Pocket Mercury-Vapour Detection System Employing a Preconcentrator Based on Au-TiO2 Nanomaterials
Abstract
:1. Introduction
2. Materials and Methods
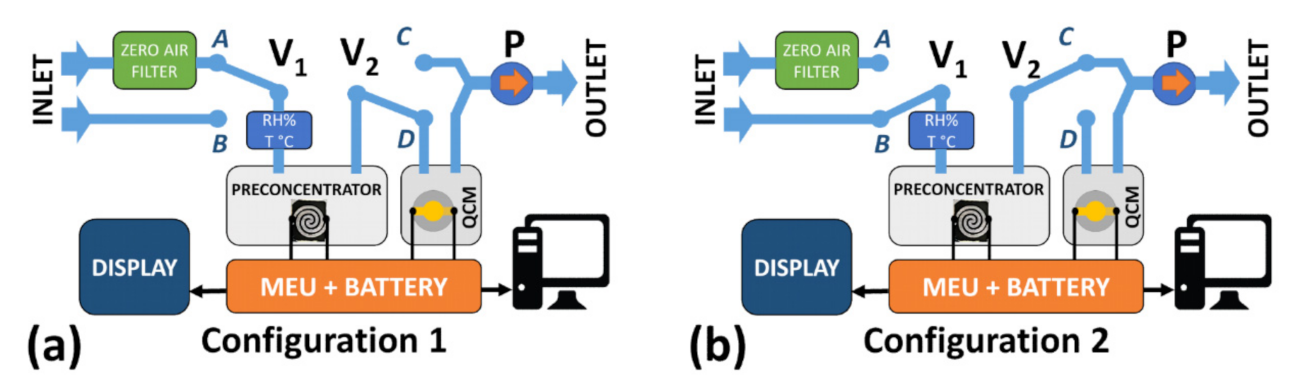
2.1. System Description
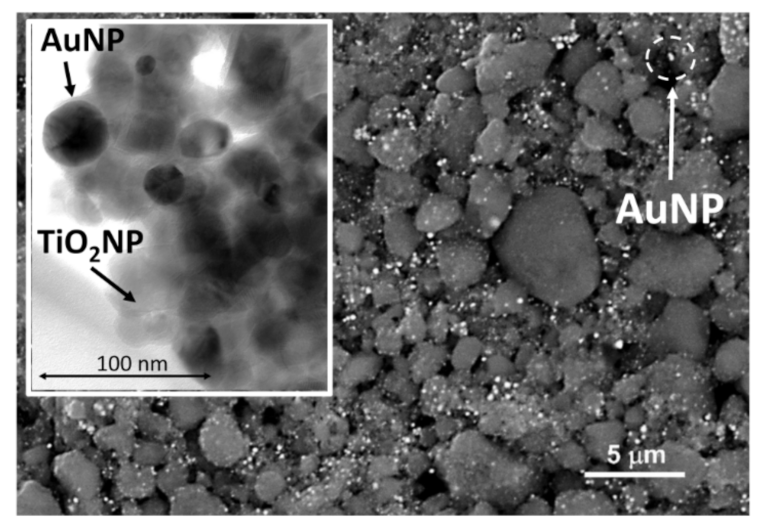
2.2. Adsorbent Material
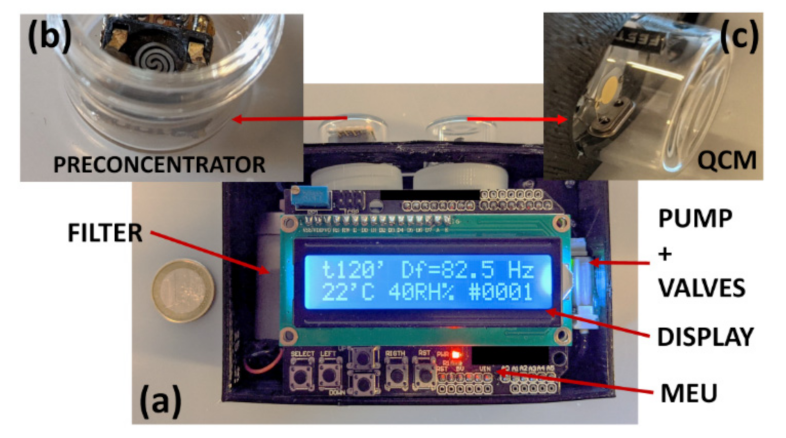
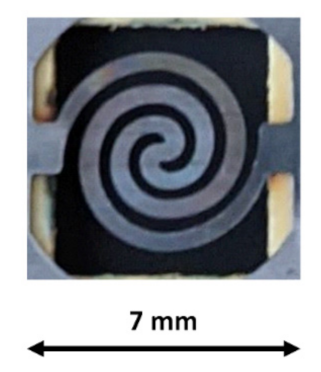
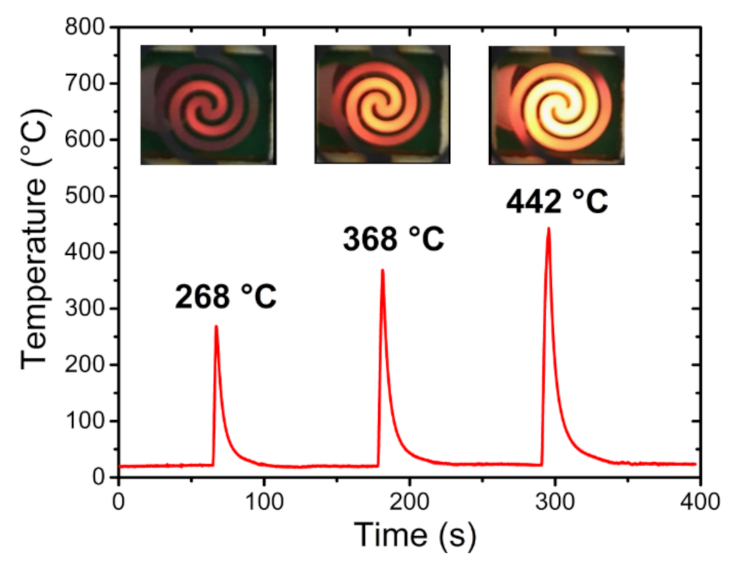
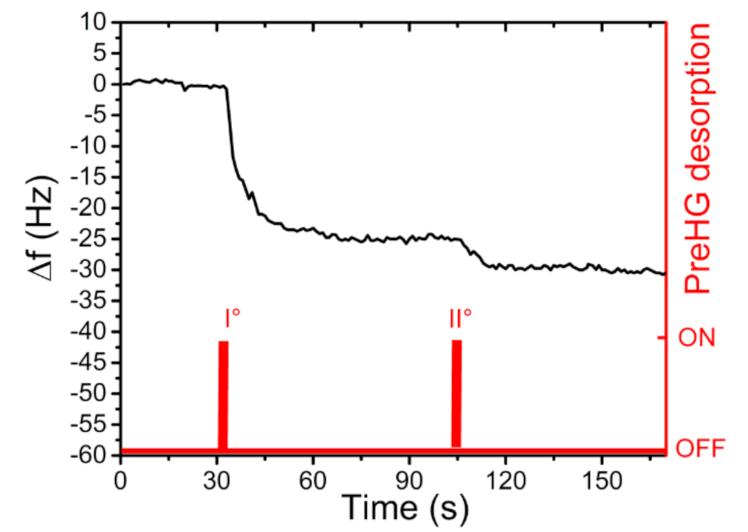
2.3. Preconcentrator
2.4. QCM Sensor
2.5. Main Electronic Unit
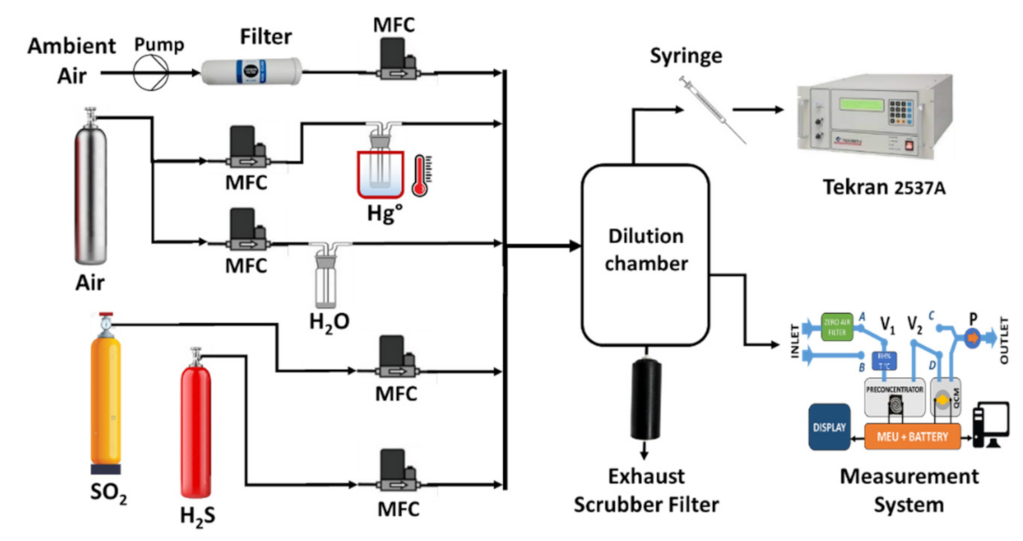
2.6. Measurement Setup
3. Results and Discussions
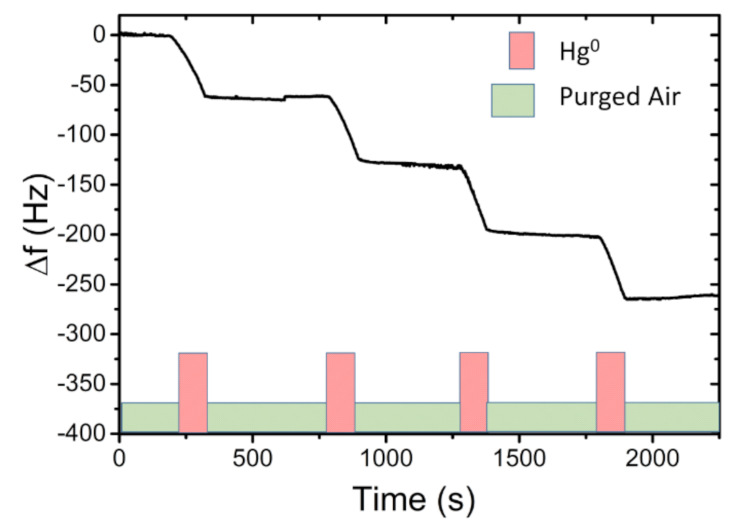
3.1. Mercury-Vapour Detection
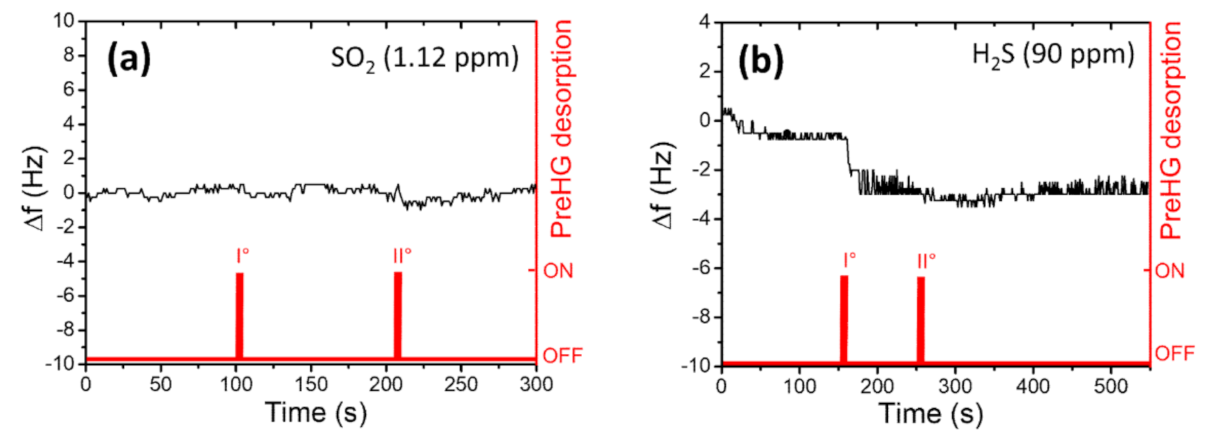
3.2. Tests of Interferers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cariccio, V.L.; Samà, A.; Bramanti, P.; Mazzon, E. Mercury Involvement in Neuronal Damage and in Neurodegenerative Diseases. Biol. Trace Elem. Res. 2018, 187, 341–356. [Google Scholar] [CrossRef]
- Park, J.D.; Wei, Z. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344. [Google Scholar] [CrossRef]
- Ratcliffe, H.E.; Swanson, G.M.; Fischer, L.J. Human Exposure to Mercury: A Critical Assessment of the Evidence of Adverse Health Effects. J. Toxicol. Environ. Health Part A 1996, 49, 221–270. [Google Scholar] [CrossRef]
- Soerensen, A.L.; Mason, R.P.; Balcom, P.H.; Jacob, D.J.; Zhang, Y.; Kuss, J.; Sunderland, E.M. Elemental mercury concentrations and fluxes in the tropical atmosphere and ocean. Environ. Sci. Technol. 2014, 48, 11312–11319. [Google Scholar] [CrossRef] [Green Version]
- Gustin, M.S.; Amos, H.M.; Huang, J.; Miller, M.B.; Heidecorn, K. Measuring and modeling mercury in the atmosphere: A critical review. Atmos. Chem. Phys. Discuss. 2015, 15, 5697–5713. [Google Scholar] [CrossRef] [Green Version]
- McLagan, D.S.; Mitchell, C.P.J.; Huang, H.; Lei, Y.D.; Cole, A.S.; Steffen, A.; Hung, H.; Wania, F. A High-Precision Passive Air Sampler for Gaseous Mercury. Environ. Sci. Technol. Lett. 2016, 3, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Macagnano, A.; Papa, P.; Avossa, J.; Perri, V.; Marelli, M.; Sprovieri, F.; Zampetti, E.; De Cesare, F.; Bearzotti, A.; Pirrone, N. Passive Sampling of Gaseous Elemental Mercury Based on a Composite TiO2NP/AuNP Layer. Nanomaterials 2018, 8, 798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLagan, D.S.; Monaci, F.; Huang, H.; Lei, Y.D.; Mitchell, C.P.J.; Wania, F. Characterization and Quantification of Atmospheric Mercury Sources Using Passive Air Samplers. J. Geophys. Res. Atmos. 2019, 124, 2351–2362. [Google Scholar] [CrossRef]
- Cordy, P.; Veiga, M.M.; Salih, I.; Al-Saadi, S.; Console, S.; Garcia, O.; Mesa, L.A.; Velásquez-López, P.C.; Roeser, M. Mercury contamination from artisanal gold mining in Antioquia, Colombia: The world’s highest per capita mercury pollution. Sci. Total Environ. 2011, 410-411, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Urba, A.; Kvietkus, K.; Sakalys, J.; Xiao, Z.; Lindqvist, O. A new sensitive and portable mercury vapor analyzer Gardis-1A. In Mercury as a Global Pollutant; Springer: Dordrecht, The Netherlands, 1995; pp. 1305–1309. [Google Scholar]
- Munthe, J.; Wängberg, I.; Pirrone, N.; Iverfeldt, Å.; Ferrara, R.; Ebinghaus, R.; Feng, X.; Gårdfeldt, K.; Keeler, G.; Lanzillotta, E.; et al. Intercomparison of methods for sampling and analysis of atmospheric mercury species. Atmos. Environ. 2001, 35, 3007–3017. [Google Scholar] [CrossRef]
- Black, O.; Chen, J.; Scircle, A.; Zhou, Y.; Cizdziel, J.V. Adaption and use of a quadcopter for targeted sampling of gaseous mercury in the atmosphere. Environ. Sci. Pollut. Res. 2018, 25, 13195–13202. [Google Scholar] [CrossRef]
- Manzo, C.; Mei, A.; Zampetti, E.; Bassani, C.; Paciucci, L.; Manetti, P. Top-down approach from satellite to terrestrial rover application for environmental monitoring of landfills. Sci. Total Environ. 2017, 584–585, 1333–1348. [Google Scholar] [CrossRef]
- Camara, E.; Breuil, P.; Briand, D.; de Rooij, N.; Pijolat, C. A micro gas preconcentrator with improved performance for pollution monitoring and explosives detection. Anal. Chim. Acta 2011, 688, 175–182. [Google Scholar] [CrossRef] [Green Version]
- Lara-Ibeas, I.; Cuevas, A.R.; Le Calvé, S. Recent developments and trends in miniaturized gas preconcentrators for portable gas chromatography systems: A review. Sens. Actuators B Chem. 2021, 346, 130449. [Google Scholar] [CrossRef]
- Rodríguez-Cuevas, A.; Lara-Ibeas, I.; Leprince, A.; Wolf, M.; Le Calvé, S. Easy-to-manufacture micro gas preconcentrator in-tegrated in a portable GC for enhanced trace detection of BTEX. Sens. Actuators B Chem. 2020, 324, 128690. [Google Scholar] [CrossRef]
- Dirri, F.; Palomba, E.; Longobardo, A.; Zampetti, E.; Saggin, B.; Scaccabarozzi, D. A review of quartz crystal microbalances for space applications. Sensors Actuators A Phys. 2019, 287, 48–75. [Google Scholar] [CrossRef]
- Sabri, Y.M.; Kandjani, A.E.; Ippolito, S.J.; Bhargava, S.K. Nanosphere Monolayer on a Transducer for Enhanced Detection of Gaseous Heavy Metal. ACS Appl. Mater. Interfaces 2015, 7, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Mortensen, J.; Komolov, A.; Denborg, J.; Møller, P.J. Polymer coated quartz crystal microbalance sensors for detection of volatile organic compounds in gas mixtures. Anal. Chim. Acta 2007, 597, 223–230. [Google Scholar] [CrossRef]
- Pascal-Delannoy, F.; Sorli, B.; Boyer, A. Quartz Crystal Microbalance (QCM) used as humidity sensor. Sens. Actuators A Phys. 2000, 84, 285–291. [Google Scholar] [CrossRef]
- Hou, T.; Chen, M.; Greene, G.W.; Horn, R.G. Mercury Vapor Sorption and Amalgamation with a Thin Gold Film. ACS Appl. Mater. Interfaces 2015, 7, 23172–23181. [Google Scholar] [CrossRef]
- Chudnenko, K.; Galina, P. Thermodynamic properties of Au–Hg binary solid solution. Thermochim. Acta 2013, 566, 175–180. [Google Scholar] [CrossRef]
- Kabir, K.M.M.; Sabri, Y.M.; Kandjani, A.E.; Matthews, G.I.; Field, M.; Jones, L.A.; Nafady, A.; Ippolito, S.; Bhargava, S.K. Mercury Sorption and Desorption on Gold: A Comparative Analysis of Surface Acoustic Wave and Quartz Crystal Microbalance-Based Sensors. Langmuir 2015, 31, 8519–8529. [Google Scholar] [CrossRef]
- Avossa, J.; De Cesare, F.; Papa, P.; Zampetti, E.; Bearzotti, A.; Marelli, M.; Pirrone, N.; Macagnano, A. Characteristics and Performances of a Nanostructured Material for Passive Samplers of Gaseous Hg. Sensors 2020, 20, 6021. [Google Scholar] [CrossRef]
- Naccarato, A.; Tassone, A.; Martino, M.; Moretti, S.; Macagnano, A.; Zampetti, E.; Papa, P.; Avossa, J.; Pirrone, N.; Nerentorp, M.; et al. A field intercomparison of three passive air samplers for gaseous mercury in ambient air. Atmos. Meas. Tech. Discuss. 2021, 14, 3657–3672. [Google Scholar] [CrossRef]
- Horvat, M. Mercury Analysis and Speciation in Environmental Samples. In Global and Regional Mercury Cycles: Sources, Fluxes and Mass Balances; Springer: Dordrecht, The Netherlands, 1996; pp. 1–31. [Google Scholar]
- Fialkowski, M.; Grzeszczak, P.; Nowakowski, A.R.; Holyst, R. Absorption of Mercury in Gold Films and Its Further Desorption: Quantitative Morphological Study of the Surface Patterns. J. Phys. Chem. B 2004, 108, 5026–5030. [Google Scholar] [CrossRef]
- Cartolano, M.; Xia, B.; Miriyev, A.; Lipson, H. Conductive Fabric Heaters for Heat-Activated Soft Actuators. Actuators 2019, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Bearzotti, A.; Macagnano, A.; Papa, P.; Venditti, I.; Zampetti, E. A study of a QCM sensor based on pentacene for the detection of BTX vapors in air. Sens. Actuators B Chem. 2017, 240, 1160–1164. [Google Scholar] [CrossRef]
- Black, P.; Richard, M.; Rossin, R.; Telmer, K. Assessing occupational mercury exposures and behaviours of artisanal and small-scale gold miners in Burkina Faso using passive mercury vapour badges. Environ. Res. 2017, 152, 462–469. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zampetti, E.; Papa, P.; Bearzotti, A.; Macagnano, A. Pocket Mercury-Vapour Detection System Employing a Preconcentrator Based on Au-TiO2 Nanomaterials. Sensors 2021, 21, 8255. https://doi.org/10.3390/s21248255
Zampetti E, Papa P, Bearzotti A, Macagnano A. Pocket Mercury-Vapour Detection System Employing a Preconcentrator Based on Au-TiO2 Nanomaterials. Sensors. 2021; 21(24):8255. https://doi.org/10.3390/s21248255
Chicago/Turabian StyleZampetti, Emiliano, Paolo Papa, Andrea Bearzotti, and Antonella Macagnano. 2021. "Pocket Mercury-Vapour Detection System Employing a Preconcentrator Based on Au-TiO2 Nanomaterials" Sensors 21, no. 24: 8255. https://doi.org/10.3390/s21248255
APA StyleZampetti, E., Papa, P., Bearzotti, A., & Macagnano, A. (2021). Pocket Mercury-Vapour Detection System Employing a Preconcentrator Based on Au-TiO2 Nanomaterials. Sensors, 21(24), 8255. https://doi.org/10.3390/s21248255






