Aging and Bimanual Effects on Finger Center of Pressure during Precision Grip: Different Strategies for Spatial Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Procedures
2.3. Materials
2.4. Clinical Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desrosiers, J.; Hébert, R.; Bravo, G.; Rochette, A. Age-related changes in upper extremity performance of elderly people: A longitudinal study. Exp. Gerontol. 1999, 34, 393–405. [Google Scholar] [CrossRef]
- Carmeli, E.; Patish, H.; Coleman, R. The Aging Hand. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, M146–M152. [Google Scholar] [CrossRef]
- Hunter, S.K.; Pereira, H.M.; Keenan, K.G. The aging neuromuscular system and motor performance. J. Appl. Physiol. 2016, 121, 982–995. [Google Scholar] [CrossRef]
- Lin, C.H.; Chou, L.W.; Wei, S.H.; Lieu, F.K.; Chiang, S.L.; Sung, W.H. Influence of aging on bimanual coordination control. Exp. Gerontol. 2014, 53, 40–47. [Google Scholar] [CrossRef]
- Temprado, J.J.; Torre, M.M.; Langeard, A.; Julien-Vintrou, M.; Devillers-Réolon, L.; Sleimen-Malkoun, R.; Berton, E. Intentional Switching Between Bimanual Coordination Patterns in Older Adults: Is It Mediated by Inhibition Processes? Front. Aging Neurosci. 2020, 12, 29. [Google Scholar] [CrossRef]
- Stanciu, I.; Biehl, R.; Bravo, S.; Hesse, C. Increased cognitive demands boost the spatial interference effect in bimanual pointing. Psychol. Res. 2017, 81, 582–595. [Google Scholar] [CrossRef][Green Version]
- Ivry, R.B.; Diedrichsen, J.; Spencer, R.; Hazeltine, E.; Semjen, A. A Cognitive Neuroscience Perspective on Bimanual Coordination and Interference. In Neuro-Behavioral Determinants of Interlimb Coordination, 1st ed.; Swinnen, S.P., Duysens, J., Eds.; Springer: Boston, MA, USA, 2004; pp. 259–295. [Google Scholar]
- Swinnen, S.P.; Wenderoth, N. Two hands, one brain: Cognitive neuroscience of bimanual skill. Trends Cogn. Sci. 2004, 8, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.B.; Ivry, R.B. The persistence of spatial interference after extended training in a bimanual drawing task. Cortex 2009, 45, 377–385. [Google Scholar] [CrossRef]
- Dimitriou, P.; Buckingham, G. Bimanual Lifting: Do Fingertip Forces Work Independently or Interactively? J. Mot. Behav. 2018, 50, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Kantak, S.; Jax, S.; Wittenberg, G. Bimanual coordination: A missing piece of arm rehabilitation after stroke. Restor. Neurol. Neurosci. 2017, 35, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Westling, G.; Johansson, R.S. Factors Influencing the Force Control during Precision Grip. Exp. Brain Res. 1984, 53, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Johansson, R.S.; Westling, G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp. Brain Res. 1984, 56, 550–564. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, W.; Santello, M. Anticipatory Planning and Control of Grasp Positions and Forces for Dexterous Two-Digit Manipulation. J. Neurosci. 2010, 30, 9117–9126. [Google Scholar] [CrossRef]
- Parikh, P.J.; Cole, K.J. Handling objects in old age: Forces and moments acting on the object. J. Appl. Physiol. 2012, 112, 1095–1104. [Google Scholar] [CrossRef]
- Kurihara, J.; Lee, B.; Hara, D.; Noguchi, N.; Yamazaki, T. Increased center of pressure trajectory of the finger during precision grip task in stroke patients. Exp. Brain Res. 2018, 237, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Augurelle, A.S.; Smith, A.M.; Lejeune, T.; Thonnard, J.L. Importance of Cutaneous Feedback in Maintaining a Secure Grip during Manipulation of Hand-Held Objects. J. Neurophysiol. 2003, 89, 665–671. [Google Scholar] [CrossRef]
- Monzee, J.; Lamarre, Y.; Smith, A.T. The Effects of Digital Anesthesia on Force Control Using a Precision Grip. J. Neurophysiol. 2003, 89, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Li, K.; Wei, N.; Yue, S.; Yin, C. Influence of Exercise-induced Local Muscle Fatigue on the Thumb and Index Finger Forces during Precision Pinch. In Proceedings of the 2017 Chinese Automation Congress, Jinan, China, 20–22 October 2017. [Google Scholar]
- Dun, S.; Kaufmann, R.A.; Li, Z.M. Lower median nerve block impairs precision grip. J. Electromyogr. Kinesiol. 2007, 17, 348–354. [Google Scholar] [CrossRef]
- Cole, K.J. Grasp Force Control in Older Adults. J. Mot. Behav. 1991, 23, 251–258. [Google Scholar] [CrossRef]
- Diermayr, G.; McIsaac, T.L.; Gordon, A.M. Finger Force Coordination Underlying Object Manipulation in the Elderly—A Mini-Review. Gerontology 2011, 57, 217–227. [Google Scholar] [CrossRef]
- Teshima, R.; Noguchi, N.; Fujii, R.; Kondo, K.; Tanaka, K.; Lee, B. Measurement of Spatial Stability in Precision Grip. J. Vis. Exp. 2020, 160, e59699. [Google Scholar] [CrossRef]
- Wettenschwiler, P.D.; Stämpfli, R.; Lorenzetti, S.; Ferguson, S.J.; Rossi, R.M.; Annaheim, S. How reliable are pressure measurements with Tekscan sensors on thebody surface of human subjects wearing load carriage systems? Int. J. Ind. Ergon. 2015, 49, 60–67. [Google Scholar] [CrossRef]
- Luo, Z.P.; Berglund, L.J.; An, K.N. Validation of F-Scan pressure sensor system: A technical note. J. Rehabil. Res. Dev. 1998, 35, 186–191. [Google Scholar]
- Tekscan Inc. Tekscan Medical Sensors-Standard Pressures; Tekscan Inc.: South Boston, MA, USA, 2004. [Google Scholar]
- Adkin, A.L.; Frank, J.S.; Carpenter, M.G.; Peysar, G.W. Postural control is scaled to level of postural threat. Gait Posture 2000, 12, 87–93. [Google Scholar] [CrossRef]
- Carpenter, M.G.; Frank, J.S.; Silcher, C.P.; Peysar, G.W. The influence of postural threat on the control of upright stance. Exp. Brain Res. 2001, 138, 210–218. [Google Scholar] [CrossRef]
- Brown, L.A.; Sleik, R.J.; Polych, M.A.; Gage, W.H. Is the Prioritization of Postural Control Altered in Conditions of Postural Threat in Younger and Older Adults? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M785–M792. [Google Scholar] [CrossRef]
- Kinoshita, H.; Francis, P.R. A comparison of prehension force control in young and elderly individuals. Eur. J. Appl. Physiol. 1996, 74, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Guillery, E.; Mouraux, A.; Thonnard, J.L. Cognitive-Motor Interference While Grasping, Lifting and Holding Objects. PLoS ONE 2013, 8, e80125. [Google Scholar] [CrossRef]
- Guillery, E.; Mouraux, A.; Thonnard, J.L.; Legrain, V. Mind Your Grip: Even Usual Dexterous Manipulation Requires High Level Cognition. Front. Behav. Neurosci. 2017, 11, 220. [Google Scholar] [CrossRef]
- Lee, B.; Miyanjo, R.; Tozato, F.; Shiihara, Y. Dual-task Interference in a Grip and Lift Task. Kita Kanto Igaku 2014, 64, 309–312. [Google Scholar] [CrossRef]
- Bagesteiro, L.B.; Sainburg, R.L. Nondominant Arm Advantages in Load Compensation during Rapid Elbow Joint Movements. J. Neurophysiol. 2003, 90, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Duff, S.V.; Sainburg, R.L. Lateralization of motor adaptation reveals independence in control of trajectory and steady-state position. Exp. Brain Res. 2007, 179, 551–561. [Google Scholar] [CrossRef]
- Ferrand, L.; Jaric, S. Force Coordination in Static Bimanual Manipulation: Effect of Handedness. Mot. Control 2006, 10, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Bangert, A.S.; Reuter-Lorenz, P.A.; Walsh, C.M.; Schachter, A.B.; Seidler, R.D. Bimanual Coordination and Aging: Neurobehavioral Implications. Neuropsychologia 2010, 48, 1165–1170. [Google Scholar] [CrossRef]
- Kim, S.J.; Cho, S.R.; Yoo, G.E. Age-Related Changes in Bimanual Instrument Playing with Rhythmic Cueing. Front. Psychol. 2017, 8, 1569. [Google Scholar] [CrossRef] [PubMed]
- Ashendorf, L.; Vanderslice-Barr, J.L.; McCaffrey, R.J. Motor Tests and Cognition in Healthy Older Adults. Appl. Neuropsychol. Adult 2009, 16, 171–176. [Google Scholar] [CrossRef]
- Rodríguez-Aranda, C.; Mittner, M.; Vasylenko, O. Association between Executive Functions, Working Memory, and Manual Dexterity in Young and Healthy Older Adults: An Exploratory Study. Percept. Mot. Ski. 2016, 122, 165–192. [Google Scholar] [CrossRef]
- Vasylenko, O.; Gorecka, M.M.; Rodríguez-Aranda, C. Manual dexterity in young and healthy older adults. 2. Association with cognitive abilities. Dev. Psychobiol. 2018, 60, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Nakashima, D.; Matsuoka, H.; Iwai, M.; Nakamura, S.; Kubo, A.; Tomiyama, N. Exploring the factor on sensory motor function of upper limb associated with executive function in communitydwelling older adults. Nagoya J. Med. Sci. 2016, 78, 285–291. [Google Scholar] [PubMed]
- Hibino, H.; Gorniak, S.L. Effects of aging on rapid grip force responses during bimanual manipulation of an active object. Exp. Brain Res. 2020, 238, 2161–2178. [Google Scholar] [CrossRef]
- Noble, J.W.; Eng, J.J.; Kokotilo, K.J.; Boyd, L.A. Aging Effects on the Control of Grip Force Magnitude: An fMRI Study. Exp. Gerontol. 2011, 46, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.; Beuting, M.; Meulenbroek, R.; Steenbergen, B. Combined effects of planning and execution constraints on bimanual task performance. Exp. Brain Res. 2008, 192, 61–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Janssen, L.; Meulenbroek, R.; Steenbergen, B. Behavioral evidence for left-hemisphere specialization of motor planning. Exp. Brain Res. 2011, 209, 65–72. [Google Scholar] [CrossRef]
- Kalisch, T.; Wilimzig, C.; Kleibel, N.; Tegenthoff, M.; Dinse, H.R. Age-Related Attenuation of Dominant Hand Superiority. PLoS ONE 2006, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Huang, C.Y. Improving posture-motor dual-task with a supraposture-focus strategy in young and elderly adults. PLoS ONE 2017, 12, e0170687. [Google Scholar] [CrossRef]
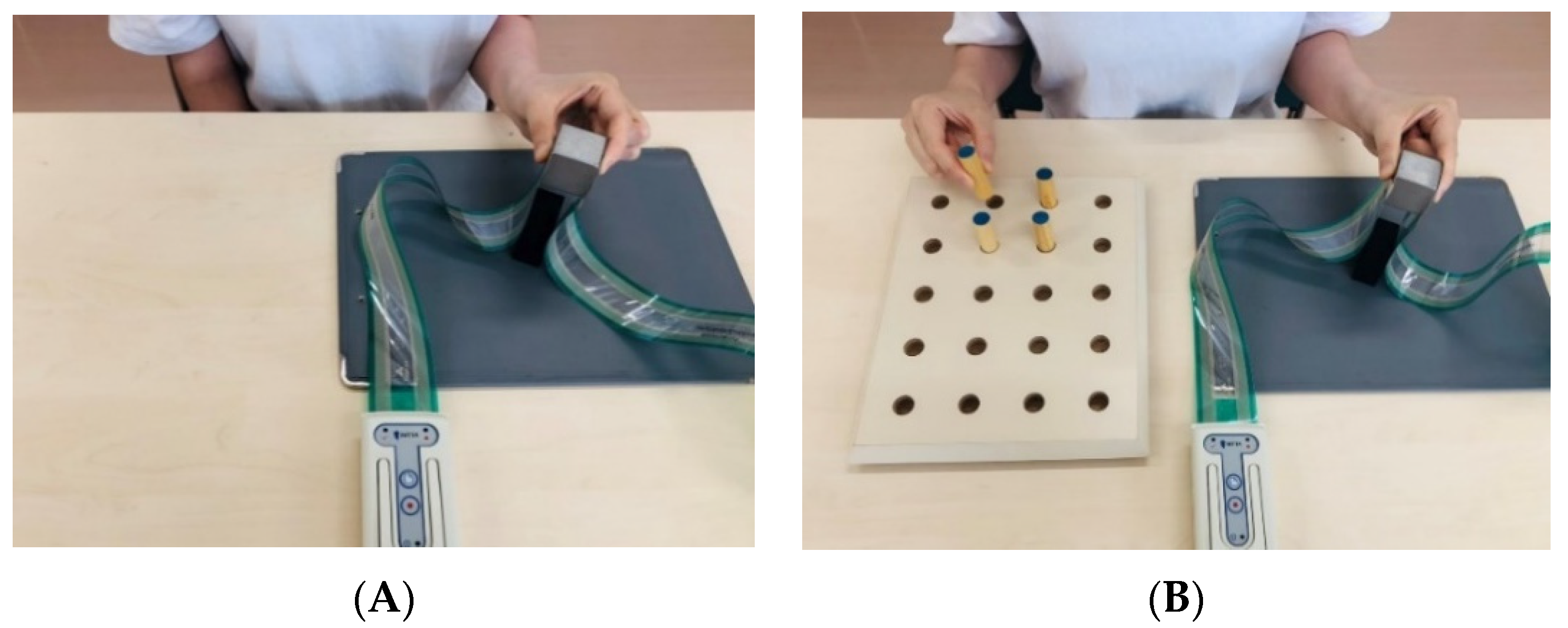
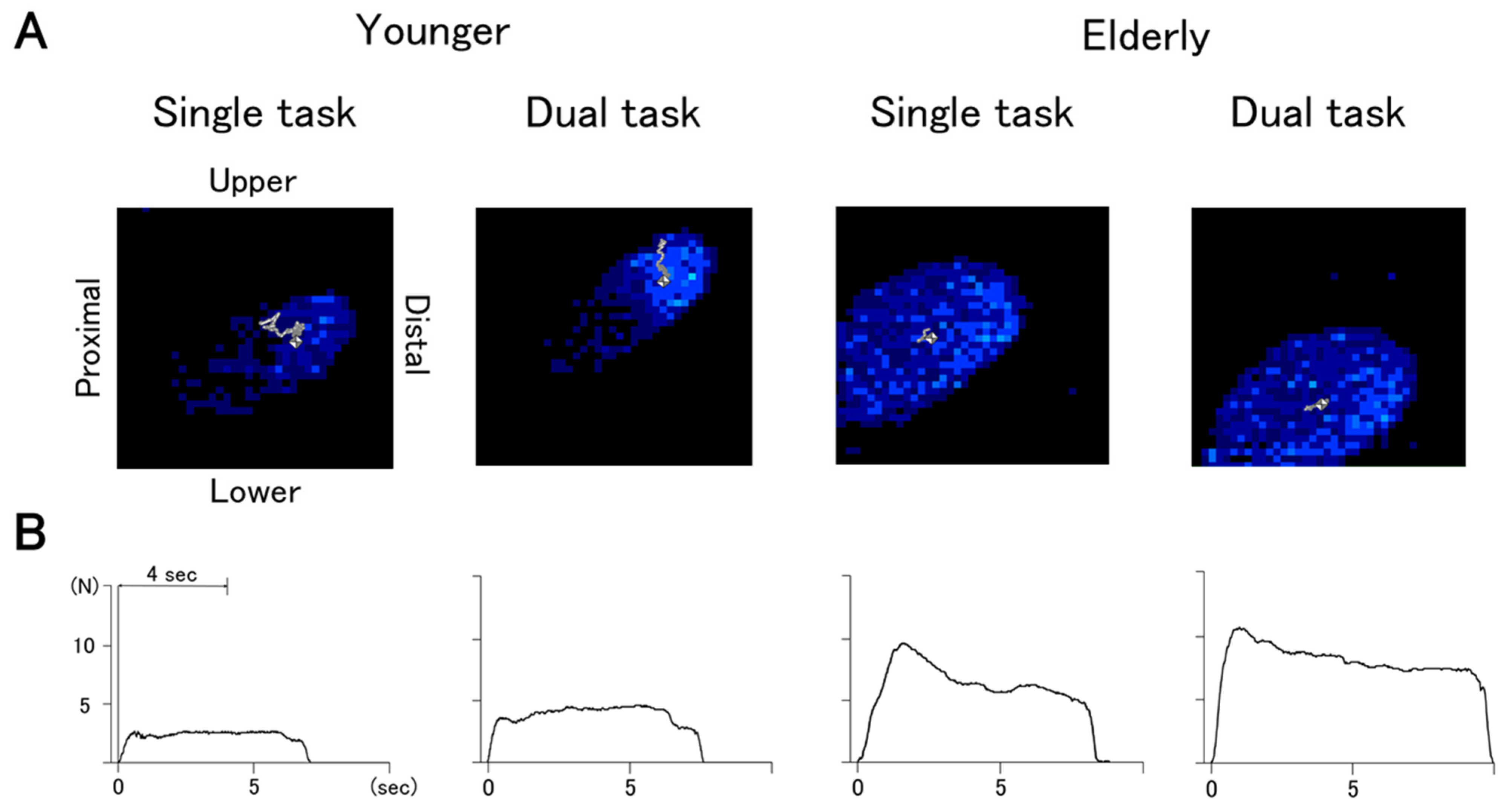
| Older (n = 21) | Younger (n = 21) | p | r | |
|---|---|---|---|---|
| Age | 78.5 ± 5.6 | 22.0 ± 2.7 | <0.01 | 0.88 |
| Sex (M/F) | 3/18 | 3/18 | 0.67 | <0.01 |
| Trail Making Test A (s) | 134.6 ± 57.4 | 65.6 ± 13.7 | <0.01 | 0.92 |
| B (s) | 164.8 ± 56.4 | 69.9 ± 22.4 | <0.01 | 0.79 |
| Δ Trail Making Test (s) | 47.6 ± 45.1 | 4.3 ± 18.6 | <0.01 | 0.58 |
| COP Trajectory | Mean Grip Force | ||||||
|---|---|---|---|---|---|---|---|
| ST (mm) a | DT (mm) b | DTC (mm) c | ST (N) a | DT (N) b | DTC (N) c | ||
| Older | |||||||
| Dominant | Thumb | 16.5 ± 4.8 | 14.8 ± 3.5 | −1.1 ± 3.2 | 2618 ± 1024 | 3310 ± 1222 | 577 ± 827 |
| Index finger | 15.5 ± 5.4 | 15.6 ± 4.9 | 0.6 ± 3.7 | 3348 ± 1107 | 3922 ± 1194 | 438 ± 795 | |
| Non- | Thumb | 15.1 ± 4.3 | 15.3 ± 4.6 | 0.3 ± 2.9 | 3157 ± 1021 | 3569 ± 1008 | 333 ± 597 |
| Index finger | 17.6 ± 5.9 | 18.2 ± 5.4 | 1.1 ± 5.0 | 2695 ± 988 | 3011 ± 1153 | 258 ± 695 | |
| Younger | |||||||
| Dominant | Thumb | 29.8 ± 10.8 | 22.7 ± 10.2 | −7.1 ± 8.3 | 1021 ± 532 | 1581 ± 705 | 561 ± 490 |
| Index finger | 28.7 ± 14.2 | 22.7 ± 11.9 | −6.0 ± 7.8 | 999 ± 553 | 1516 ± 722 | 517 ± 490 | |
| Non- | Thumb | 28.8 ± 9.1 | 23.7 ± 9.0 | −5.1 ± 8.8 | 962 ± 433 | 1321 ± 1153 | 359 ± 430 |
| Index finger | 24.8 ± 8.8 | 20.8 ± 7.4 | −4.0 ± 7.5 | 1095 ± 459 | 1475 ± 607 | 380 ± 497 | |
| Source | Sum of Squares | df | F Value | p Value | ηp2 | |
|---|---|---|---|---|---|---|
| COP trajectory (mm) | ||||||
| Thumb | Age | 2876.16 | 1 | 46.17 | <0.01 | 0.25 |
| Task | 403.04 | 1 | 6.47 | 0.01 | 0.04 | |
| Hand | 1.46 | 1 | 0.02 | 0.87 | <0.01 | |
| Age × Task | 284.78 | 1 | 4.57 | 0.03 | 0.03 | |
| Age × Hand | 1.00 | 1 | 0.02 | 0.89 | <0.01 | |
| Task × Hand | 23.67 | 1 | 0.38 | 0.53 | <0.01 | |
| Age × Task × Hand | 1.24 | 1 | 0.02 | 0.88 | <0.01 | |
| Index finger | Age | 1698.597 | 1 | 22.14 | <0.01 | 0.14 |
| Task | 185.656 | 1 | 2.42 | 0.12 | 0.02 | |
| Hand | 1.00 | 1 | 0.01 | 0.90 | <0.01 | |
| Age × Task | 272.13 | 1 | 3.54 | 0.06 | 0.03 | |
| Age × Hand | 263.786 | 1 | 3.44 | 0.06 | 0.02 | |
| Task × Hand | 5.530 | 1 | 0.07 | 0.78 | <0.01 | |
| Age × Task × Hand | 12.67 | 1 | 0.17 | 0.68 | <0.01 | |
| Mean grip force (N) | ||||||
| Thumb | Age | 69,304,377.38 | 1 | 96.58 | <0.01 | 0.41 |
| Task | 8,438,874.61 | 1 | 11.76 | <0.01 | 0.08 | |
| Hand | 288,028.74 | 1 | 0.40 | 0.52 | <0.01 | |
| Age × Task | 15,099.02 | 1 | 0.02 | 0.88 | <0.01 | |
| Age × Hand | 2,253,441.26 | 1 | 3.14 | 0.08 | 0.02 | |
| Task × Hand | 364,154.87 | 1 | 0.50 | 0.47 | <0.01 | |
| Age × Task × Hand | 34.48 | 1 | <0.01 | 0.99 | <0.01 | |
| Index finger | Age | 81,822,564.45 | 1 | 107.72 | <0.01 | 0.44 |
| Task | 6,228,928.25 | 1 | 8.20 | <0.01 | 0.06 | |
| Hand | 5,819,038.81 | 1 | 7.66 | <0.01 | 0.05 | |
| Age × Task | 47,802.17 | 1 | 0.06 | 0.80 | <0.01 | |
| Age × Hand | 6,658,118.63 | 1 | 8.77 | <0.01 | 0.06 | |
| Task × Hand | 207,754.248 | 1 | 0.27 | 0.60 | <0.01 | |
| Age × Task × Hand | 1792.31 | 1 | <0.01 | 0.96 | <0.01 |
| ΔTMT a | ||||
|---|---|---|---|---|
| Older b | Younger c | |||
| DTC | ||||
| COP trajectory | Dominant | Thumb | −0.22 | −0.46 * |
| Index finger | −0.06 | −0.37 | ||
| Non- | Thumb | 0.17 | 0.05 | |
| Index finger | −0.32 | 0.04 | ||
| Mean grip force | Dominant | Thumb | −0.26 | 0.56 ** |
| Index finger | −0.21 | 0.50 * | ||
| Non- | Thumb | 0.58 * | −0.02 | |
| Index finger | 0.55 * | 0.04 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akiyama, R.; Noguchi, N.; Kondo, K.; Tanaka, K.; Lee, B. Aging and Bimanual Effects on Finger Center of Pressure during Precision Grip: Different Strategies for Spatial Stability. Sensors 2021, 21, 8396. https://doi.org/10.3390/s21248396
Akiyama R, Noguchi N, Kondo K, Tanaka K, Lee B. Aging and Bimanual Effects on Finger Center of Pressure during Precision Grip: Different Strategies for Spatial Stability. Sensors. 2021; 21(24):8396. https://doi.org/10.3390/s21248396
Chicago/Turabian StyleAkiyama, Ryoto, Naoto Noguchi, Ken Kondo, Koji Tanaka, and Bumsuk Lee. 2021. "Aging and Bimanual Effects on Finger Center of Pressure during Precision Grip: Different Strategies for Spatial Stability" Sensors 21, no. 24: 8396. https://doi.org/10.3390/s21248396
APA StyleAkiyama, R., Noguchi, N., Kondo, K., Tanaka, K., & Lee, B. (2021). Aging and Bimanual Effects on Finger Center of Pressure during Precision Grip: Different Strategies for Spatial Stability. Sensors, 21(24), 8396. https://doi.org/10.3390/s21248396






