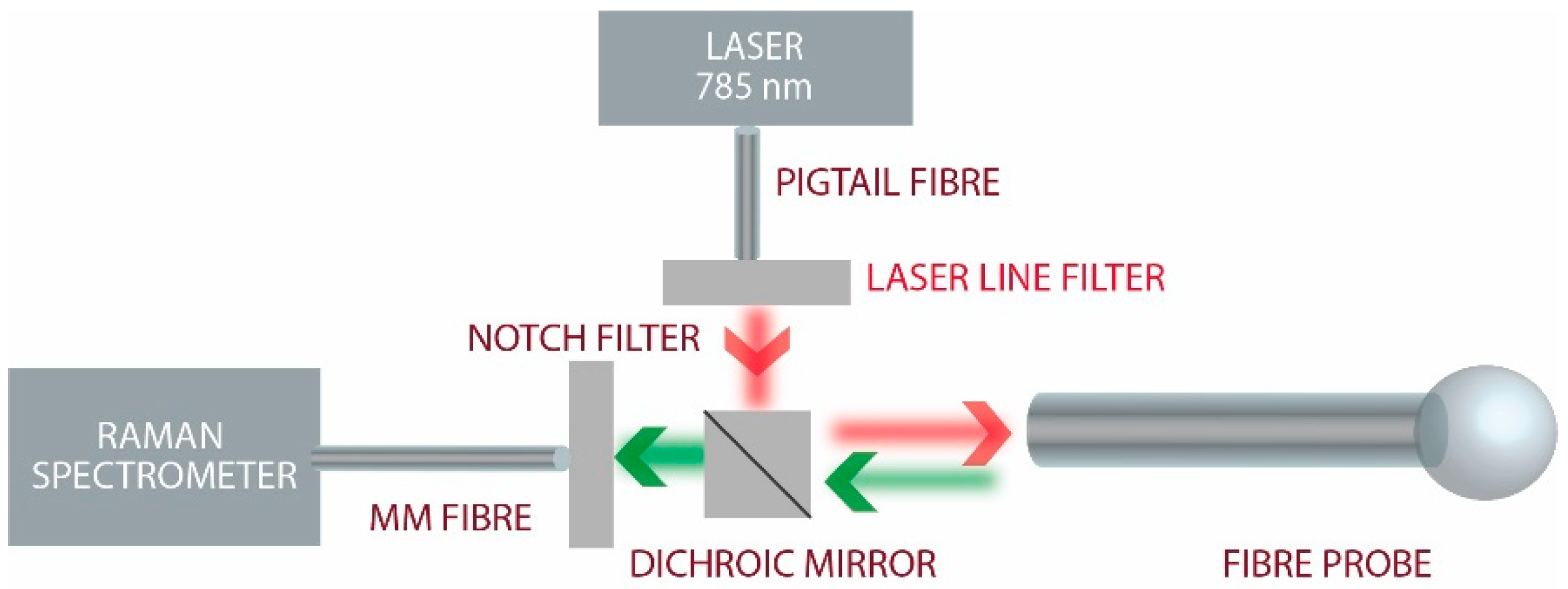
2.2. Experimental Setup
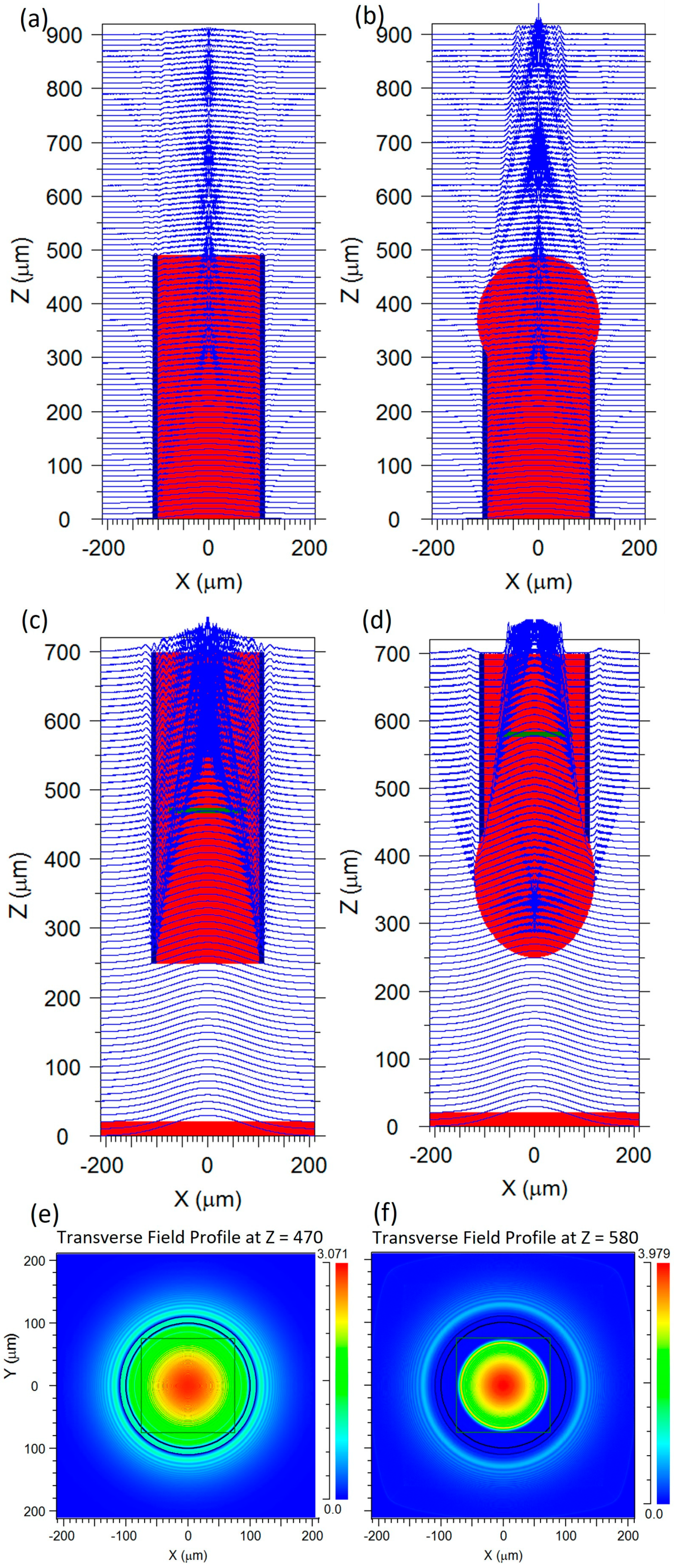
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.D.; Wolfbeis, O.S. Fiber-Optic Chemical Sensors and Biosensors (2013–2015). Anal. Chem. 2016, 88, 203–227. [Google Scholar] [CrossRef]
- Sieburg, A.; Schneider, S.; Yan, D.; Popp, J.; Frosch, T. Monitoring of gas composition in a laboratory biogas plant using cavity enhanced Raman spectroscopy. Analyst 2018, 143, 1358–1366. [Google Scholar] [CrossRef]
- Keiner, R.; Frosch, T.; Massad, T.; Trumbore, S.; Popp, J. Enhanced Raman multigas sensing—A novel tool for control and analysis of 13CO2 labeling experiments in environmental research. Analyst 2014, 139, 3879–3884. [Google Scholar] [CrossRef] [Green Version]
- Knebl, A.; Yan, D.; Popp, J.; Frosch, T. Fiber enhanced Raman gas spectroscopy. TrAC Trends Anal. Chem. 2018, 103, 230–238. [Google Scholar] [CrossRef]
- Premasiri, W.R.; Clarke, R.H.; Womble, M.E. Urine analysis by laser Raman spectroscopy. Lasers Surg. Med. 2001, 28, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Enejder, A.M.K.; Koo, T.-W.; Oh, J.; Hunter, M.; Sasic, S.; Feld, M.S.; Horowitz, G.L. Blood analysis by Raman spectroscopy. Opt. Lett. 2002, 27, 2004–2006. [Google Scholar] [CrossRef]
- Domes, C.; Domes, R.; Popp, J.; Pletz, M.W.; Frosch, T. Ultrasensitive Detection of Antiseptic Antibiotics in Aqueous Media and Human Urine Using Deep UV Resonance Raman Spectroscopy. Anal. Chem. 2017, 89, 9997–10003. [Google Scholar] [CrossRef]
- Cîntǎ Pînzaru, S.; Pavel, I.; Leopold, N.; Kiefer, W. Identification and characterization of pharmaceuticals using Raman and surface-enhanced Raman scattering. J. Raman Spectrosc. 2004, 35, 338–346. [Google Scholar] [CrossRef]
- Cordero, E.; Latka, I.; Matthaus, C.; Schie, I.; Popp, J. In-vivo Raman spectroscopy: From basics to applications. J. Biomed. Opt. 2018, 23, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Heng, H.P.S.; Shu, C.; Zheng, W.; Lin, K.; Huang, Z. Advances in real-time fiber-optic Raman spectroscopy for early cancer diagnosis: Pushing the frontier into clinical endoscopic applications. Transl. Biophotonics 2021, 3, e202000018. [Google Scholar] [CrossRef]
- Stevens, O.; Iping Petterson, I.E.; Day, J.C.C.; Stone, N. Developing fibre optic Raman probes for applications in clinical spectroscopy. Chem. Soc. Rev. 2016, 45, 1919–1934. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhang, P.; Leung, C.; Xie, Y.-H. SERS optical fiber probe with plasmonic end-facet. J. Raman Spectrosc. 2017, 48, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Pence, I.J.; O’Brien, C.M.; Masson, L.E.; Mahadevan-Jansen, A. Application driven assessment of probe designs for Raman spectroscopy. Biomed. Opt. Express 2021, 12, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; MacLachlan, D.G.; Smith, B.J.E.; Beck, R.J.; Shephard, J.D.; Weston, N.; Thomson, R.R. A Miniature Fibre-Optic Raman Probe Fabricated by Ultrafast Laser-Assisted Etching. Micromachines 2020, 11, 185. [Google Scholar] [CrossRef] [Green Version]
- Milenko, K.; Fuglerud, S.S.; Kjeldby, S.B.; Ellingsen, R.; Aksnes, A.; Hjelme, D.R. Micro-lensed optical fibers for a surface-enhanced Raman scattering sensing probe. Opt. Lett. 2018, 43, 6029–6032. [Google Scholar] [CrossRef]
- Yu, F.; Knight, J.C. Negative Curvature Hollow-Core Optical Fiber. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Yerolatsitis, S.; Yu, F.; McAughtrie, S.; Tanner, M.G.; Fleming, H.; Stone, J.M.; Campbell, C.J.; Birks, T.A.; Knight, J.C. Ultra-low background Raman sensing using a negative-curvature fibre and no distal optics. J. Biophotonics 2019, 12, e201800239. [Google Scholar] [CrossRef]
- Konorov, S.O.; Addison, C.J.; Schulze, H.G.; Turner, R.F.B.; Blades, M.W. Hollow-core photonic crystal fiber-optic probes for Raman spectroscopy. Opt. Lett. 2006, 31, 1911–1913. [Google Scholar] [CrossRef]
- Kudlinski, A.; Cassez, A.; Vanvincq, O.; Septier, D.; Pastre, A.; Habert, R.; Baudelle, K.; Douay, M.; Mytskaniuk, V.; Tsvirkun, V.; et al. Double clad tubular anti-resonant hollow core fiber for nonlinear microendoscopy. Opt. Express 2020, 28, 15062–15070. [Google Scholar] [CrossRef] [PubMed]
- McAnally, M.O.; Phelan, B.T.; Young, R.M.; Wasielewski, M.R.; Schatz, G.C.; Van Duyne, R.P. Quantitative Determination of the Differential Raman Scattering Cross Sections of Glucose by Femtosecond Stimulated Raman Scattering. Anal. Chem. 2017, 89, 6931–6935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.; Lin, M.; Li, Y.; Li, X.; Liu, J.; Liang, J.; Yao, H. In Vivo Blood Glucose Quantification Using Raman Spectroscopy. PLoS ONE 2012, 7, e48127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.W.; Park, Y.S.; Chang, H.; Lee, W.; Singh, S.P.; Choi, W.; Galindo, L.H.; Dasari, R.R.; Nam, S.H.; Park, J.; et al. Direct observation of glucose fingerprint using in vivo Raman spectroscopy. Sci. Adv. 2020, 6, eaay5206. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milenko, K.; Yerolatsitis, S.; Aksnes, A.; Hjelme, D.R.; Stone, J.M. Micro-Lensed Negative-Curvature Fibre Probe for Raman Spectroscopy. Sensors 2021, 21, 8434. https://doi.org/10.3390/s21248434
Milenko K, Yerolatsitis S, Aksnes A, Hjelme DR, Stone JM. Micro-Lensed Negative-Curvature Fibre Probe for Raman Spectroscopy. Sensors. 2021; 21(24):8434. https://doi.org/10.3390/s21248434
Chicago/Turabian StyleMilenko, Karolina, Stephanos Yerolatsitis, Astrid Aksnes, Dag Roar Hjelme, and James M. Stone. 2021. "Micro-Lensed Negative-Curvature Fibre Probe for Raman Spectroscopy" Sensors 21, no. 24: 8434. https://doi.org/10.3390/s21248434
APA StyleMilenko, K., Yerolatsitis, S., Aksnes, A., Hjelme, D. R., & Stone, J. M. (2021). Micro-Lensed Negative-Curvature Fibre Probe for Raman Spectroscopy. Sensors, 21(24), 8434. https://doi.org/10.3390/s21248434






