Evaluation of Remote Photoplethysmography Measurement Conditions toward Telemedicine Applications
Abstract
:1. Introduction
2. Materials and Methods
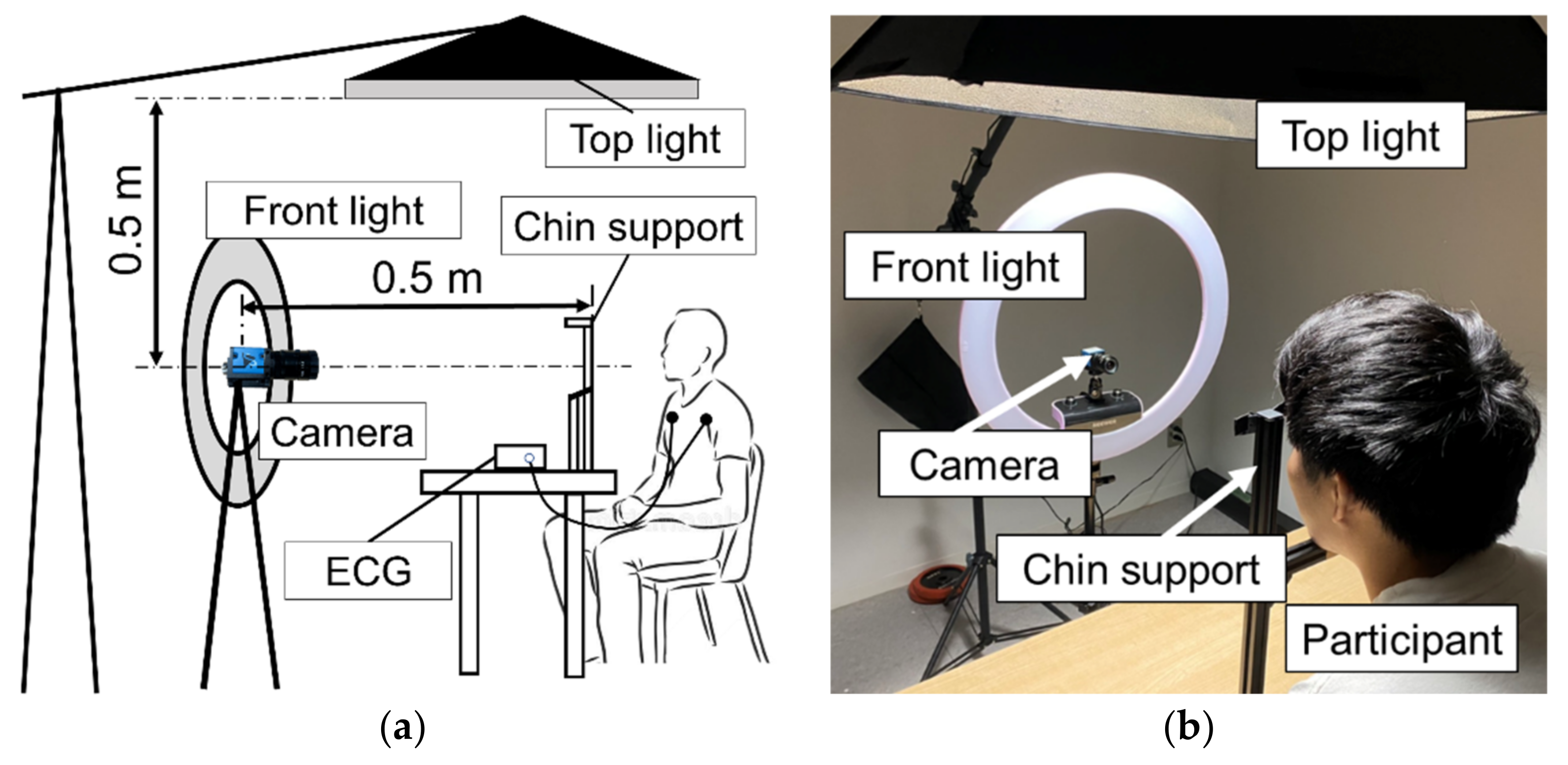
2.1. Measurement Equipment
2.2. Experimental Setup and Protocol
2.2.1. Experiment on Effect of Light Source
2.2.2. Experiment on the Effect of Camera’s Frame Rate
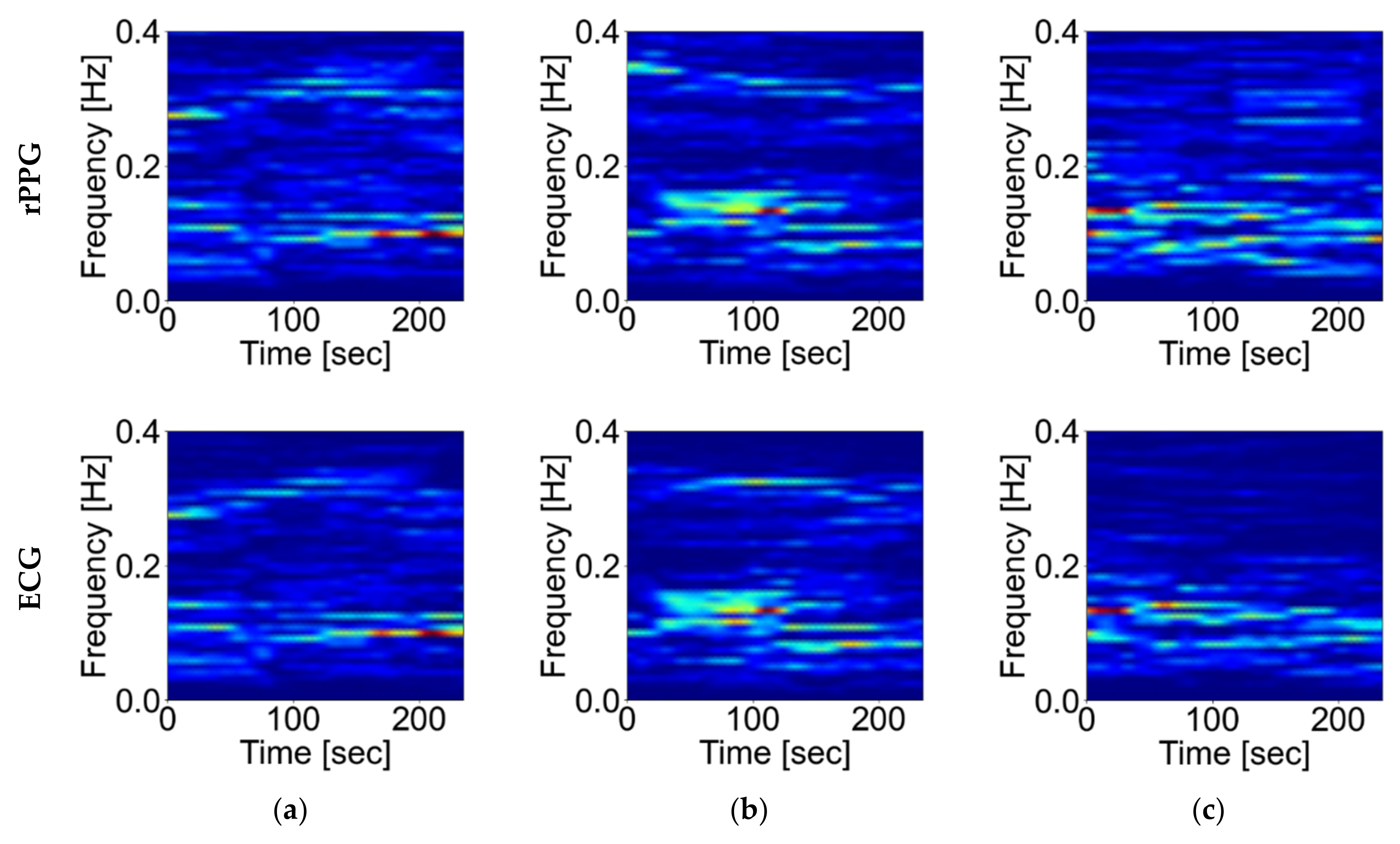
2.2.3. Experiment on the Effect of Body Motion
2.3. Image and Signal Processing
2.3.1. Region of Interest (RoI) Detection
2.3.2. Pulse Extraction
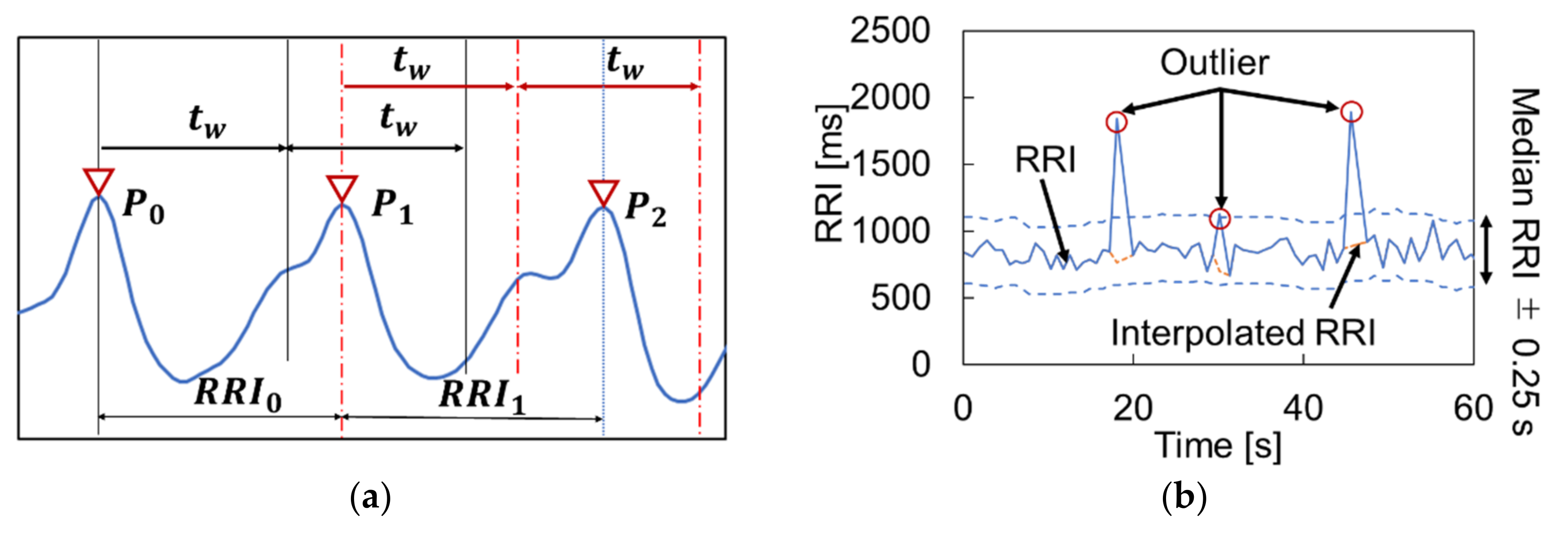
2.3.3. Peak Detection
2.3.4. HRV Calculation
3. Results
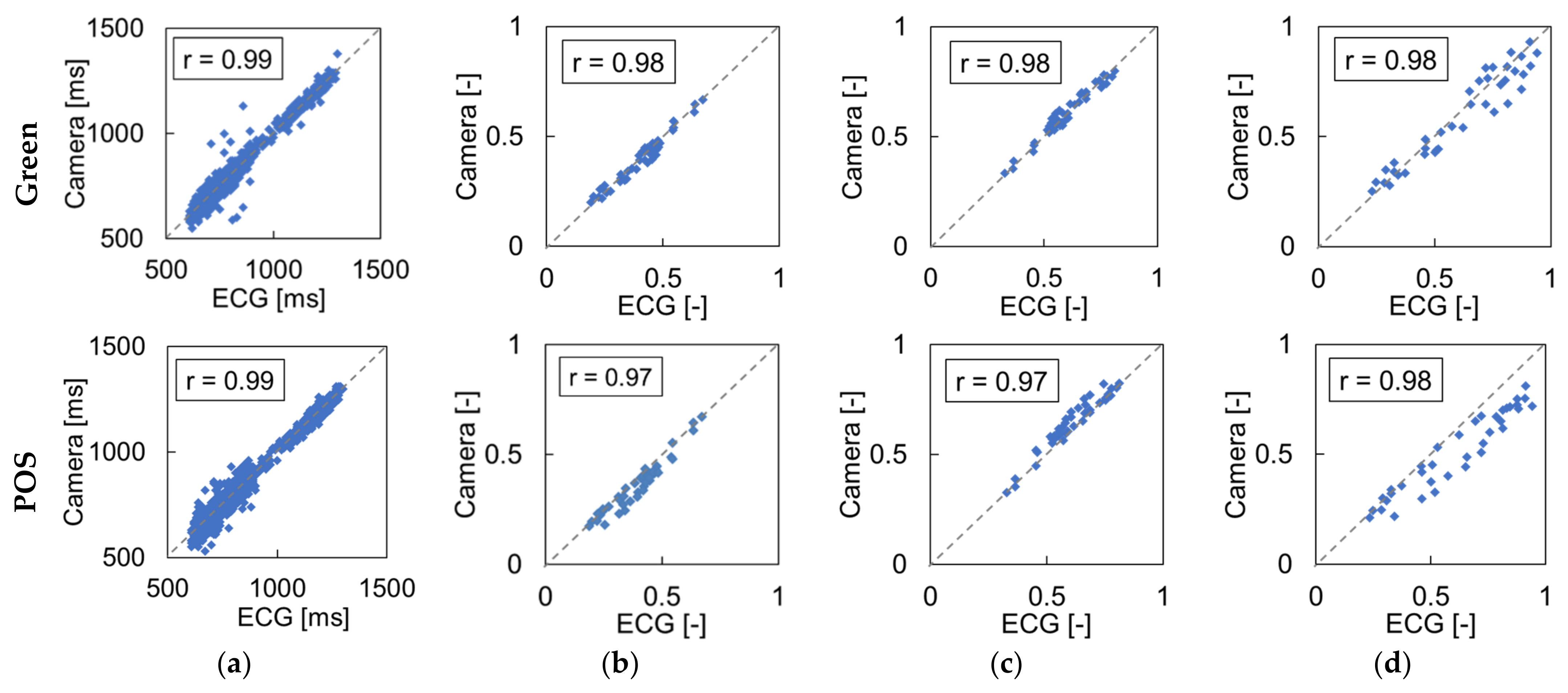
3.1. Effect of Light Source
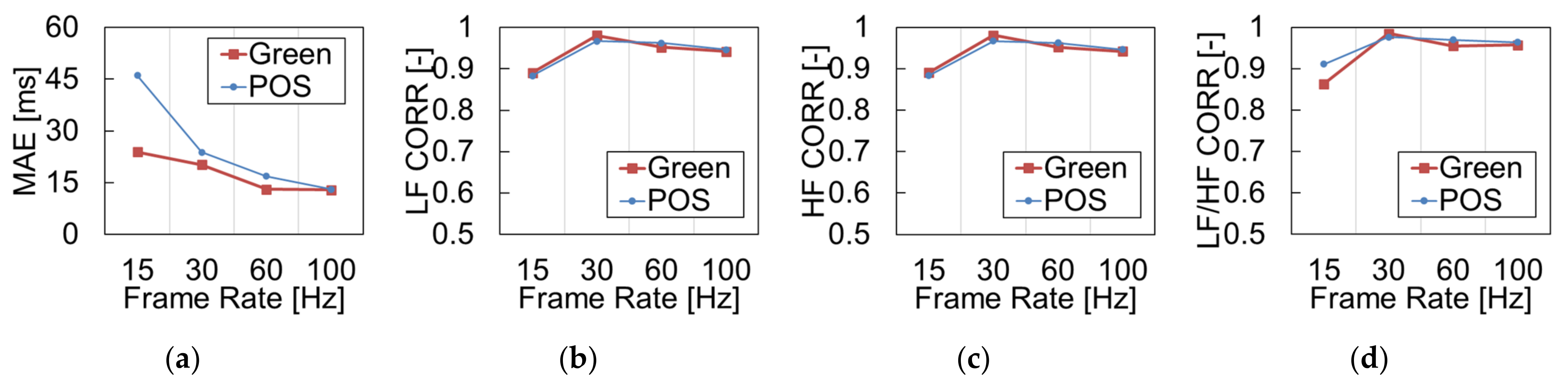
3.2. Effects of Camera’s Frame Rate
3.3. Effects of Body Motion
4. Discussion
4.1. Influence of Light Source
4.2. Influence of Camera’s Frame Rate
4.3. Influence of Body Motion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kichloo, A.; Albosta, M.; Dettloff, K.; Wani, F.; El-Amir, Z.; Singh, J.; Aljadah, M.; Chakinala, R.C.; Kanugula, A.K.; Solanki, S.; et al. Telemedicine, the current COVID-19 pandemic and the future: A narrative review and perspectives moving forward in the USA. Fam. Med. Community Health 2020, 8, e000530. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Thouheed, S.S.; Thanuja, K.; Guptha, N.S.; Narasimha, S. Telemedicine approach for remote patient monitoring system using smart phones with an economical hardware kit. In Proceedings of the International Conference on Computing Technologies and Intelligent Data Engineering Conference (ICCTIDE’16), Kovilpatti, India, 7–9 January 2016; pp. 1–4. [Google Scholar]
- Yamazaki, Y.; Ishii, M.; Ito, T.; Hashimoto, T. Frailty Care Robot for Elderly and its Application for Physical and Psychological Support. J. Adv. Comput. Intell. Intell. Inform. 2021, 25(6), 944–952. [Google Scholar] [CrossRef]
- Remote Patient Monitoring for Diabetes and Related Chronic Conditions Glooko. Available online: https://www.glooko.com (accessed on 7 October 2021).
- Abo-Zahhad, M.; Ahmed, M.A.; Elnahas, O. A Wireless Emergency Telemedicine System for Patients Monitoring and Diagnosis. Int. J. Telemed. Appl. 2014, 2014, 380787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamath, M.V.; Ghista, D.N.; Fallen, E.L.; Fitchett, D.; Miller, D.; McKelvie, R. Heart rate variability power spectrogram as a potential noninvasive signature of cardiac regulatory system response, mechanisms, and disorders. Heart Vessel. 1987, 3, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, A.; Otsuka, A.; Kohara, K.; Mikami, H.; Katahira, K.; Tsunetoshi, T.; Higashimori, K.; Ohishi, M.; Yo, Y.; Ogihara, T. Spectral change in heart rate variability in response to mental arithmetic before and after the beta-adrenoceptor blocker, carteolol. Clin. Auton. Res. 1992, 2, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Verkruysse, W.; Svaasand, L.O.; Nelson, J.S. Remote plethysmographic imaging using ambient light. Opt. Express 2008, 16, 21434–21445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, Y.; Wang, L. A review of non-contact, low-cost physiological information measurement based on photoplethysmographic imaging. In Proceedings of the 34th Annual International Conference of the IEEE Engineering in Medicine and Biology Society Conference (EMBC 2012), San Diego, CA, USA, 28 August–1 September 2012; pp. 2088–2091. [Google Scholar]
- Takano, C.; Ohta, Y. Heart rate measurement based on a time-lapse image. Med. Eng. Phys. 2007, 29, 853–857. [Google Scholar] [CrossRef]
- Alian, A.A.; Shelley, K.H. Photoplethysmography. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 395–406. [Google Scholar] [CrossRef]
- Tarassenko, L.; Villarroel, M.; Guazzi, A.; Jorge, J.; A Clifton, D.; Pugh, C. Non-contact video-based vital sign monitoring using ambient light and auto-regressive models. Physiol. Meas. 2014, 35, 807–831. [Google Scholar] [CrossRef]
- Vogels, T.; van Gastel, M.; Wang, W.; de Haan, G. Fully-automatic camera-based pulse-oximetry during sleep. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition Workshops (CVPRW2018), Salt Lake City, UT, USA, 18–22 June 2018; pp. 1349–1357. [Google Scholar]
- Cobos-Torres, J.-C.; Abderrahim, M.; Martinez-Orgado, J. Non-contact, simple neonatal monitoring by photoplethysmography. Sensors 2018, 18, 4362. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, A.; de Haan, G. Adaptive Gain Tuning for Robust Remote Pulse Rate Monitoring under Changing Light Conditions. Master’s Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 31 August 2014. [Google Scholar]
- Wang, W.W.; Stuijk, S.; De Haan, G.G. Exploiting Spatial Redundancy of Image Sensor for Motion Robust rPPG. IEEE Trans. Biomed. Eng. 2014, 62, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Ethan, B.; Justin, E. Effects of frame rate and image resolution on pulse rate measured using multiple camera imaging photoplethysmography. In Medical Imaging 2015: Biomedical Applications in Molecular, Structural, and Functional Imaging; International Society for Optics and Photonics: Bellingham, WA, USA; Volume 9417, p. 94172D.
- Poh, M.-Z.; McDuff, D.J.; Picard, R.W. Advancements in Noncontact, Multiparameter Physiological Measurements Using a Webcam. IEEE Trans. Biomed. Eng. 2010, 58, 7–11. [Google Scholar] [CrossRef] [Green Version]
- De Haan, G.; Jeanne, V. Robust pulse rate from chrominance-based rppg. IEEE Trans. Biomed. Eng. 2013, 60, 2878–2886. [Google Scholar] [CrossRef]
- De Haan, G.; Jeanne, V. Improved motion robustness of remote-PPG by using the blood volume pulse signature. Physiol. Meas. 2014, 35, 1913–1926. [Google Scholar] [CrossRef]
- Moco, A.V.; Stuijk, S.; de Haan, G. Ballistocardiographic Artifacts in PPG Imaging. IEEE Trans. Biomed. Eng. 2016, 63, 1804–1811. [Google Scholar] [CrossRef] [Green Version]
- Camm, A.J. Heart rate variability Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Kumar, M.; Veeraraghavan, A.; Sabharwal, A. DistancePPG: Robust non-contact vital signs monitoring using a camera. Biomed. Opt. Express 2015, 6, 1565–1588. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Brinker, A.; Stuijk, S.; de Haan, G. Algorithmic principles of remote PPG. IEEE Trans. Biomed. Eng. 2017, 64, 1479–1491. [Google Scholar] [CrossRef] [Green Version]
- Baltrusaitis, T.; Zadeh, A.; Lim, Y.C.; Morency, L.-P. OpenFace 2.0: Facial Behavior Analysis Toolkit. In Proceedings of the 13th IEEE International Conference on Automatic Face & Gesture Recognition Conference (FG 2018), Xi’an, China, 10–15 May 2018; pp. 59–66. [Google Scholar]
- Vezhnevets, V.; Sazonov, V.; Andreeva, A. A Survey on Pixel-Based Skin Color Detection Techniques. In Proceedings of the International Conference on Computer Graphics and Vision Conference, Moscow, Russia, 5–10 September 2003; pp. 85–92. [Google Scholar]
- Lin, Y.; Yuan-Hsiang, L. A study of color illumination effect on the SNR of rPPG signals. In Proceedings of the 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society Conference (EMBC2017), Jeju Island, Korea, 11–15 July 2017; pp. 4301–4304. [Google Scholar]
- Pai, A.; Veeraraghavan, A.; Sabharwal, A. CameraHRV Robust measurement of Heart Rate Variability using a Camera. Opt. Diagn. Sens. XVIII: Towar. Point-Care Diagn. 2018, 10501, 1–9. [Google Scholar]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV–Heart Rate Variability Analysis Software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Wang, W.; den Brinker, A.C.; Stuijk, S.; de Haan, G. Robust heart rate from fitness videos. Physiol. Meas. 2017, 38, 1023–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Gastel, M.; Stuijk, S.; de Haan, G. Motion robust remote-PPG in infrared. IEEE Trans. Biomed. Eng. 2015, 62, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, S.; Azorin-Peris, V.; Kalawsky, R.; Greenwald, S. Noncontact imaging photoplethysmography to effectively access pulse rate variability. J. Biomed. Opt. 2013, 18, 061205. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Condition | MAE of RRI [ms] | CORR of LF [-] | CORR of HF [-] | CORR of LF/HF [-] |
|---|---|---|---|---|
| FIX (fixed) | 23.9 | 0.967 | 0.967 | 0.976 |
| nFIX (stationary) | 26.1 | 0.796 | 0.796 | 0.643 |
| SPK (speaking) | 53.9 | 0.254 | 0.254 | 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohma, A.; Nishikawa, M.; Hashimoto, T.; Yamazaki, Y.; Sun, G. Evaluation of Remote Photoplethysmography Measurement Conditions toward Telemedicine Applications. Sensors 2021, 21, 8357. https://doi.org/10.3390/s21248357
Tohma A, Nishikawa M, Hashimoto T, Yamazaki Y, Sun G. Evaluation of Remote Photoplethysmography Measurement Conditions toward Telemedicine Applications. Sensors. 2021; 21(24):8357. https://doi.org/10.3390/s21248357
Chicago/Turabian StyleTohma, Akito, Maho Nishikawa, Takuya Hashimoto, Yoichi Yamazaki, and Guanghao Sun. 2021. "Evaluation of Remote Photoplethysmography Measurement Conditions toward Telemedicine Applications" Sensors 21, no. 24: 8357. https://doi.org/10.3390/s21248357
APA StyleTohma, A., Nishikawa, M., Hashimoto, T., Yamazaki, Y., & Sun, G. (2021). Evaluation of Remote Photoplethysmography Measurement Conditions toward Telemedicine Applications. Sensors, 21(24), 8357. https://doi.org/10.3390/s21248357






