3D Electrochemical Sensor and Microstructuration Using Aerosol Jet Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Design and Fabrication
2.2. Physical and Electrochemical Evaluations
3. Results and Discussion
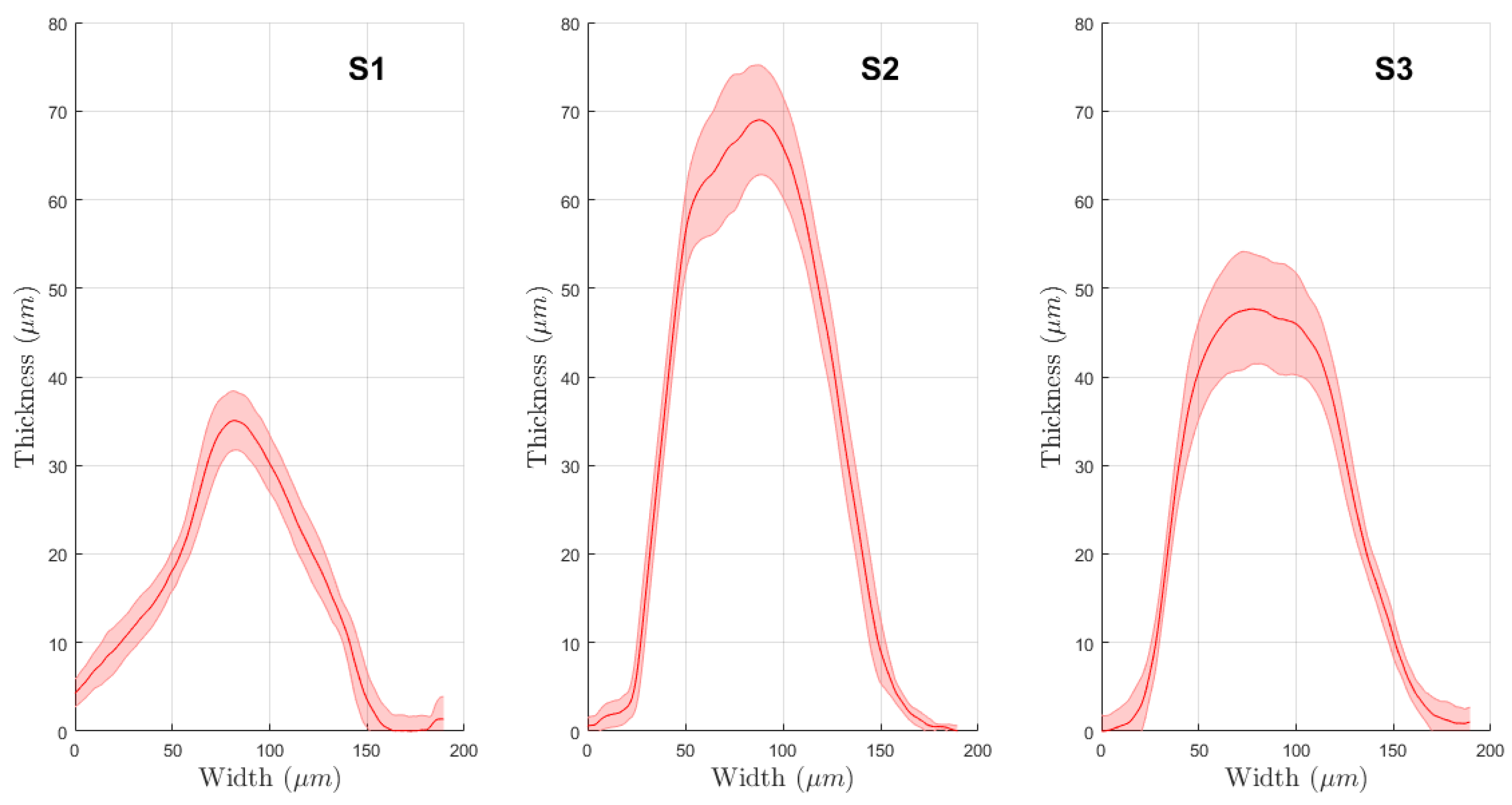
3.1. Physical Evaluations
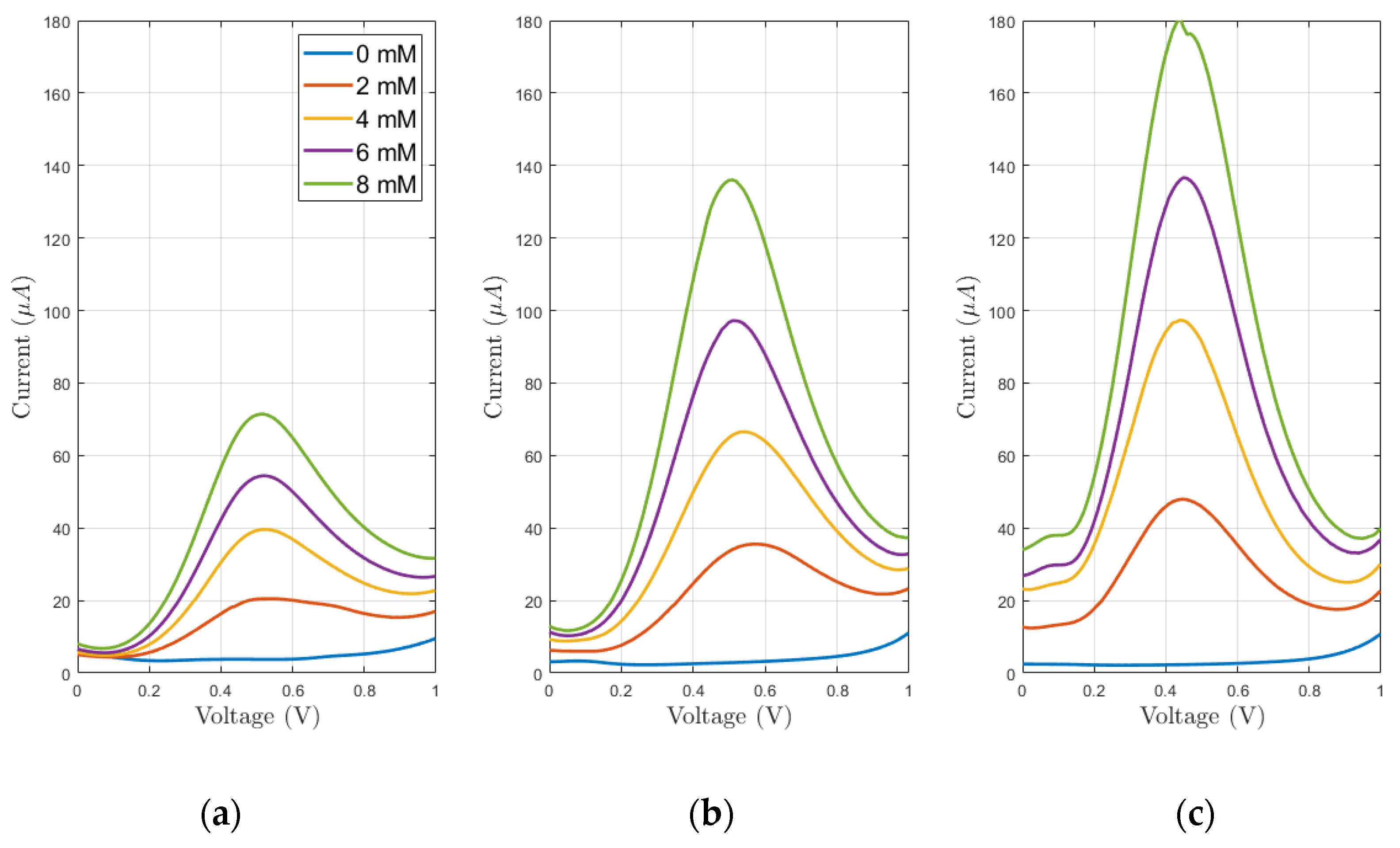
3.2. Preliminary Electrochemical Tests
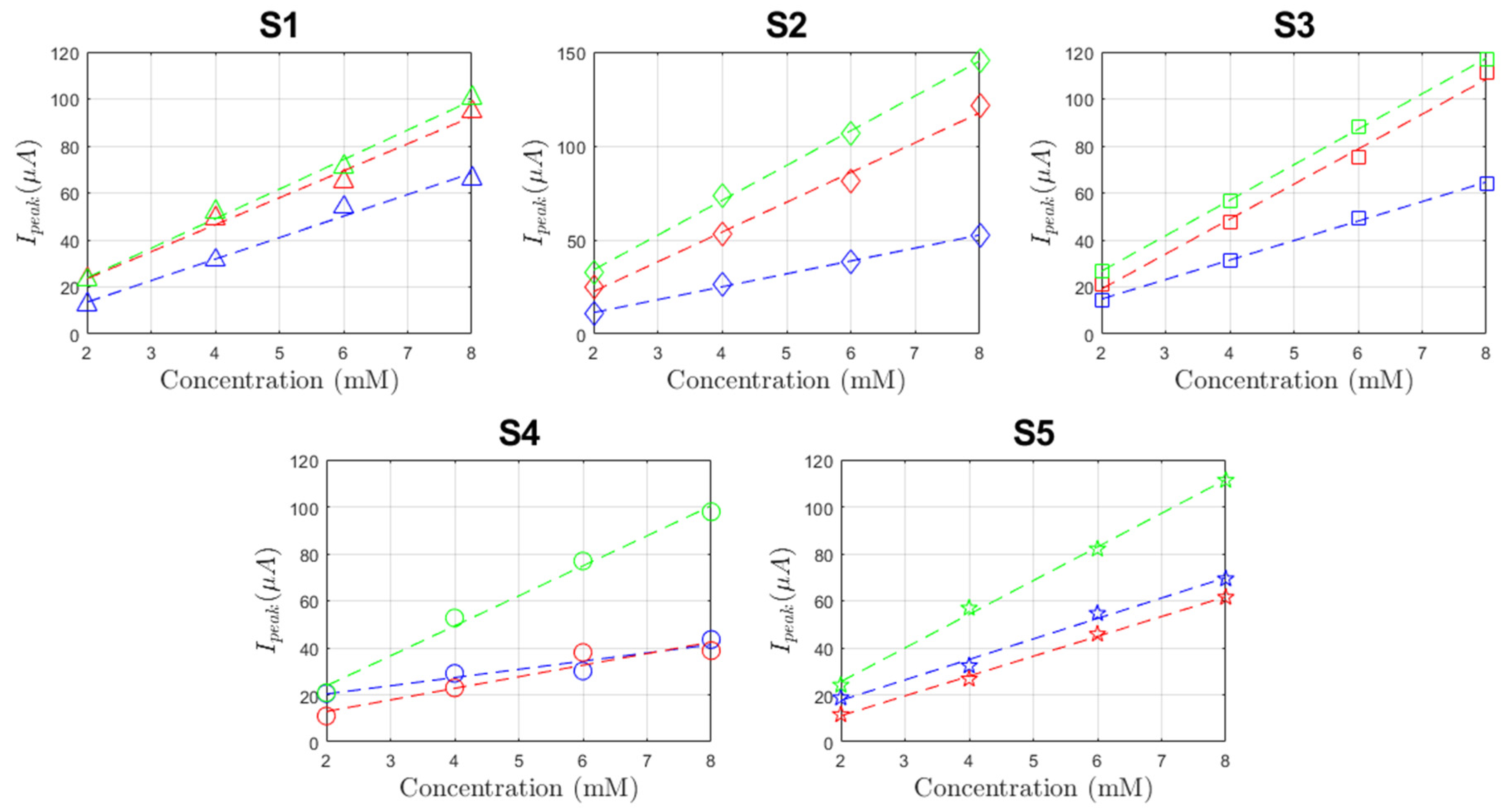
3.3. Electrochemical Evaluations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, J.; Zhang, Z. Application of electrochemical sensors based on carbon nanomaterials for detection of flavonoids. Nanomaterials 2020, 10, 2020. [Google Scholar] [CrossRef]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: A review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Vargas, G.; Sosa-Hernández, J.E.; Saldarriaga-Hernandez, S.; Villalba-Rodríguez, A.M.; Parra-Saldivar, R.; Iqbal, H.M.N. Electrochemical biosensors: A solution to pollution detection with reference to environmental contaminants. Biosensors 2018, 8, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majdinasab, M.; Daneshi, M.; Louis Marty, J. Recent developments in non-enzymatic (bio)sensors for detection of pesticide residues: Focusing on antibody, aptamer and molecularly imprinted polymer. Talanta 2021, 232, 122397. [Google Scholar] [CrossRef]
- Torrinha, Á.; Morais, S. Electrochemical (bio)sensors based on carbon cloth and carbon paper: An overview. TrAC-Trends Anal. Chem. 2021, 142, 116324. [Google Scholar] [CrossRef]
- Sardini, E.; Serpelloni, M.; Tonello, S. Printed electrochemical biosensors: Opportunities and metrological challenges. Biosensors 2020, 10, 166. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Gamella, M.; Serafín, V.; Yáñez-Sedeño, P.; Pingarrón, J.M. Beyond sensitive and selective electrochemical biosensors: Towards continuous, real-time, antibiofouling and calibration-free devices. Sensors 2020, 20, 3376. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G. Bionanomaterial-based electrochemical biosensing platforms for biomedical applications. Anal. Methods 2020, 12, 1688–1701. [Google Scholar] [CrossRef]
- Piroozmand, F.; Mohammadipanah, F.; Faridbod, F. Emerging biosensors in detection of natural products. Synth. Syst. Biotechnol. 2020, 5, 293–303. [Google Scholar] [CrossRef]
- Fan, L.; Ji, X.; Lin, G.; Liu, K.; Chen, S.; Ma, G.; Xue, W.; Zhang, X.; Wang, L. Green synthesis of stable platinum nanoclusters with enhanced peroxidase-like activity for sensitive detection of glucose and glutathione. Microchem. J. 2021, 166, 106202. [Google Scholar] [CrossRef]
- Tonello, S.; Stradolini, F.; Abate, G.; Uberti, D.; Serpelloni, M.; Carrara, S.; Sardini, E. Electrochemical detection of different p53 conformations by using nanostructured surfaces. Sci. Rep. 2019, 9, 17347. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maghsoudi, A.S.; Hassani, S.; Akmal, M.R.; Ganjali, M.R.; Mirnia, K.; Norouzi, P.; Abdollahi, M. An electrochemical aptasensor platform based on flower-like gold microstructure-modified screen-printed carbon electrode for detection of serpin A12 as a type 2 diabetes biomarker. Int. J. Nanomed. 2020, 15, 2219–2230. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, M.; Wu, Q.; Wei, W.; Liu, X. Photolithographic 3D microarray electrode-based high-performance non-enzymatic H2O2 sensor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127249. [Google Scholar] [CrossRef]
- Krejci, J.; Sajdlova, Z.; Nedela, V.; Flodrova, E.; Sejnohova, R.; Vranova, H.; Plicka, R. Effective Surface Area of Electrochemical Sensors. J. Electrochem. Soc. 2014, 161, B147–B150. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kaewjunlakan, C.; Saichanapan, J. Electrochimica Acta Poly(phenol red) hierarchical micro-structure interface enhanced electrode kinetics for adsorption and determination of hydroquinone. Electrochim. Acta 2021, 377, 138072. [Google Scholar] [CrossRef]
- Liu, B.; Lv, C.; Chen, C.; Ran, B.; Lan, M.; Chen, H.; Zhu, Y. Electrochemical Performance of Micropillar Array Electrodes in Microflows. Micromachines 2020, 11, 858. [Google Scholar] [CrossRef]
- Chen, C.; Ran, B.; Wang, Z.; Zhao, H. Development of micropillar array electrodes for highly sensitive detection of biomarkers. RSC Adv. 2020, 10, 41110–41119. [Google Scholar] [CrossRef]
- Surface, M.B.; Movilli, J.; Kolkman, R.W.; Rozzi, A.; Corradini, R.; Segerink, L.I.; Huskens, J. Increasing the Sensitivity of Electrochemical DNA Detection by a Micropillar-Structured Biosensing Surface. Langmuir 2020, 36, 4272–4279. [Google Scholar]
- Cao, Q.; Puthongkham, P.; Venton, B.J. Review: New insights into optimizing chemical and 3D surface structures of carbon electrodes for neurotransmitter detection. Anal. Methods 2019, 11, 247–261. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, R.M.; Kalinke, C.; Rocha, R.G.; dos Santos, P.L.; Rocha, D.P.; Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A.; Richter, E.M.; Munoz, R.A.A. Additive-manufactured (3D-printed) electrochemical sensors: A critical review. Anal. Chim. Acta 2020, 1118, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, M.; Cantù, E.; Sardini, E.; Serpelloni, M. Future sensors for smart objects by printing technologies in Industry 4.0 scenario. Energies 2020, 13, 5916. [Google Scholar] [CrossRef]
- Di Novo, N.G.; Cantù, E.; Tonello, S.; Sardini, E.; Serpelloni, M. Support-Material-Free Microfluidics on an Electrochemical Sensors Platform by Aerosol Jet Printing. Sensors 2019, 19, 1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeber, R.; Zanardi, C.; Inzelt, G. The inherent coupling of charge transfer and mass transport processes: The curious electrochemical reversibility. ChemTexts 2016, 2, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Gomathl, H. Chemistry and electrochemistry of iron complexes. Bull. Electrochem. 2000, 16, 459–465. [Google Scholar]
- Morrin, A.; Smyth, M.R. Electrochemical Characterization of Commercial and Home-Made Screen-Printed Carbon Electrodes Electrochemical Characterization of Commercial and Home-Made Screen-Printed Carbon Electrodes. Anal. Lett. 2003, 36, 2021–2039. [Google Scholar] [CrossRef] [Green Version]
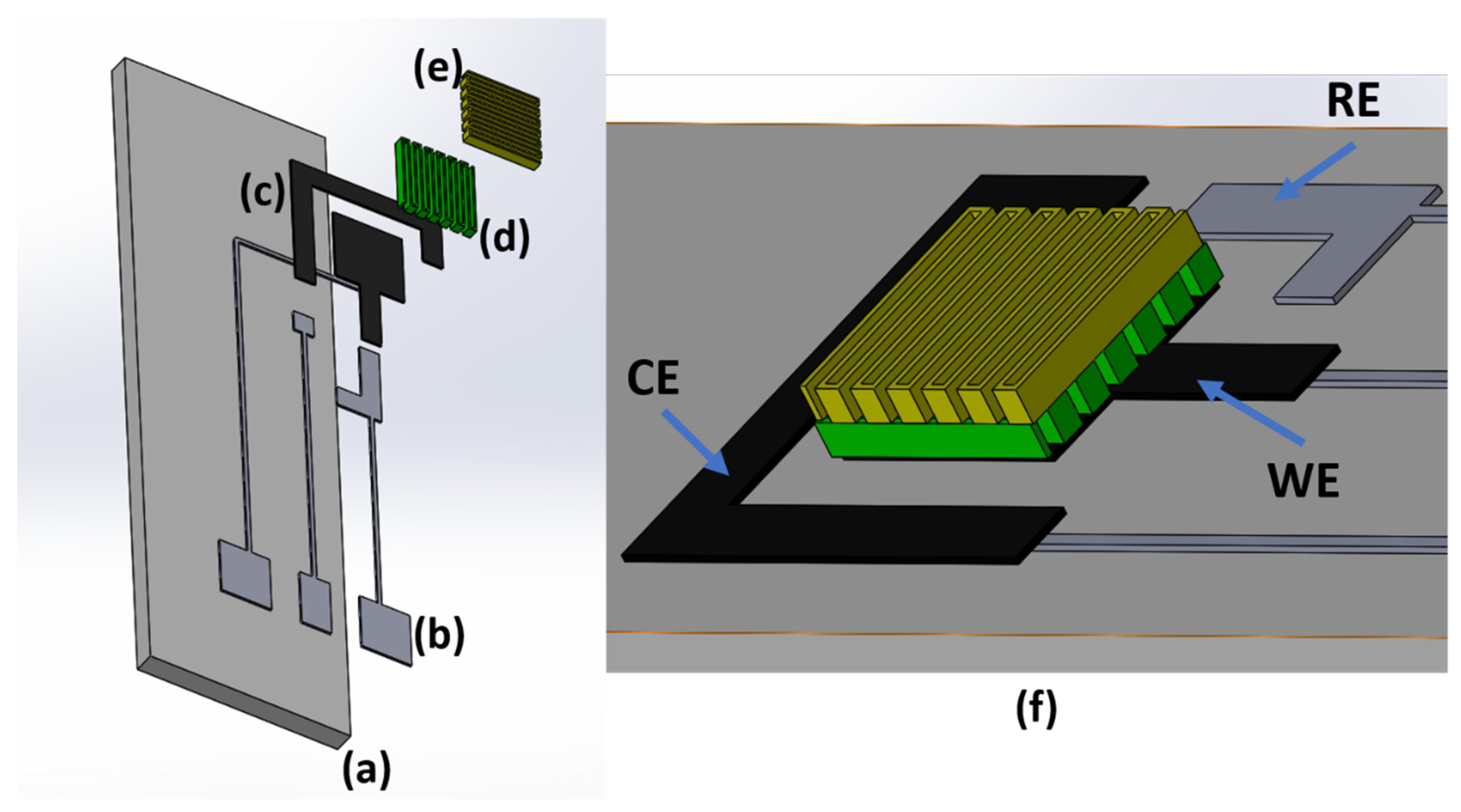
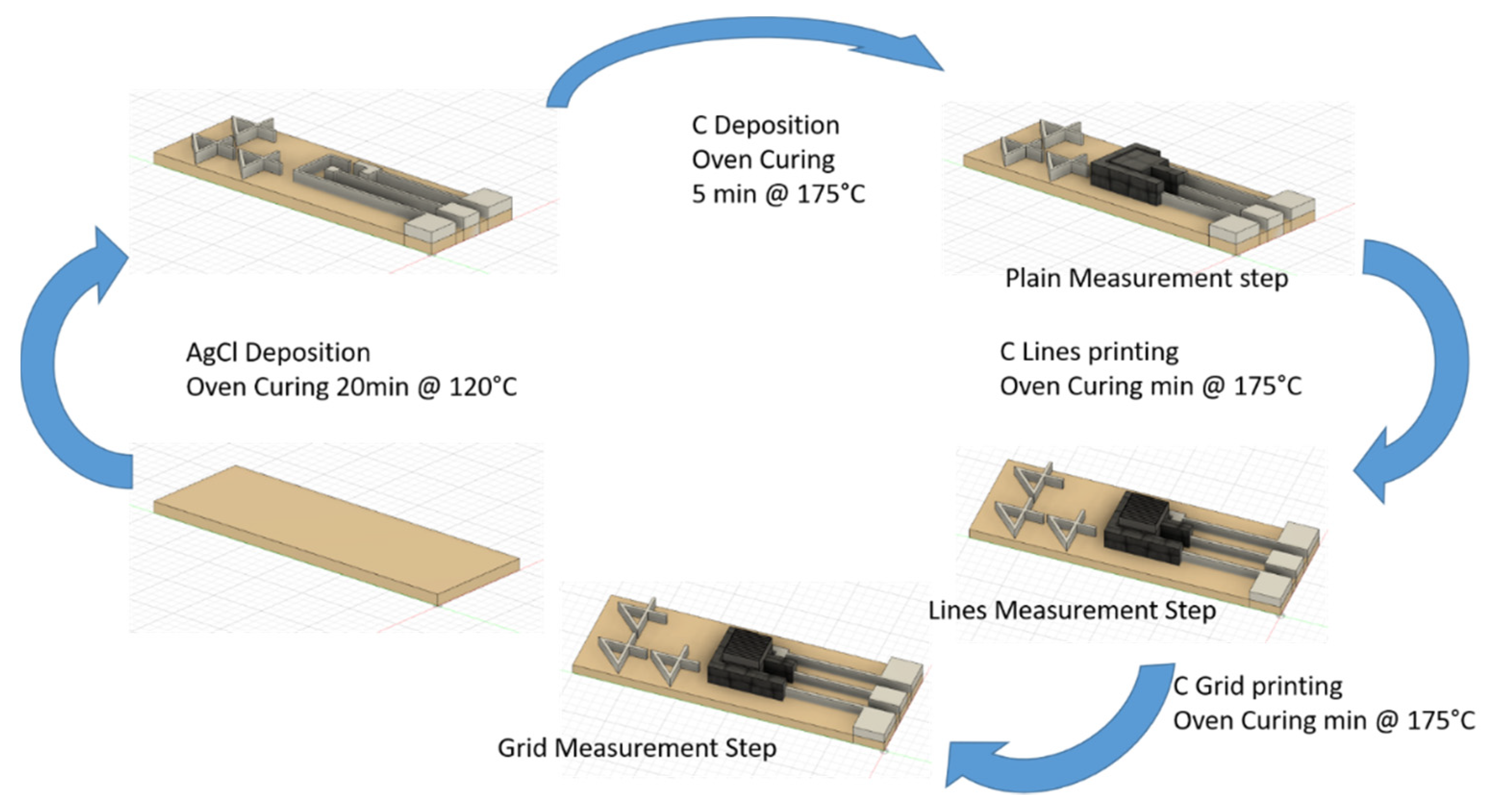
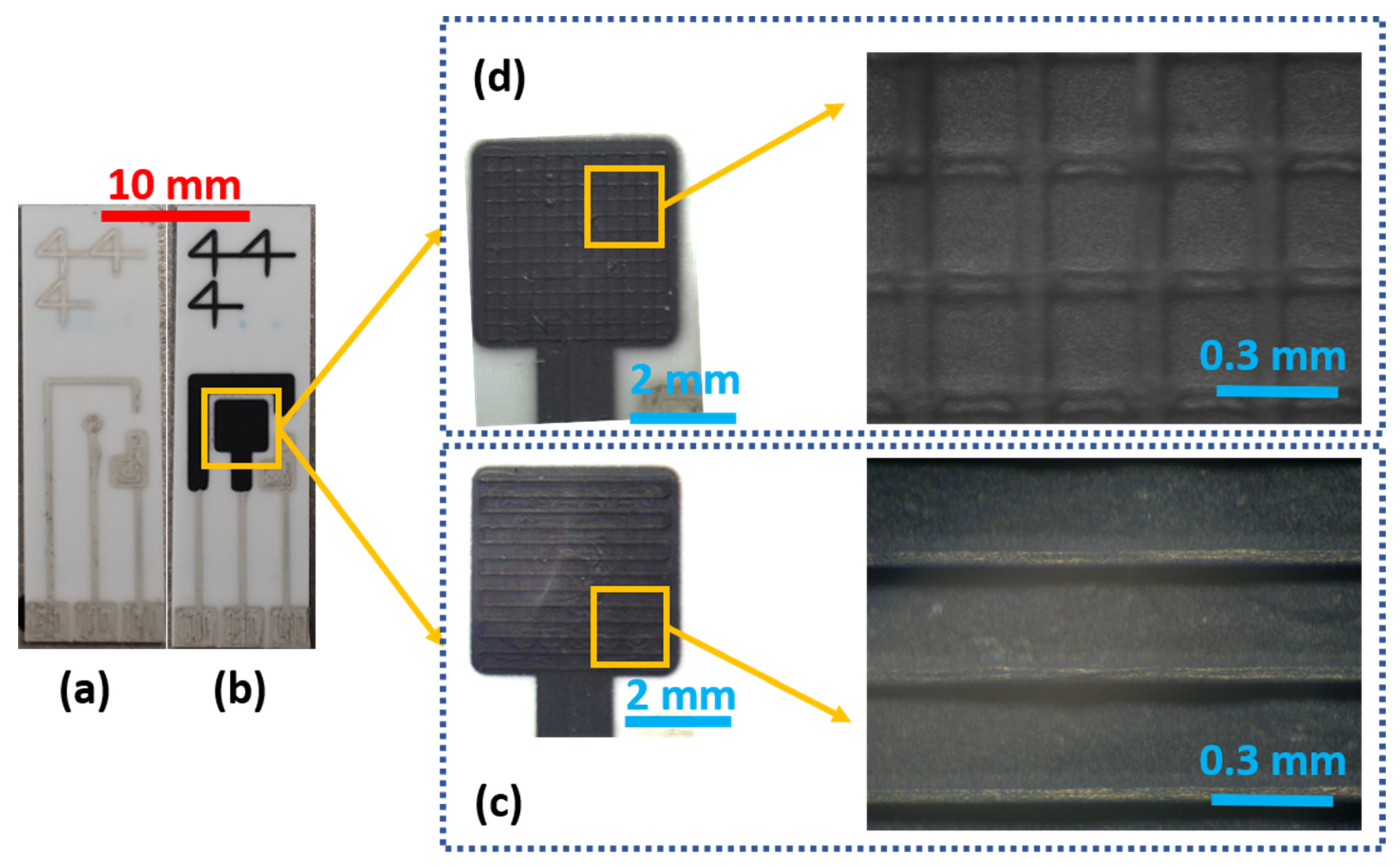
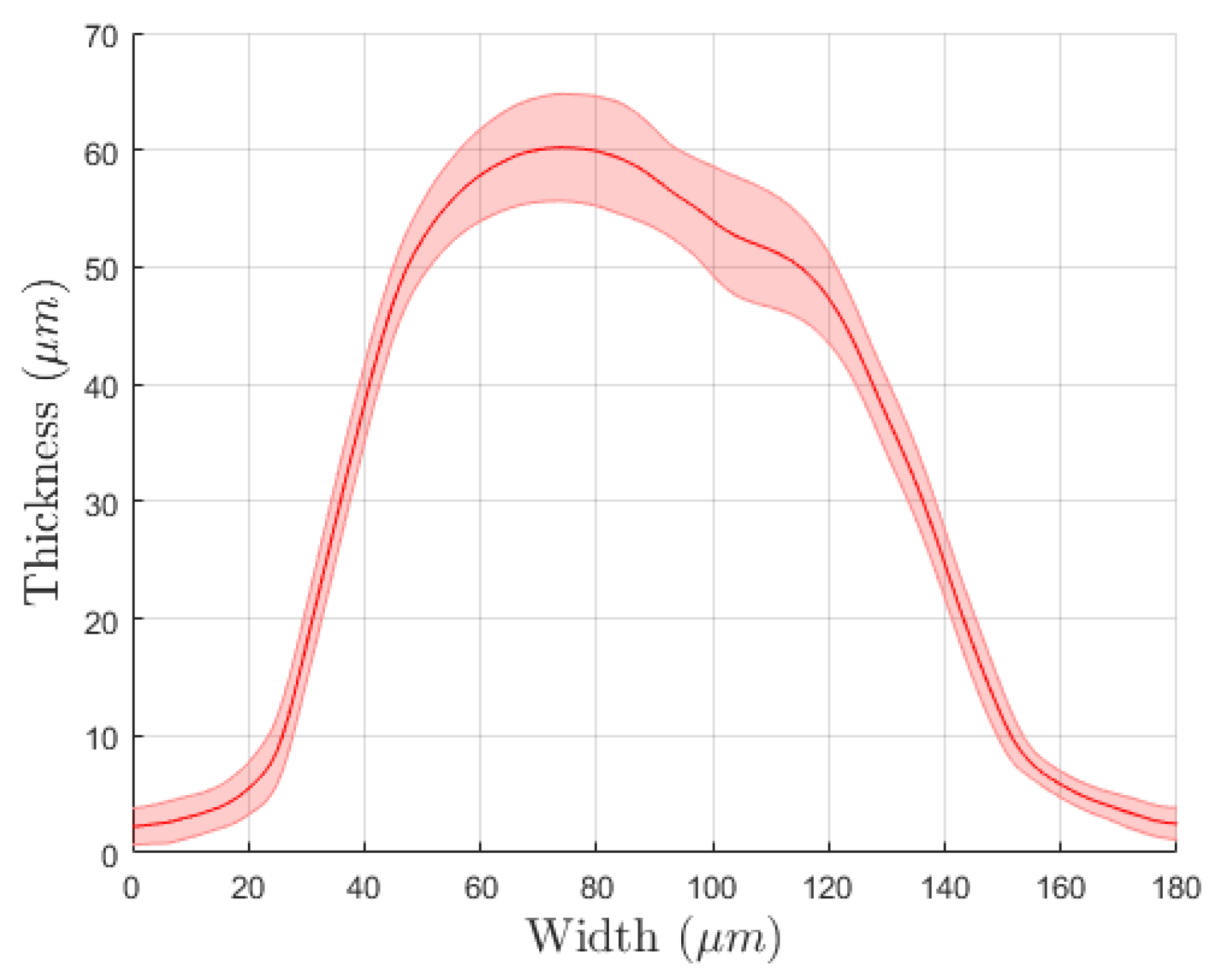




| Ink | Sheath Flow (SCCM) | Atm Flow (SCCM) | Exhaust Flow (SCCM) | Substrate Temperature | PROCESS SPEED | Number of Depositions | |
|---|---|---|---|---|---|---|---|
| Conductive tracks, RE | AgCl | 250 | 1100 | 1030 | 50 °C | 3 mm/s | 1 |
| CE and WE coating | C | 400 | 1150 | 1030 | 75 °C | 3 mm/s | 6 |
| Microstructuration | C | 40 | 805 | 790 | 70 °C | 2 mm/s | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fapanni, T.; Sardini, E.; Serpelloni, M.; Tonello, S. 3D Electrochemical Sensor and Microstructuration Using Aerosol Jet Printing. Sensors 2021, 21, 7820. https://doi.org/10.3390/s21237820
Fapanni T, Sardini E, Serpelloni M, Tonello S. 3D Electrochemical Sensor and Microstructuration Using Aerosol Jet Printing. Sensors. 2021; 21(23):7820. https://doi.org/10.3390/s21237820
Chicago/Turabian StyleFapanni, Tiziano, Emilio Sardini, Mauro Serpelloni, and Sarah Tonello. 2021. "3D Electrochemical Sensor and Microstructuration Using Aerosol Jet Printing" Sensors 21, no. 23: 7820. https://doi.org/10.3390/s21237820
APA StyleFapanni, T., Sardini, E., Serpelloni, M., & Tonello, S. (2021). 3D Electrochemical Sensor and Microstructuration Using Aerosol Jet Printing. Sensors, 21(23), 7820. https://doi.org/10.3390/s21237820









