Differences in the Asymmetry of Beat-to-Beat Fetal Heart Rate Accelerations and Decelerations at Preterm and Term Active Labor
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
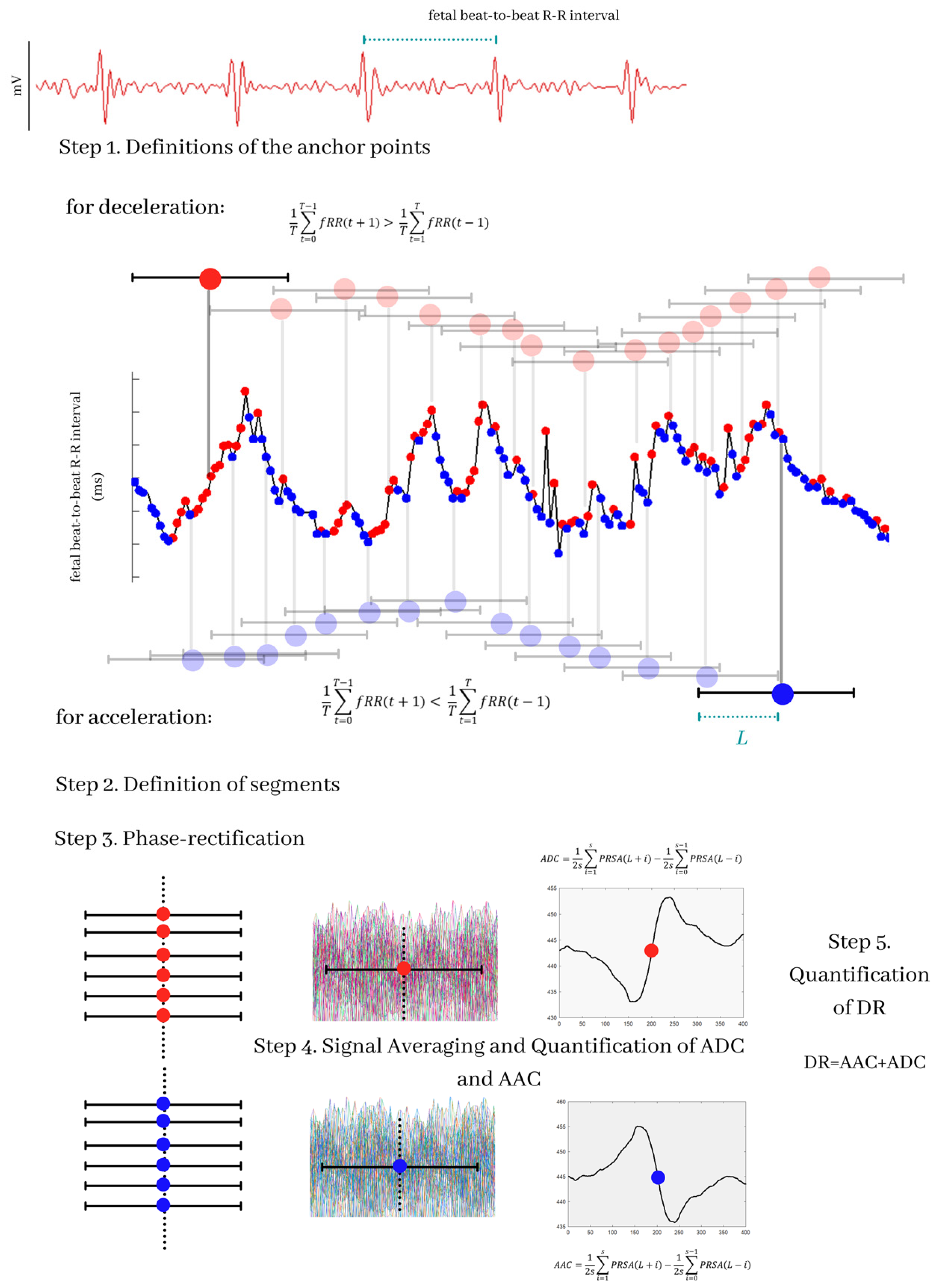
2.2. Segmentation of Fetal RR Time Series and Preprocessing
2.3. Definition of AAC and ADC
2.4. Deceleration Reserve (DR)
2.5. Multiscale Asymmetry Indices
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics
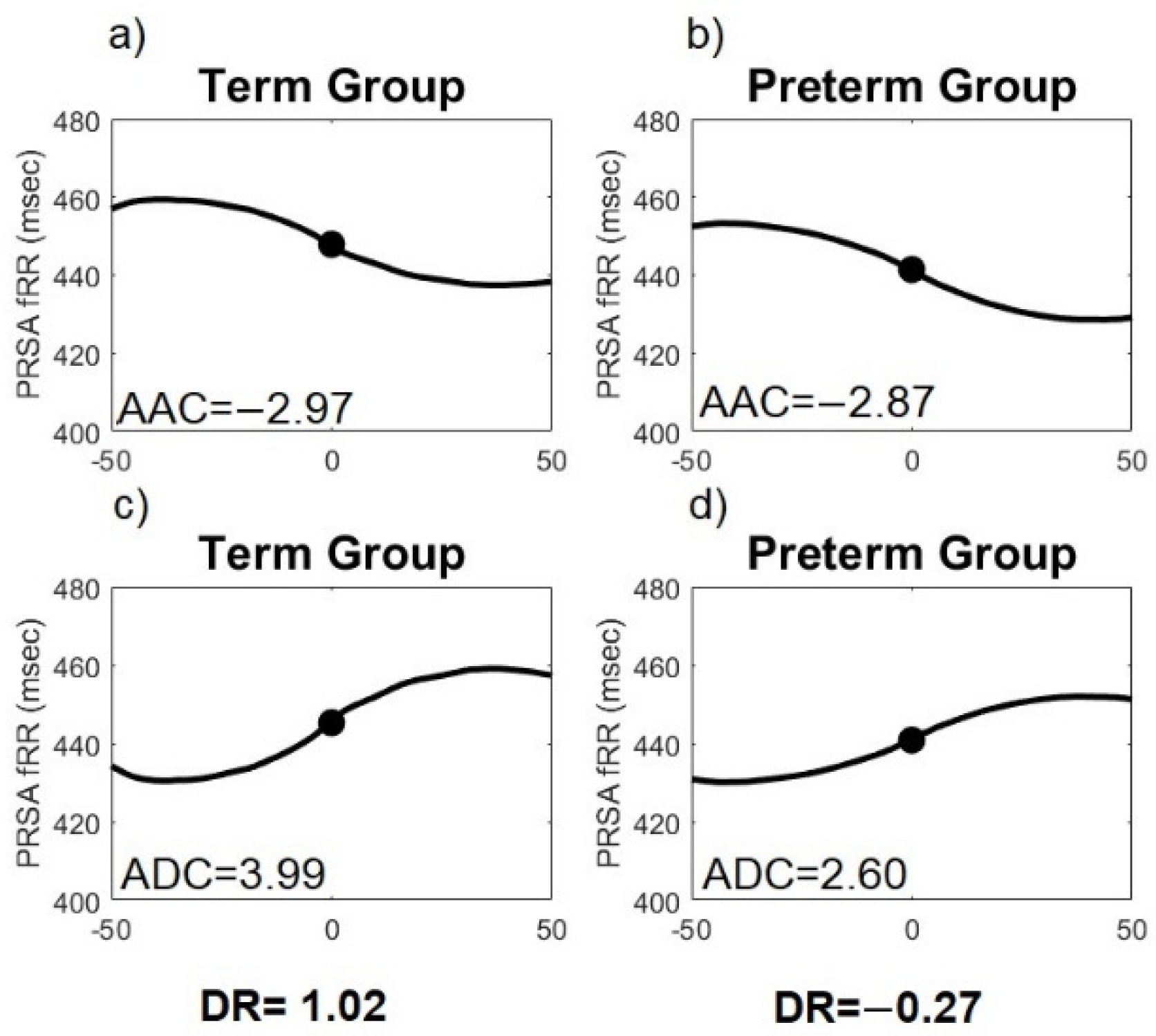
3.2. PRSA
3.3. Multiscale Asymmetry Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jelinek, H.F.; Cornforth, D.J.; Khandoker, A.H. Heart rate variability Standards of measurement, physiological interpretation, and clinical use. ECG Time Ser. Var. Anal Eng. Med. 2017, 1–12. [Google Scholar] [CrossRef]
- Ayres-De-Campos, D.; Spong, C.Y.; Chandraharan, E. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int. J. Gynecol. Obstet. 2015, 131, 13–24. [Google Scholar] [CrossRef]
- Sameni, R.; Clifford, G.D. A Review of Fetal ECG Signal Processing Issues and Promising Directions. Open Pacing Electrophysiol. Ther. J. 2010, 3, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M.; Mitchell, M.D.; Kumar, S.S. The physiology of intrapartum fetal compromise at term. Am. J. Obstet. Gynecol. 2020, 222, 17–26. [Google Scholar] [CrossRef]
- Cunningham, F.G.; Williams, J.W. Parto prematuro. In Williams Obstetricia; McGraw-Hill Interamericana: Ciudad de Mexico, Mexico, 2011; ISBN 9786071512772. [Google Scholar]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef]
- Donker, D.K.; van Geijn, H.P.; Hasman, A. Interobserver variation in the assessment of fetal heart rate recordings. Eur. J. Obstet. Gynecol. Reprod. Biol. 1993, 52, 21–28. [Google Scholar] [CrossRef]
- Beard, R.W.; Filshie, G.M.; Knight, C.A.; Roberts, G.M. The significance of the changes in the continuous fetal heart rate in the first stage of labour. BJOG An Int. J. Obstet. Gynaecol. 1971, 78, 865–881. [Google Scholar] [CrossRef]
- Campana, L.M.; Owens, R.L.; Clifford, G.D.; Pittman, S.D.; Malhotra, A. Phase-rectified signal averaging as a sensitive index of autonomic changes with aging. J. Appl. Physiol. 2010, 108, 1668–1673. [Google Scholar] [CrossRef]
- Kantelhardt, J.W.; Bauer, A.; Schumann, A.Y.; Barthel, P.; Schneider, R.; Malik, M.; Schmidt, G. Phase-rectified signal averaging for the detection of quasi-periodicities and the prediction of cardiovascular risk. Chaos 2007, 17, 015112. [Google Scholar] [CrossRef]
- Rivolta, M.W.; Stampalija, T.; Casati, D.; Richardson, B.S.; Ross, M.G.; Frasch, M.G.; Bauer, A.; Ferrazzi, E.; Sassi, R. Acceleration and deceleration capacity of fetal heart rate in an in-vivo sheep model. PLoS ONE 2014, 9, e104193. [Google Scholar] [CrossRef]
- Huhn, E.A.; Lobmaier, S.; Fischer, T.; Schneider, R.; Bauer, A.; Schneider, K.T.; Schmidt, G. New computerized fetal heart rate analysis for surveillance of intrauterine growth restriction. Prenat. Diagn. 2011, 31, 509–514. [Google Scholar] [CrossRef]
- Stampalija, T.; Casati, D.; Montico, M.; Sassi, R.; Rivolta, M.W.; Maggi, V.; Bauer, A.; Ferrazzi, E. Parameters influence on acceleration and deceleration capacity based on trans-abdominal ECG in early fetal growth restriction at different gestational age epochs. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 188, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.; Stampalija, T.; Casati, D.; Ferrazzi, E.; Bauer, A.; Rivolta, M.W. A methodological assessment of phase-rectified signal averaging through simulated beat-to-beat interval time series. Comput. Cardiol. 2014, 41, 601–604. [Google Scholar]
- Pan, Q.; Zhou, G.; Wang, R.; Cai, G.; Yan, J.; Fang, L.; Ning, G. Do the deceleration/acceleration capacities of heart rate reflect cardiac sympathetic or vagal activity? A model study. Med. Biol. Eng. Comput. 2016, 54, 1921–1933. [Google Scholar] [CrossRef] [PubMed]
- Rivolta, M.W.; Stampalija, T.; Frasch, M.G.; Sassi, R. Theoretical Value of Deceleration Capacity Points to Deceleration Reserve of Fetal Heart Rate. IEEE Trans. Biomed. Eng. 2019, 67, 1176–1185. [Google Scholar] [CrossRef]
- Rivolta, M.W.; Barbieri, M.; Stampalija, T.; Sassi, R.; Martin, G. Relationship between Deceleration Morphology and Phase Rectified Signal Averaging-based Parameters during Labor. bioRxiv 2021, 1–10. [Google Scholar] [CrossRef]
- Prigogine, I. Laws of nature, probability and time symmetry breaking. Phys. A Stat. Mech. Its Appl. 1999, 263, 528–539. [Google Scholar] [CrossRef]
- Chialvo, D.R.; Millonas, M.M. Asymmetric unbiased fluctuations are sufficient for the operation of a correlation ratchet. Phys. Lett. A 1995, 209, 26–30. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Broken asymmetry of the human heartbeat: Loss of time irreversibility in aging and disease. Phys. Rev. Lett. 2005, 95, 198102. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef]
- Karmakar, C.; Kimura, Y.; Palaniswami, M.; Khandoker, A. Analysis of fetal heart rate asymmetry before and after 35 weeks of gestation. Biomed. Signal. Process. Control 2015, 21, 43–48. [Google Scholar] [CrossRef]
- Wacker-Gussmann, A.; Plankl, C.; Sewald, M.; Schneider, K.T.M.; Oberhoffer, R.; Lobmaier, S.M. Fetal cardiac time intervals in healthy pregnancies—An observational study by fetal ECG (Monica Healthcare System). J. Perinat. Med. 2018, 46, 587–592. [Google Scholar] [CrossRef]
- Rauf, Z.; Alfirevic, Z. 666: Continuous remote fetal monitoring with MONICA AN24 during home induction of labor. Am. J. Obstet. Gynecol. 2011, 204, S263. [Google Scholar] [CrossRef]
- Reinhard, J.; Hayes-Gill, B.R.; Schiermeier, S.; Hatzmann, H.; Heinrich, T.M.; Louwen, F. Intrapartum heart rate ambiguity: A comparison of cardiotocogram and abdominal fetal electrocardiogram with maternal electrocardiogram. Gynecol. Obstet. Investig. 2013, 75, 101–108. [Google Scholar] [CrossRef]
- Wessel, N.; Voss, A.; Malberg, H.; Ziehmann, C.; Voss, H.U.; Schirdewan, A.; Meyerfeldt, U.; Kurths, J. Nonlinear analysis of complex phenomena in cardiological data. Herzschrittmacherther. Elektrophysiol. 2000, 11, 159–173. [Google Scholar] [CrossRef]
- Bauer, A.; Kantelhardt, J.W.; Barthel, P.; Schneider, R.; Mäkikallio, T.; Ulm, K.; Hnatkova, K.; Schömig, A.; Huikuri, H.; Bunde, A.; et al. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: Cohort study. Lancet 2006, 367, 1674–1681. [Google Scholar] [CrossRef]
- Lobmaier, S.M.; Mensing van Charante, N.; Ferrazzi, E.; Giussani, D.A.; Shaw, C.J.; Müller, A.; Ortiz, J.U.; Ostermayer, E.; Haller, B.; Prefumo, F.; et al. Phase-rectified signal averaging method to predict perinatal outcome in infants with very preterm fetal growth restriction—A secondary analysis of TRUFFLE-trial. Am. J. Obstet. Gynecol. 2016, 215, 630.e1–630.e7. [Google Scholar] [CrossRef]
- Pan, Q.; Zhou, G.; Wang, R.; Yu, Y.; Li, F.; Fang, L.; Yan, J.; Ning, G. The degree of heart rate asymmetry is crucial for the validity of the deceleration and acceleration capacity indices of heart rate: A model-based study. Comput. Biol. Med. 2016, 76, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.E.V.; Fazan, R.; Marin-Neto, J.A. PyBioS: A freeware computer software for analysis of cardiovascular signals. Comput. Methods Programs Biomed. 2020, 197, 105718. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Casali, K.R.; Casali, A.G.; Gnecchi-Ruscone, T.; Tobaldini, E.; Montano, N.; Lange, S.; Geue, D.; Cysarz, D.; Van Leeuwen, P. Temporal asymmetries of short-term heart period variability are linked to autonomic regulation. Am. J. Physiol.–Regul. Integr. Comp. Physiol. 2008, 295, 550–557. [Google Scholar] [CrossRef]
- Garabedian, C.; Butruille, L.; Servan-Schreiber, E.; Ficheur, G.; Storme, L.; Deruelle, P.; De Jonckheere, J.; Houfflin-Debarge, V. Fetal Heart-Rate Variability: Validation of a New Continuous, Noninvasive Computerized Analysis. Gynecol. Obstet. Investig. 2017, 82, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Van Scheepen, J.A.M.; Koster, M.P.H.; Vasak, B.; Redman, C.; Franx, A.; Georgieva, A. Effect of signal acquisition method on the fetal heart rate analysis with phase rectified signal averaging. Physiol. Meas. 2016, 37, 2245–2259. [Google Scholar] [CrossRef]
- Weyrich, J.; Setter, A.; Müller, A.; Schmidt, G.; Brambs, C.E.; Ortiz, J.U.; Lobmaier, S.M. Longitudinal progression of fetal short-term variation and average acceleration and deceleration capacity after antenatal maternal betamethasone application. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 85–90. [Google Scholar] [CrossRef]
- Montalvo-Jaramillo, C.I.; Pliego-Carrillo, A.C.; Peña-Castillo, M.Á.; Echeverría, J.C.; Becerril-Villanueva, E.; Pavón, L.; Ayala-Yáñez, R.; González-Camarena, R.; Berg, K.; Wessel, N.; et al. Comparison of fetal heart rate variability by symbolic dynamics at the third trimester of pregnancy and low-risk parturition. Heliyon 2020, 6, e03485. [Google Scholar] [CrossRef] [PubMed]
- Nagel, C.; Aurich, J.; Trenk, L.; Ille, N.; Drillich, M.; Pohl, W.; Aurich, C. Stress response and cardiac activity of term and preterm calves in the perinatal period. Theriogenology 2016, 86, 1498–1505. [Google Scholar] [CrossRef]
- Kramarić, K.; Šapina, M.; Garcin, M.; Milas, K.; Pirić, M.; Brdarić, D.; Lukić, G.; Milas, V.; Pušeljić, S. Heart rate asymmetry as a new marker for neonatal stress. Biomed. Signal. Process Control 2019, 47, 219–223. [Google Scholar] [CrossRef]
- Burykin, A.; Costa, M.D.; Peng, C.-K.; Goldberger, A.L.; Buchman, T.G. Generating signals with multiscale time irreversibility: The asymmetric weierstrass function. Complexity 2011, 16, 29–38. [Google Scholar] [CrossRef][Green Version]
- Frank, J.; Seifert, G.; Schroeder, R.; Gruhn, B.; Stritter, W.; Jeitler, M.; Steckhan, N.; Kessler, C.S.; Michalsen, A.; Voss, A. Yoga in school sports improves functioning of autonomic nervous system in young adults: A non-randomized controlled pilot study. PLoS ONE 2020, 15, e0231299. [Google Scholar] [CrossRef]
- Hurtado-Sánchez, M.F.; Pérez-Melero, D.; Pinto-Ibáñez, A.; González-Mesa, E.; Mozas-Moreno, J.; Puertas-Prieto, A. Characteristics of heart rate tracings in preterm fetus. Medicina 2021, 57, 528. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, C.K.; Jelinek, H.F.; Warner, P.; Khandoker, A.H.; Palaniswami, M. Effect of gender and diabetes on major depressive disorder using heart rate asymmetry. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 6679–6682. [Google Scholar] [CrossRef]
- Lucchini, M.; Widjaja, D.; Varon, C.; Jansen, K.; Van Huffel, S. Phase-rectified signal averaging to evaluate ANS development in premature infants. In Proceedings of the International Conference on Bio-Inspired Systems and Signal Processing, Barcelona, Spain, 11–14 February 2013; pp. 203–208. [Google Scholar] [CrossRef]
- Weyrich, J.; Ortiz, J.U.; Müller, A.; Schmidt, G.; Brambs, C.E.; Graupner, O.; Kuschel, B.; Lobmaier, S.M. Intrapartum PRSA: A new method to predict fetal acidosis?-a case-control study. Arch. Gynecol. Obstet. 2020, 301, 137–142. [Google Scholar] [CrossRef]
- Sholapurkar, S.L. The present and future of intrapartum computerized cardiotocography: Role of pattern recognition incorporating single vs. multiple parameters. J. Matern. Neonatal Med. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dia, N.; Fontecave-Jallon, J.; Resendiz, M.; Faisant, M.-C.; Equy, V.; Riethmuller, D.; Gumery, P.-Y.; Rivet, B. Fetal heart rate estimation by non-invasive single abdominal electrocardiography in real clinical conditions. Biomed. Signal Process Control 2022, 71, 103187. [Google Scholar] [CrossRef]
- Furukawa, A.; Neilson, D.; Hamilton, E. Cumulative deceleration area: A simplified predictor of metabolic acidemia. J. Matern. Neonatal Med. 2021, 34, 3104–3111. [Google Scholar] [CrossRef]

| Term | Preterm | |
|---|---|---|
| (n = 27) | (n = 17) | |
| Maternal age (years) | 21 ± 4 | 21 ± 4 |
| Weeks of gestation (weeks, USG) | a 39 ± 1 | 34 ± 2 |
| Maternal BMI (kg/cm2) | 24.3 ± 1.3 | 25 ± 2.8 |
| Cervical dilatation (cm) | 5.9 ± 1.6 | 5.0 ± 1.7 |
| Cervical effacement (%) | 71 ± 12 | 62 ± 13 |
| Newborn birth weight (kg) | a 2.9 ± 0.4 | 2.4 ± 0.6 |
| APGAR score 1 min (>7) | 96% | 80% |
| APGAR score 5 min (>7) | 96% | 70% |
| Head circumference (cm) | a 33.7 ± 1.73 | 32.0 ± 2.29 |
| Fetal size (cm) | a 49.5 ± 2.1 | 45.1 ± 6.0 |
| Gender (male percentage) | 52% | 50% |
| R-R mean (ms) | a 431.2 ± 31.0 | 413.2 ± 26.9 |
| T | s | Term | Preterm | p-Value |
|---|---|---|---|---|
| n = 27 | n = 17 | |||
| 40 | 1 | 0.02 (−0.00, 0.05) | 0.01 (−0.02, 0.04) | 0.0470 |
| 40 | 2 | 0.04 (−0.00, 0.09) | 0.00 (−0.03, 0.07) | 0.0487 |
| 40 | 3 | 0.07 (0.00, 0.14) | 0.00 (−0.05, 0.11) | 0.0483 |
| 40 | 4 | 0.09 (0.00, 0.19) | 0.01 (−0.07, 0.14) | 0.0496 |
| 45 | 1 | 0.02 (−0.01, 0.04) | −0.00 (−0.02, 0.03) | 0.0296 |
| 45 | 2 | 0.03 (−0.01, 0.09) | −0.01 (−0.04, 0.06) | 0.0277 |
| 45 | 3 | 0.05 (−0.02, 0.13) | −0.01 (−0.06, 0.08) | 0.0294 |
| 45 | 4 | 0.07 (−0.02, 0.02) | −0.02 (−0.08, 0.11) | 0.0324 |
| 45 | 5 | 0.08 (−0.03, 0.21) | −0.02 (−0.10, 0.13) | 0.0342 |
| 45 | 6 | 0.09 (−0.04, 0.25) | −0.02 (−0.12, 0.15) | 0.0359 |
| 45 | 7 | 0.11 (−0.05, 0.28) | −0.03 (−0.14, 0.18) | 0.0380 |
| 45 | 8 | 0.13 (−0.05, 0.31) | −0.03 (−0.16, 0.20) | 0.0397 |
| 45 | 9 | 0.14 (−0.06, 0.34) | −0.04 (−0.17, 0.22) | 0.0413 |
| 45 | 10 | 0.17 (−0.04, 0.48) | −0.04 (−0.19, 0.23) | 0.0191 |
| 50 | 1 | 0.02 (−0.01, 0.05) | −0.00 (−0.02, 0.02) | 0.0147 |
| 50 | 2 | 0.03 (−0.02, 0.10) | −0.01 (−0.05, 0.05) | 0.0162 |
| 50 | 3 | 0.04 (−0.03, 014) | −0.01 (−0.09, 0.08) | 0.0186 |
| 50 | 4 | 0.06 (−0.04, 0.19) | −0.02 (−0.11, 0.10) | 0.0208 |
| 50 | 5 | 0.07 (−0.04, 0.23) | −0.02 (−0.13, 0.12) | 0.0223 |
| 50 | 6 | 0.07 (−0.04, 0.28) | −0.03 (−0.15, 0.14) | 0.0236 |
| 50 | 7 | 0.06 (−0.05, 0.31) | −0.02 (−0.17, 0.16) | 0.0252 |
| 50 | 8 | 0.06 (−0.06, 0.35) | −0.03 (−0.19, 0.18) | 0.0266 |
| 50 | 9 | 0.06 (−0.06, 0.38) | −0.03 (−0.21, 0.21) | 0.0280 |
| 50 | 10 | 0.09 (−0.05, 0.49) | −0.03 (−0.23, 0.22) | 0.0131 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Justo, C.; Pliego-Carrillo, A.C.; Ledesma-Ramírez, C.I.; Mendieta-Zerón, H.; Peña-Castillo, M.Á.; Echeverría, J.C.; Rodríguez-Arce, J.; Reyes-Lagos, J.J. Differences in the Asymmetry of Beat-to-Beat Fetal Heart Rate Accelerations and Decelerations at Preterm and Term Active Labor. Sensors 2021, 21, 8249. https://doi.org/10.3390/s21248249
López-Justo C, Pliego-Carrillo AC, Ledesma-Ramírez CI, Mendieta-Zerón H, Peña-Castillo MÁ, Echeverría JC, Rodríguez-Arce J, Reyes-Lagos JJ. Differences in the Asymmetry of Beat-to-Beat Fetal Heart Rate Accelerations and Decelerations at Preterm and Term Active Labor. Sensors. 2021; 21(24):8249. https://doi.org/10.3390/s21248249
Chicago/Turabian StyleLópez-Justo, Carolina, Adriana Cristina Pliego-Carrillo, Claudia Ivette Ledesma-Ramírez, Hugo Mendieta-Zerón, Miguel Ángel Peña-Castillo, Juan Carlos Echeverría, Jorge Rodríguez-Arce, and José Javier Reyes-Lagos. 2021. "Differences in the Asymmetry of Beat-to-Beat Fetal Heart Rate Accelerations and Decelerations at Preterm and Term Active Labor" Sensors 21, no. 24: 8249. https://doi.org/10.3390/s21248249
APA StyleLópez-Justo, C., Pliego-Carrillo, A. C., Ledesma-Ramírez, C. I., Mendieta-Zerón, H., Peña-Castillo, M. Á., Echeverría, J. C., Rodríguez-Arce, J., & Reyes-Lagos, J. J. (2021). Differences in the Asymmetry of Beat-to-Beat Fetal Heart Rate Accelerations and Decelerations at Preterm and Term Active Labor. Sensors, 21(24), 8249. https://doi.org/10.3390/s21248249







