Electrical Properties of Two Types of Membrane Component Used in Taste Sensors
Abstract
:1. Introduction
2. Materials and Methods
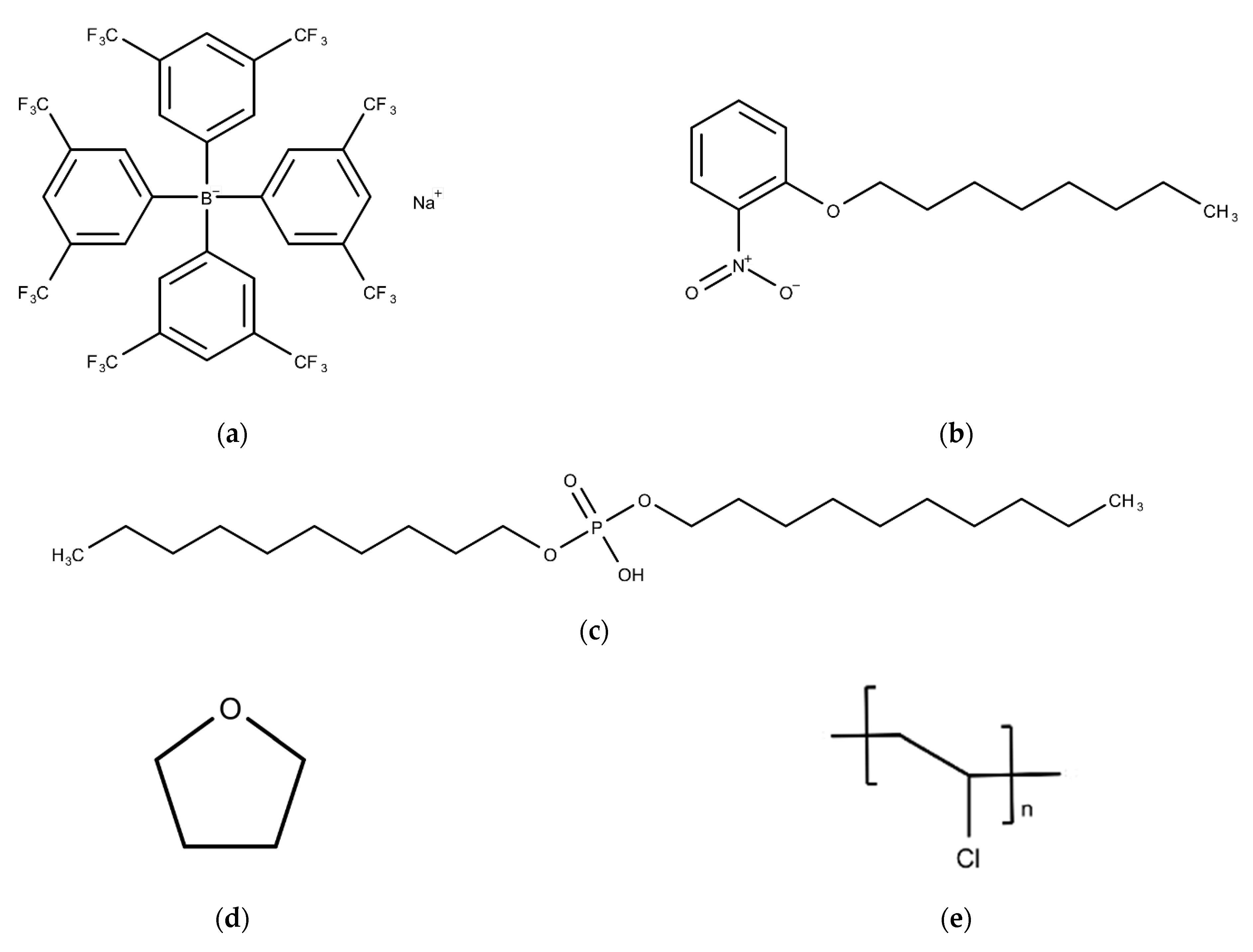
2.1. Reagents
2.2. Lipid Polymer Membrane
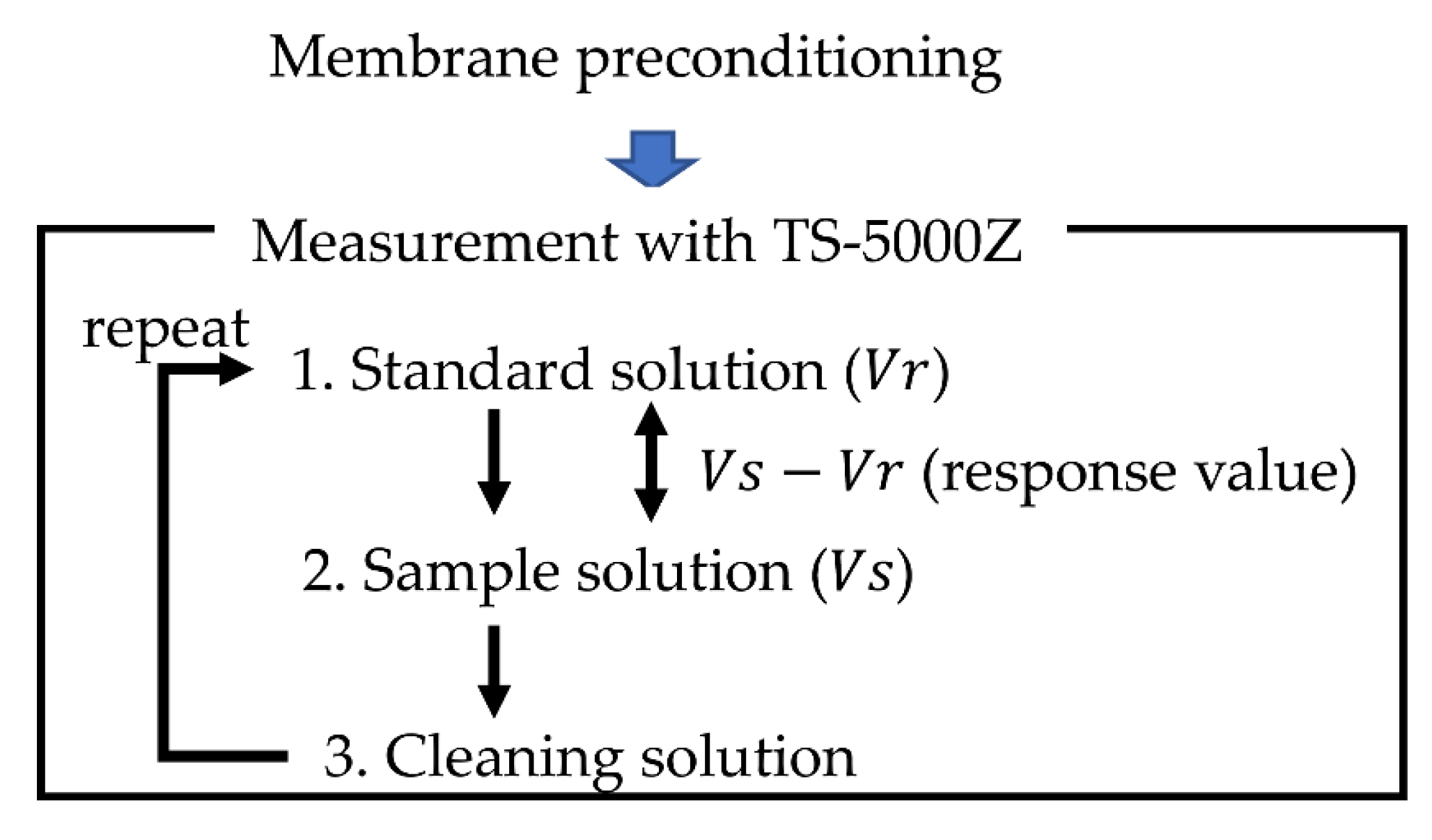
2.3. Measurement Procedure of Taste Sensor
2.4. Measurement of the Selectivity Coefficient
2.5. Measurement of Membrane Impedance
3. Results and Discussion
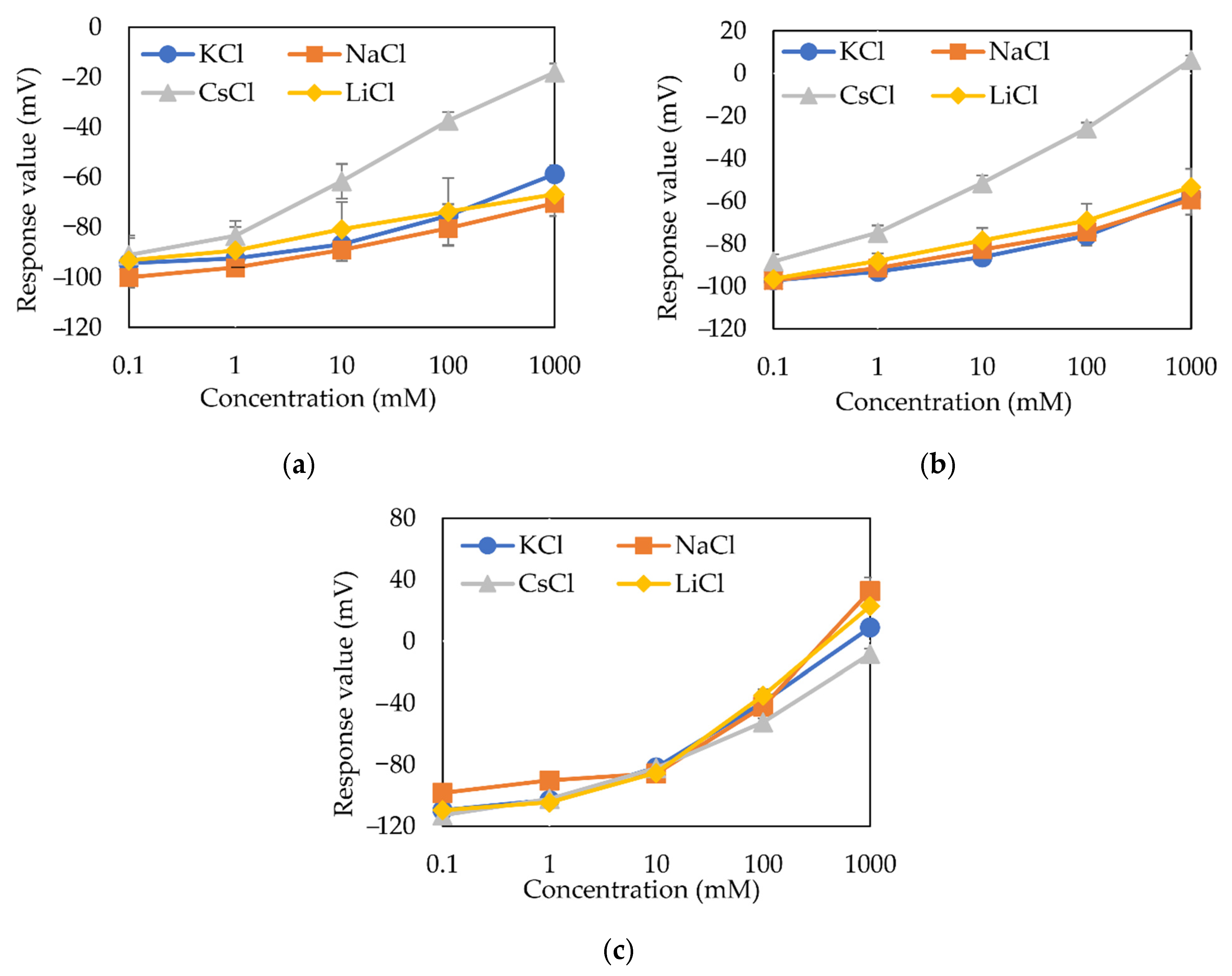
3.1. Sensor Response to Different Alkali Metals in the PADE Membrane
3.2. Sensor Response to Different Alkali Metals in the TFPB Membrane
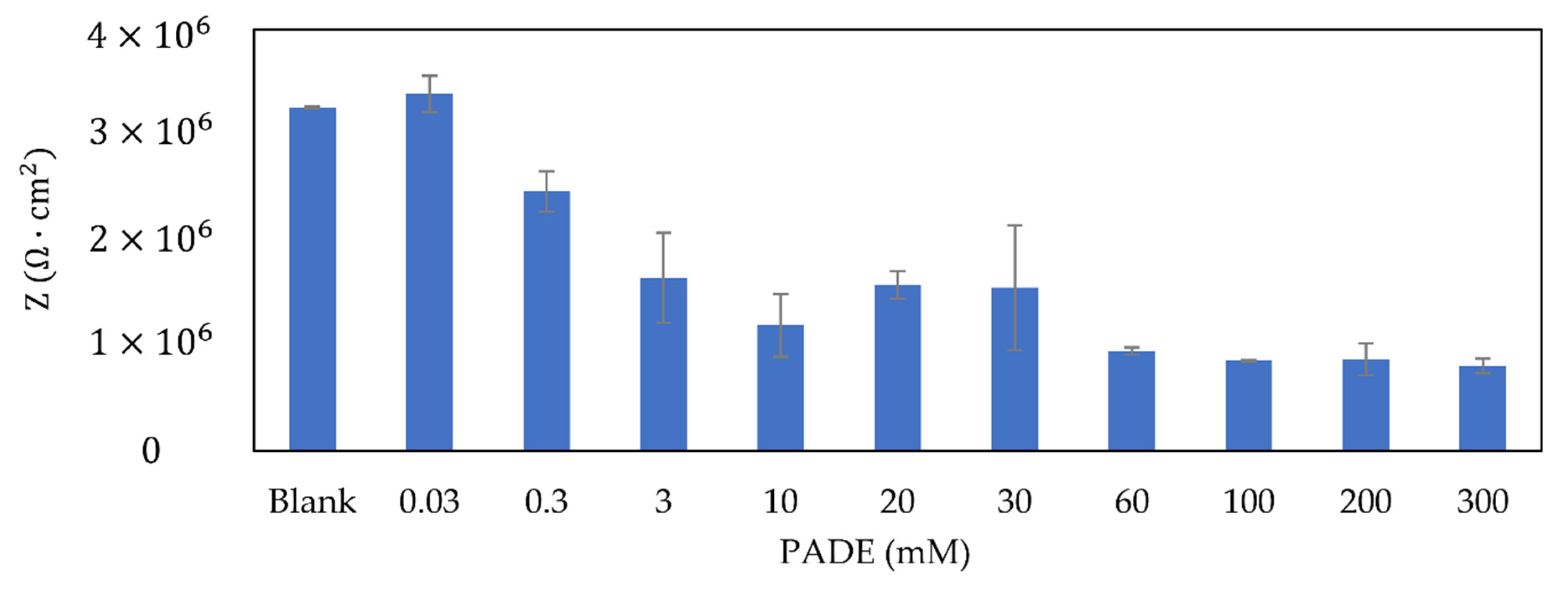
3.3. Selectivity Coefficient and Impedance of Different Concentrations of PADE Membrane
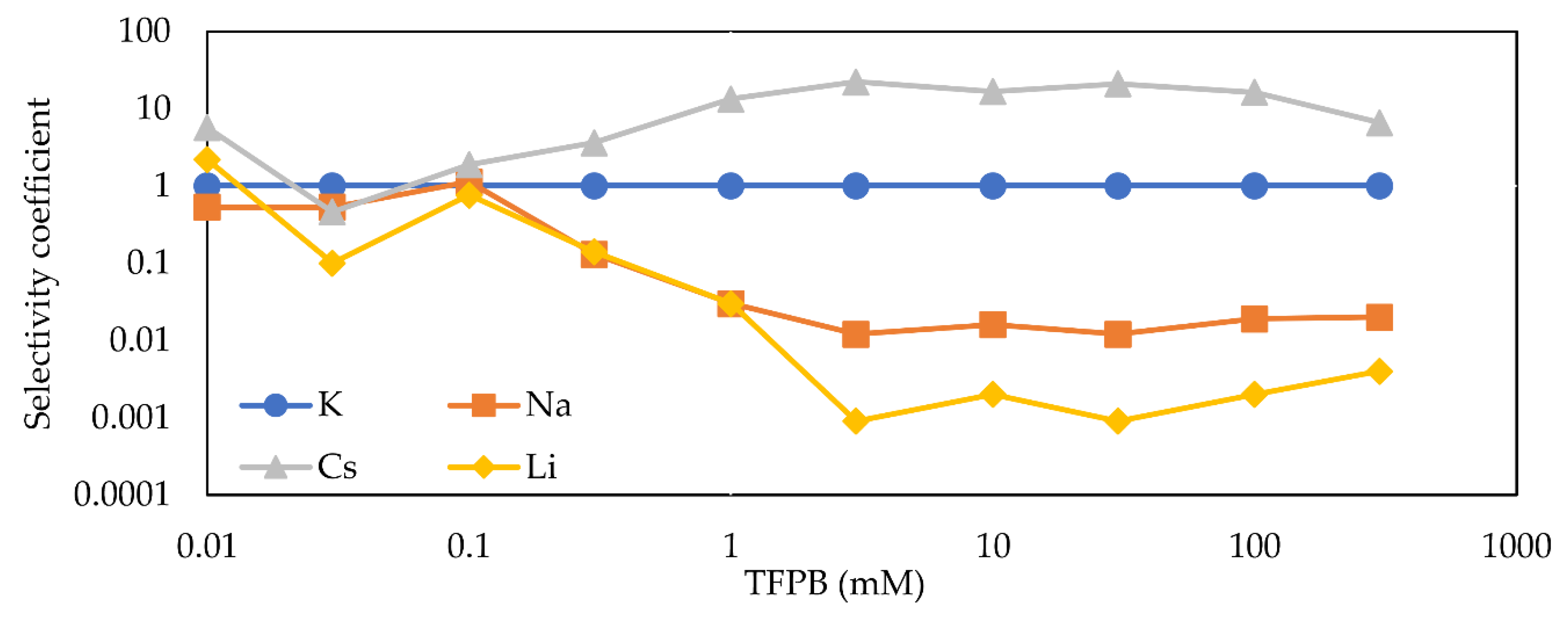
3.4. Selectivity Coefficient and Impedance of Different Concentrations of TFPB Membrane
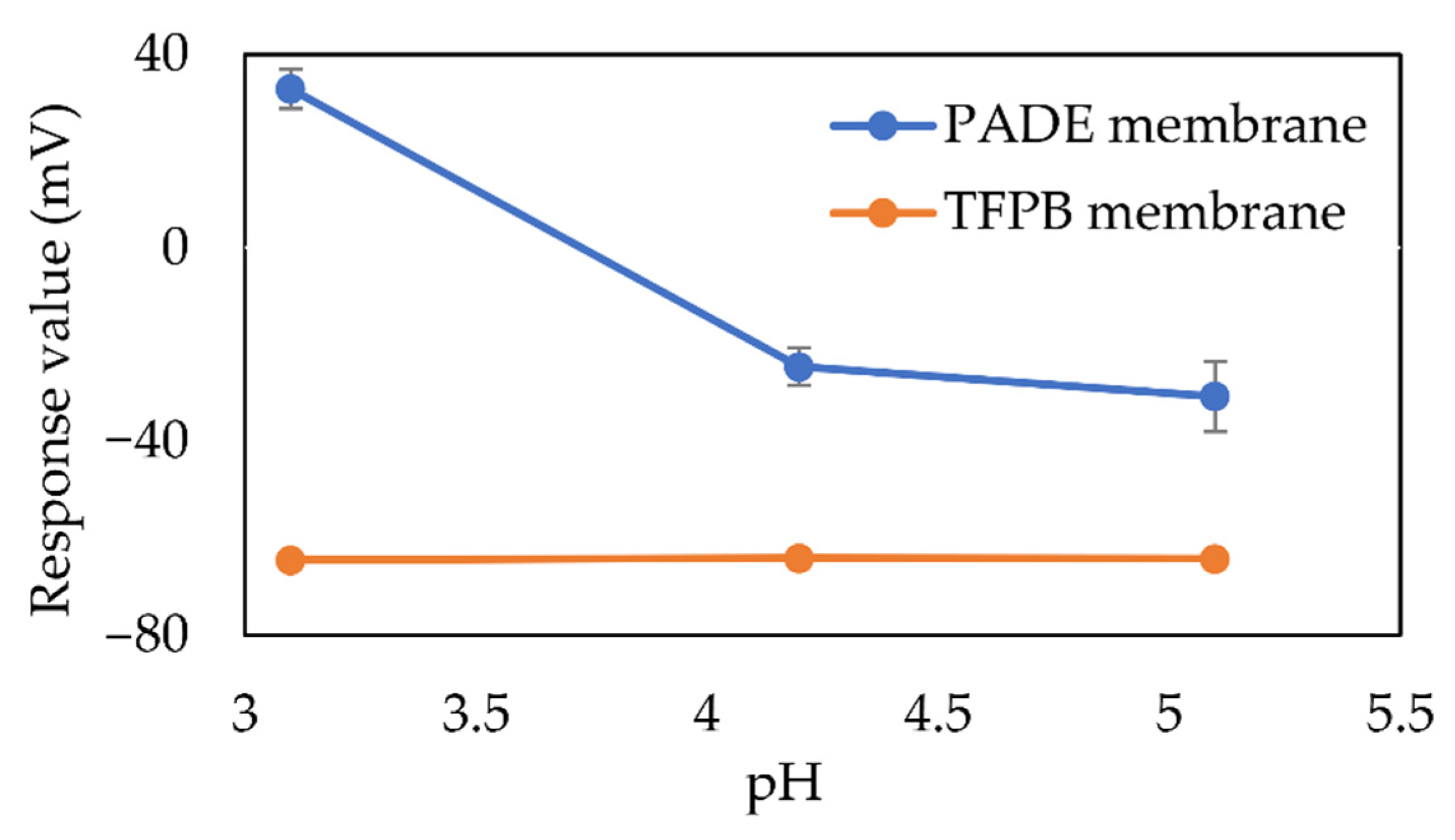
3.5. The Advantage of Using TFPB in the Taste Sensing System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Marco, R.; Clarke, G. Electrodes | Ion-selective electrodes. Encycl. Electrochem. Power Sources 2009, 103–109. [Google Scholar] [CrossRef]
- Kimura, K.; Maeda, T.; Tamura, H.; Shono, T. Potassium-selective PVC membrane electrodes based on bis- and poly(crown ether)s. J. Electroanal. Chem. 1979, 95, 91–101. [Google Scholar] [CrossRef]
- Suzuki, K.; Watanabe, K.; Matsumoto, Y.; Kobayashi, M.; Sato, S.; Siswanta, D.; Hisamoto, H. Design and synthesis of calcium and magnesium ionophores based on double-armed diazacrown ether compounds and their application to an ion sensing component for an ion-selective electrode. Anal. Chem. 1995, 67, 324–334. [Google Scholar] [CrossRef]
- Nishizawa, S.; Bühlmann, P.; Xiao, K.P.; Umezawa, Y. Application of a bis-thiourea ionophore for an anion selective electrode with a remarkable sulfate selectivity. Anal. Chim. Acta 1998, 358, 35–44. [Google Scholar] [CrossRef]
- Sutton, P.G.; Braven, J.; Ebdon, L.; Scholefield, D. Development of a sensitive nitrate-selective electrode for on-site use in fresh waters. Analyst 1999, 124, 877–882. [Google Scholar] [CrossRef]
- Vadgama, P. Multifunctional biosensor development and manufacture. Compr. Biotechnol. 2011, 81–94. [Google Scholar] [CrossRef]
- Schaller, U.; Bakker, E.; Pretsch, E. Carrier mechanism of acidic ionophores in solvent polymeric membrane ion-selective electrodes. Anal. Chem. 1995, 67, 3123–3132. [Google Scholar] [CrossRef]
- Dimeski, G.; Badrick, T.; John, A.S. Ion selective electrodes (ISEs) and interferences—A review. Clin. Chim. Acta 2010, 411, 309–317. [Google Scholar] [CrossRef]
- Frag, E.Y.Z.; Ali, T.A.; Mohamed, G.G.; Awad, Y.H.H. Construction of different types of ion-selective electrodes. characteristic performances and validation for direct potentiometric determination of orphenadrine citrate. Int. J. Electrochem. Sci. 2012, 7, 4443–4464. [Google Scholar]
- Wu, X.; Onitake, H.; Huang, Z.; Shiino, T.; Tahara, Y.; Yatabe, R.; Ikezaki, H.; Toko, K. Improved durability and sensitivity of bitterness-sensing membrane for medicines. Sensors 2017, 17, 2541. [Google Scholar] [CrossRef] [Green Version]
- Tahara, Y.; Toko, K. Electronic tongues-a review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Wu, X.; Tahara, Y.; Yatabe, R.; Toko, K. Taste sensor: Electronic tongue with lipid membranes. Anal. Sci. 2020, 36, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Yamanaka, M.; Toko, K.; Yamafuji, K. Multichannel taste sensor using lipid membranes. Sens. Actuators B Chem. 1990, 2, 205–213. [Google Scholar] [CrossRef]
- Toko, K. Taste sensor. Sens. Actuators B Chem. 2000, 64, 205–215. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, G.; Liu, W.; Zhou, Z. Beer taste detection based on electronic tongue. Sens. Mater. 2020, 32, 2949–2958. [Google Scholar] [CrossRef]
- Hayashi, N.; Ujihara, T.; Hayakawa, F.; Nakano, Y.; Kawakami, T.; Ikezaki, H. Standardization of tomato juice tastes using a taste sensor approach. Biosci. Biotechnol. Biochem. 2020, 84, 2569–2575. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, Y.; Tahara, Y.; Habara, M.; Ikezaki, H.; Toko, K. Reusability enhancement of taste sensor using lipid polymer membranes by surfactant cleaning treatment. IEEE Sens. J. 2020, 20, 4579–4586. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hamada, H.; Yamaguchi, Y.; Ikezaki, H.; Toko, K. Development of an artificial lipid-based membrane sensor with high selectivity and sensitivity to the bitterness of drugs and with high correlation with sensory score. IEEJ Trans. Electr. Electron. Eng. 2009, 4, 710–719. [Google Scholar] [CrossRef]
- Uchida, T.; Kobayashi, Y.; Miyanaga, Y.; Toukubo, R.; Ikezaki, H.; Taniguchi, A.; Nishikata, M.; Matsuyama, K. A new method for evaluating the bitterness of medicines by semi-continuous measurement of adsorption using a taste sensor. Chem. Pharm. Bull. 2001, 49, 1336–1339. [Google Scholar] [CrossRef]
- Nakatani, F.; Ienaga, T.; Wu, X.; Tahara, Y.; Ikezaki, H.; Sano, H.; Muto, Y.; Kaneda, Y.; Toko, K. Development of a sensor with a lipid/polymer membrane comprising Na+ ionophores to evaluate the saltiness enhancement effect. Sensors 2019, 19, 5251. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Shiino, T.; Tahara, Y.; Ikezaki, H.; Toko, K. Quantification of pharmaceutical bitterness using a membrane electrode based on a hydrophobic tetrakis [3,5-bis (Trifluoromethyl) phenyl] borate. Chemosensors 2021, 9, 28. [Google Scholar] [CrossRef]
- Strauss, S.H. The search for larger and more weakly coordinating anions. Chem. Rev. 1993, 93, 927–942. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sonoda, T.; Iwamoto, H.; Yoshimura, M. Tetrakis[3,5-di (F-methyl) phenyl]borate as the first efficient negatively charged phase transfer catalyst. kinetic evidences. Chem. Lett. 1981, 10, 579–580. [Google Scholar] [CrossRef] [Green Version]
- Nishida, H.; Takada, N.; Yoshimura, M.; Sonoda, T.; Kobayashi, H. Tetrakis[3,5-bis (trifluoromethyl) phenyl]borate. Highly lipophilic stable anionic agent for solvent-extraction of cations. Bull. Chem. Soc. Jpn. 1984, 57, 2600–2604. [Google Scholar] [CrossRef] [Green Version]
- Peper, S.; Gonczy, C. Potentiometric response characteristics of membrane-based Cs+ -selective electrodes containing ionophore-functionalized polymeric microspheres. Int. J. Electrochem. 2011, 2011, 276896. [Google Scholar] [CrossRef] [Green Version]
- Ying, K.S.; Heng, L.Y.; Hassan, N.I.; Hasbullah, S.A. A new copper ionophore N 1, N 3-bis [[3,5-bis (trifluoromethyl) phenyl] carbamothioyl] isophtalamide for potentiometric sensor. Sains Malays. 2018, 47, 2657–2666. [Google Scholar] [CrossRef]
- Yoshimatsu, J.; Toko, K.; Tahara, Y.; Ishida, M.; Habara, M.; Ikezaki, H.; Kojima, H.; Ikegami, S.; Yoshida, M.; Uchida, T. Development of taste sensor to detect non-charged bitter substances. Sensors 2020, 20, 3455. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recomendations for nomenclature of ion-selective electrodes (IUPAC recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Van den Berg, A.; Van der Wal, P.D.; Skowronska-Ptasińska, M.; Sudhölter, E.J.; Reinhoudt, D.N.; Bergveld, P. Nature of anionic sites in plasticized poly(vinyl chloride) membranes. Anal. Chem. 1987, 59, 2827–2829. [Google Scholar] [CrossRef] [Green Version]
- Cutler, S.G.; Meares, P.; Hall, D.G. Surfactant-sensitive polymeric membrane electrodes. J. Electroanal. Chem. 1977, 85, 145–161. [Google Scholar] [CrossRef]
- Watanabe, M.; Toko, K.; Sato, K.; Kina, K.; Takahashi, Y.; Iiyama, S. Charged impurities of plasticizer used for ion-selective electrode and taste sensor. Sens. Mater. 1998, 10, 103–112. [Google Scholar]
- Teorell, T. Transport processes and electrical phenomena in ionic membranes. Prog. Biophys. Biophys. Chem. 1953, 3, 305. [Google Scholar] [CrossRef]
- Kobatake, Y.; Toyoshima, Y.; Takeguchi, N. Studies of membrane phenomena. II. Theoretical study of membrane potentials. J. Phys. Chem. 1966, 70, 1187. [Google Scholar] [CrossRef]
- Kamo, N.; Oikawa, M.; Kobatake, Y. Effective fixed charge density governing membrane phenomena. V. A reduced expression of permselectivity. J. Phys. Chem. 1973, 77, 92–95. [Google Scholar] [CrossRef]
- Jyo, A.; Torikai, M.; Ishibashi, N. The selectivity evaluation of an ion-selective electrode with a liquid membrane by electrode response to a foreign ion. Bull. Chem. Soc. Jpn. 1974, 47, 2862–2868. [Google Scholar] [CrossRef] [Green Version]
- Helfferich, F. Equilibria. In Ion Exchange; Dover Publications: Mineola, NY, USA, 1995; p. 624. ISBN 9780486687841. [Google Scholar]
- Wegmann, D.; Weiss, H.; Ammann, D.; Morf, W.E.; Pretsch, E.; Sugahara, K.; Simon, W. Anion-selective liquid membrane electrodes based on lipophilic quaternary ammonium compounds. Microchim. Acta 1984, 84, 1–16. [Google Scholar] [CrossRef]
- Goddard, E.D.; Kao, O.; Kung, H.C. Counterion effects in charged monolayers. J. Colloid Interface Sci. 1968, 27, 616–624. [Google Scholar] [CrossRef]
- Rais, J. Some regularities in the free enthalpies of transfer of univalent ions from water to organic polar solvents. Collect. Czechoslov. Chem. Commun. 1971, 36, 3080–3087. [Google Scholar] [CrossRef]
- Toko, K.; Hara, D.; Tahara, Y.; Yasuura, M.; Ikezaki, H. Relationship between the amount of bitter substances adsorbed onto lipid/polymer membrane and the electric response of taste sensors. Sensors 2014, 14, 16274–16286. [Google Scholar] [CrossRef] [PubMed]






| Membrane | Concentration |
|---|---|
| Blank membrane | 0 (NPOE + PVC) |
| PADE membrane | 0.03, 0.3, 3, 10, 20, 30, 60, 100, 200, 300 mM |
| TFPB membrane | 0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, 100, 300 mM |
| Sample | Concentration |
|---|---|
| KCl | 0.1, 1, 10, 100, 1000 mM |
| NaCl | 0.1, 1, 10, 100, 1000 mM |
| LiCl | 0.1, 1, 10, 100, 1000 mM |
| CsCl | 0.1, 1, 10, 100, 1000 mM |
| Details | Parameter |
|---|---|
| Measure procedure | NOVA FRA impedance potentiostatic |
| Electrode configuration | Three electrode system |
| Electrolyte | 30 mM KCl + 0.3 mM tartaric acid |
| Sweeping frequencies | 0.1 Hz, 0.223 Hz, 0.5 Hz |
| Voltage | 200 mV |
| Membrane Component | Blank Membrane | PADE 0.03 mM | PADE 30 mM |
|---|---|---|---|
| Slope for KCl (mV) | 9.0 | 9.7 | 30.1 |
| Vr (mV) | 28.5 | 25.7 | −29.9 |
| Selectivity coefficients ( | |||
| 0 | 0 | 0 | |
| −0.3 | 0.2 | −0.2 | |
| 0.5 | 0.5 | −0.1 | |
| 1.2 | 1.2 | −0.1 | |
| Membrane Component | TFPB 0.03 mM | TFPB 30 mM |
|---|---|---|
| Slope for KCl (mV) | 14.9 | 51.4 |
| Vr (mV) | 78.5 | −109.9 |
| Selectivity coefficients ( | ||
| 0 | 0 | |
| −0.3 | −1.8 | |
| −1 | −2.7 | |
| −0.3 | 1.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Z.; Jing, Y.; Ikezaki, H.; Toko, K. Electrical Properties of Two Types of Membrane Component Used in Taste Sensors. Sensors 2021, 21, 8343. https://doi.org/10.3390/s21248343
Xiang Z, Jing Y, Ikezaki H, Toko K. Electrical Properties of Two Types of Membrane Component Used in Taste Sensors. Sensors. 2021; 21(24):8343. https://doi.org/10.3390/s21248343
Chicago/Turabian StyleXiang, Zhanyi, Yifei Jing, Hidekazu Ikezaki, and Kiyoshi Toko. 2021. "Electrical Properties of Two Types of Membrane Component Used in Taste Sensors" Sensors 21, no. 24: 8343. https://doi.org/10.3390/s21248343
APA StyleXiang, Z., Jing, Y., Ikezaki, H., & Toko, K. (2021). Electrical Properties of Two Types of Membrane Component Used in Taste Sensors. Sensors, 21(24), 8343. https://doi.org/10.3390/s21248343






