The Biomechanical Mechanism of Upper Airway Collapse in OSAHS Patients Using Clinical Monitoring Data during Natural Sleep
Abstract
1. Introduction
- Lack of clinical data on OSAHS patients in natural sleep for model construction
- Lack of clinical monitoring data for modelling boundary conditions
- Obscurity caused by airway collapse and the phase
2. Data and Methods
2.1. Data
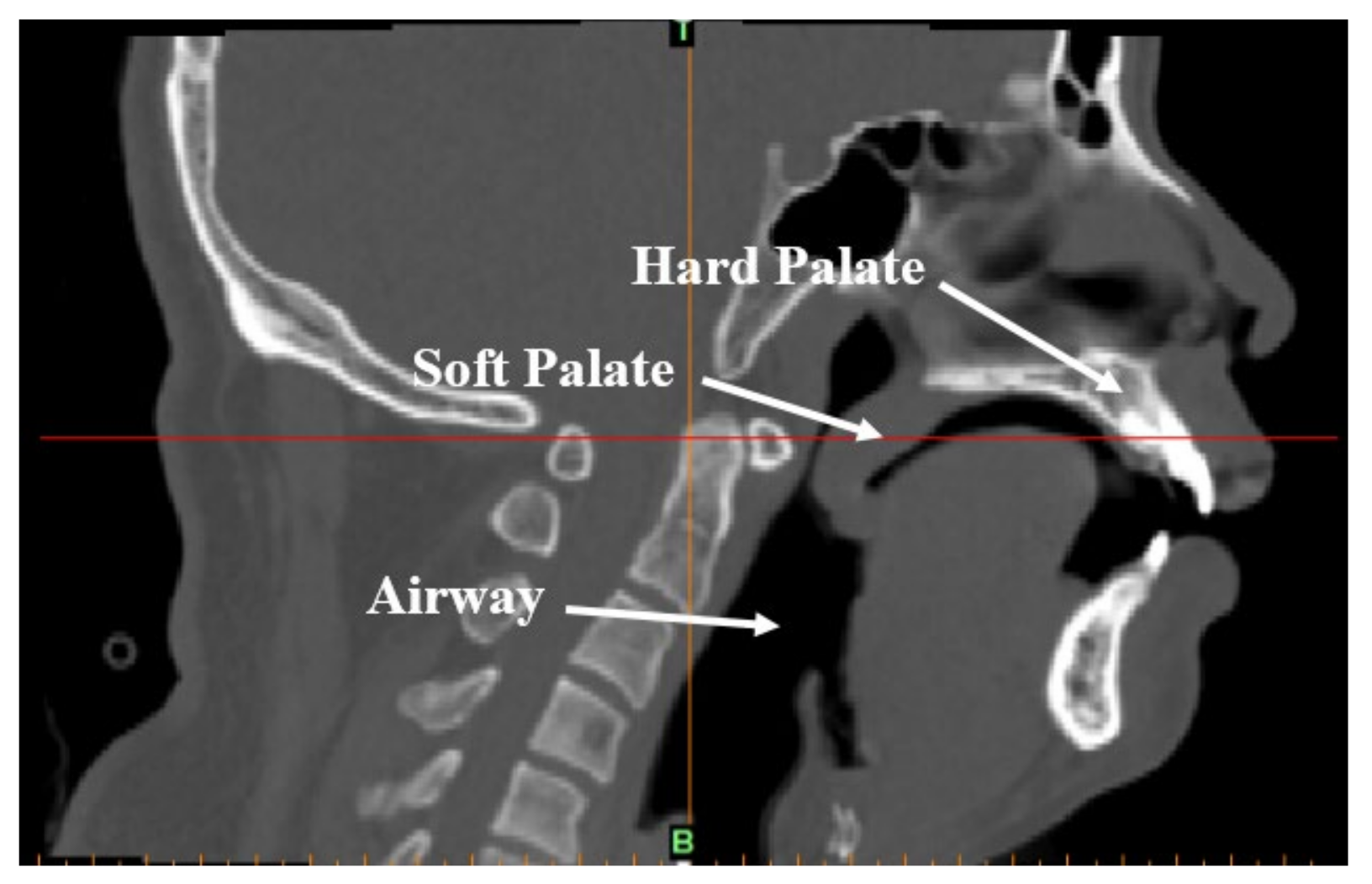
2.1.1. Clinical CT Data and Establishment of Finite Element Model
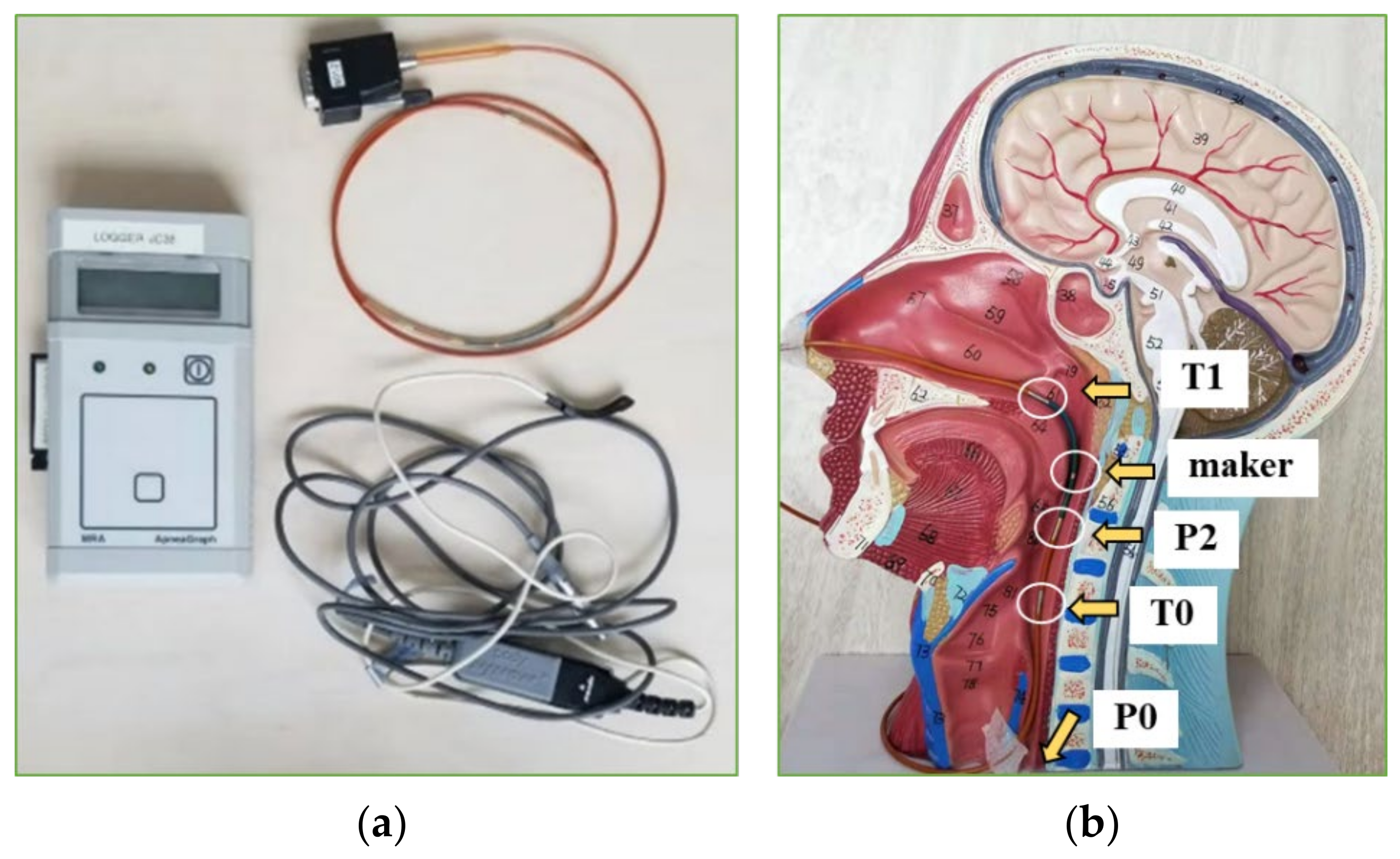
2.1.2. Clinical Monitoring of Cavity Pressure
2.2. Methods
2.2.1. One-Way Valve Structure of the Soft Palate
2.2.2. Fluid-Solid Coupling and Arbitrary Lagrangian Eulerian Algorithm
- Navier-Stokes equations
- Mass conservation equation
3. Results
3.1. One-Way Valve Effect of Soft Palate
3.2. Mechanical Characteristics of the Fluid Field in the Upper Airway
3.2.1. The Pressure Field of the Cavity
3.2.2. Velocity Field of the Cavity
4. Discussions
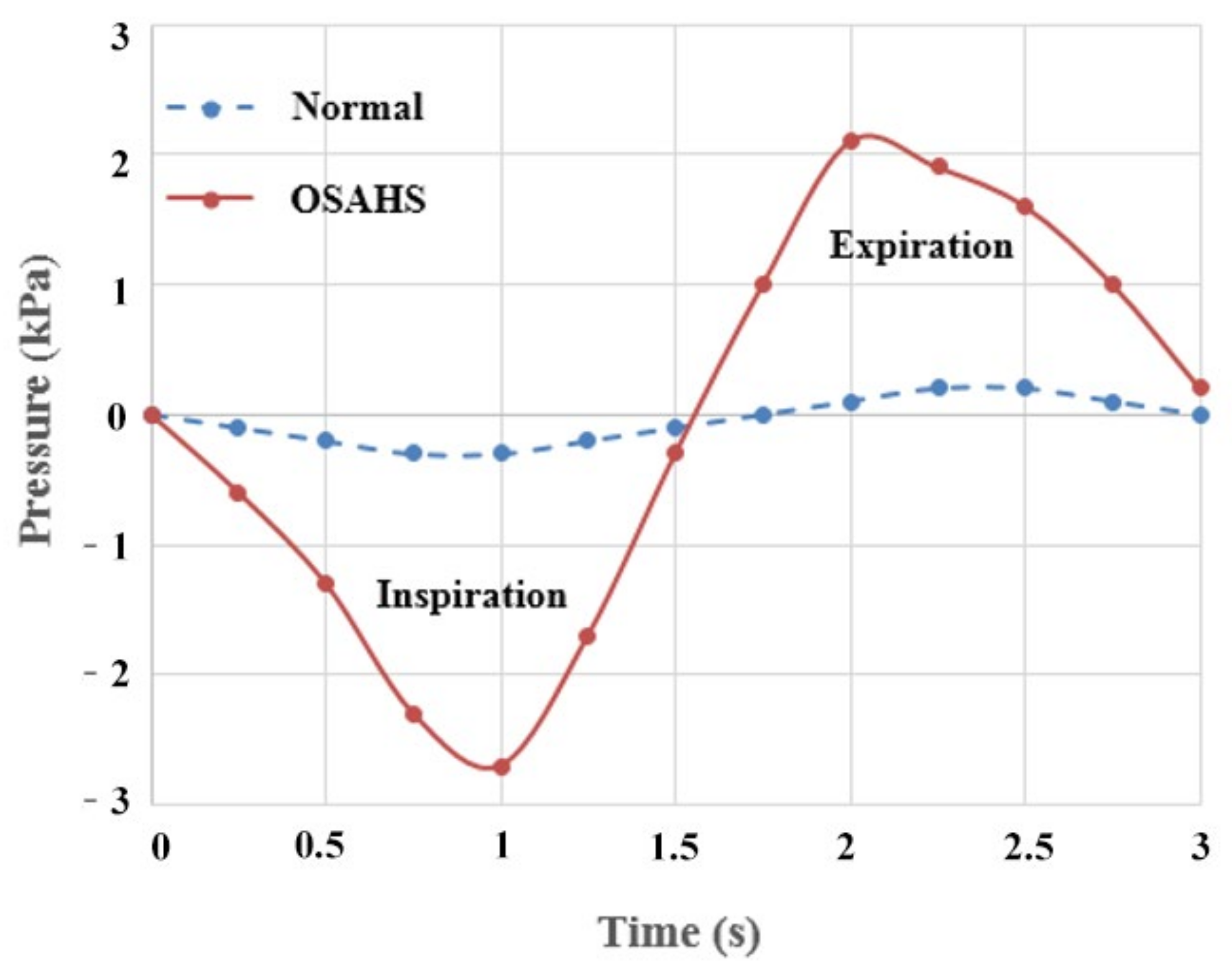
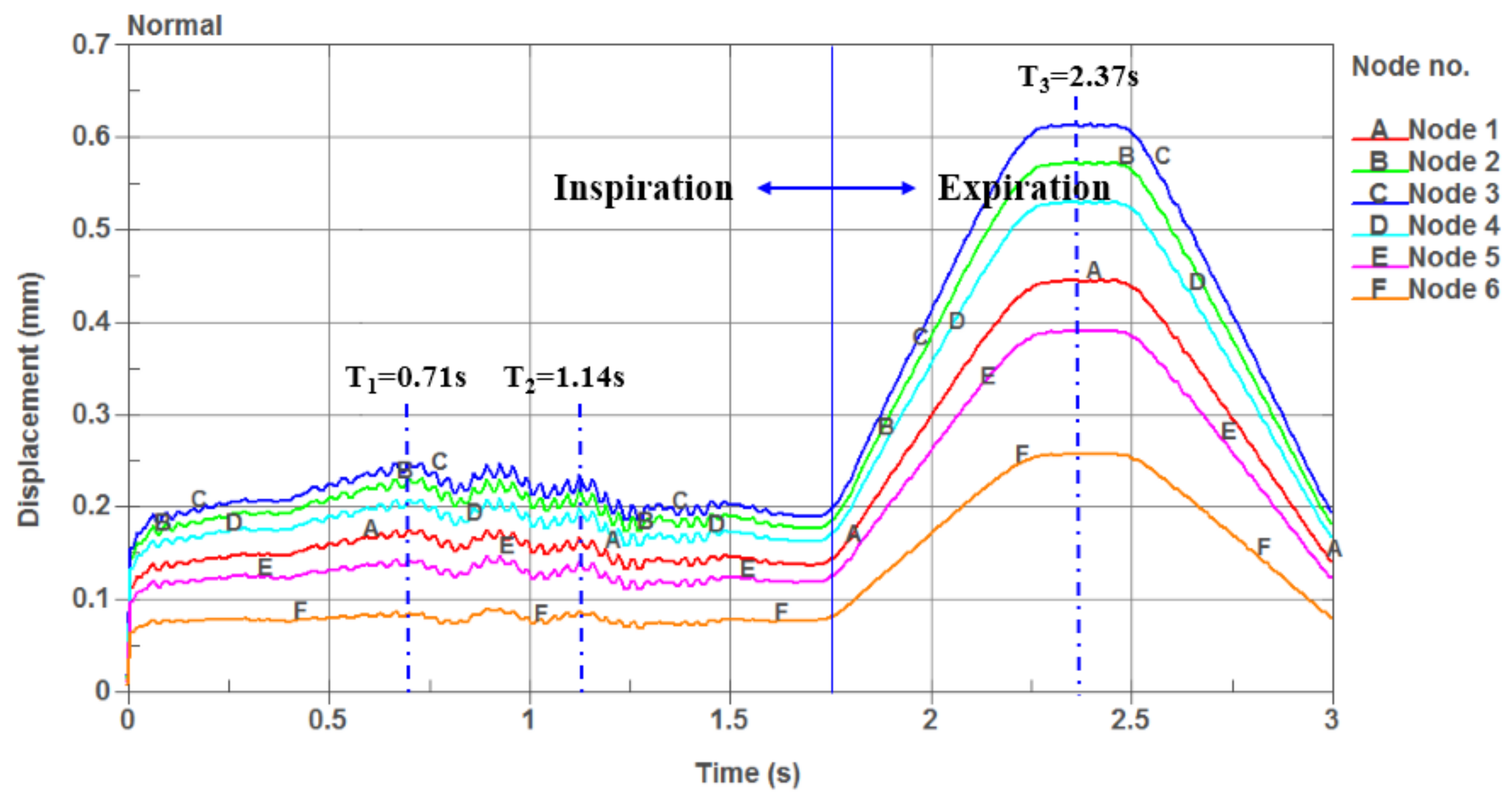
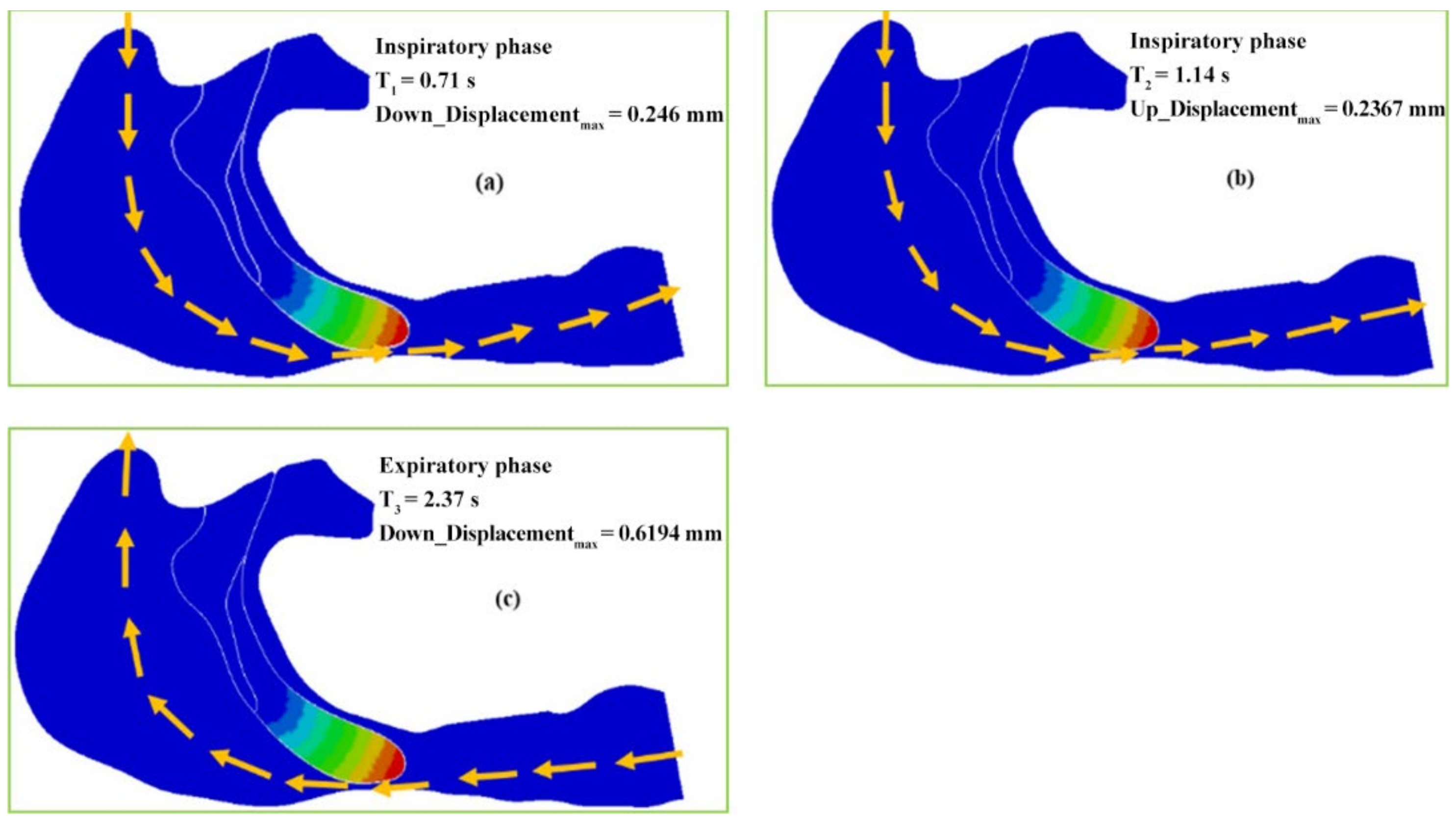
- The airflow characteristics during the inhalation phase are noticeably different from those in the exhalation phase for both eupnea and apnea. As the OSAHS patient breathes, the minimum pressure occurs alternately between the soft palate and the anteroposterior wall of pharynx, which causes the soft palate to vibrate during respiration, and therefore, the patient snores in sleep.
- The soft palate in pharyngeal cavity would exerts a one-way valve effect and collapses in the exhalation phase if the patient sleeps in the supine position, regardless of eupnea or apnea.
- The mechanical environment of the airway is directly dependent on the action of the airflow. If the mechanical properties of the soft palate remain unchanged, the pressure makes the soft palate collapse in apnea. In eupnea, the pressure allows the airflow to pass freely through the airway.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyons, M.M.; Bhatt, N.Y.; Pack, A.I.; Magalang, U.J. Global burden of sleep-disordered breathing and its implications. Respirology 2020, 25, 690–702. [Google Scholar] [CrossRef]
- Benjafield, A.; Ayas, N.; Eastwood, P.; Heinzer, R.; Ip, M.; Morrell, M.; Nunez, C.; Patel, S.; Penzel, T.; Pepin, J. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Resp. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Kun-Ying, Y.; Chao-Chi, Y.; Wu, C.C.; Tang, K.; Wu, J.Y.; Chen, Y.T.; Xu, M.X.; Chen, Y.J.; Yang, Y.J.; Lu, S.S. A wireless monitoring system using a tunneling sensor array in a smart oral appliance for sleep apnea treatment. Sensors 2017, 17, 2358. [Google Scholar] [CrossRef]
- Xia, F.; Sawan, M. Clinical and research solutions to manage obstructive sleep apnea: A review. Sensors 2021, 21, 1784. [Google Scholar] [CrossRef] [PubMed]
- Mam, A.; Fpca, B. A systematic review of COVID-19 and obstructive sleep apnoea. Sleep Med. Rev. 2020, 55, 101382. [Google Scholar] [CrossRef]
- Caples, S.M.; Gami, A.S.; Somers, V.K. Obstructive sleep apnea. Ann. Intern. Med. 2005, 142, 187–197. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Su, J.; Wang, Y. Mindfulness may be a novel factor associated with CPAP adherence in OSAHS patients. Sleep Breath. 2019, 24, 183–190. [Google Scholar] [CrossRef]
- Tan, J.; Huang, J.; Yang, J.; Wang, D.; Liu, J.; Liu, J.; Lin, S.; Li, C.; Lai, H.; Zhu, H. Numerical simulation for the upper airway flow characteristics of Chinese patients with OSAHS using CFD models. Eur. Arch. Oto-Rhino-Laryn. 2013, 270, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Yeh, C.Y.; Lee, C.T.; Lin, C.C. A sleep apnea detection system based on a one-dimensional deep convolution neural network model using single-lead electrocardiogram. Sensors 2020, 20, 4157. [Google Scholar] [CrossRef]
- Yu, C.C.; Hsiao, H.D.; Lee, L.C.; Yao, C.M.; Chen, N.H.; Wang, C.J.; Chen, Y. Computational fluid dynamic study on obstructive sleep apnea syndrome treated with maxillomandibular advancement. J. Craniofac. Surg. 2009, 20, 426–430. [Google Scholar] [CrossRef]
- Na, J.S.; Jung, H.D.; Cho, H.J.; Choi, Y.J.; Lee, J.S. Computational analysis of airflow dynamics for predicting collapsible sites in the upper airways: A preliminary study. J. Appl. Physiol. 2019, 126, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; James Quinn, S.; Ellis, P.D.M.; Williams, J.E.F. Biomechanics of snoring. Endeavour 1995, 19, 96–100. [Google Scholar] [CrossRef]
- Farré, R.; Rigau, J.; Montserrat, J.M.; Buscemi, L.; Ballester, E.; Navajas, D. Static and dynamic upper airway obstruction in sleep apnea role of the breathing gas properties. Am. J. Respir. Crit. Care Med. 2003, 168, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Wootton, D.M.; Sin, S.Y.; Luo, H.Y.; Yazdani, A.; McDonough, J.M.; Wagshul, M.E.; Isasi, C.R.; Arens, R. Computational fluid dynamics upper airway effective compliance, critical closing pressure, and obstructive sleep apnea severity in obese adolescent girls. J. Appl. Physiol. 2016, 121, 925–931. [Google Scholar] [CrossRef]
- Zhu, J.H.; Lee, H.P.; Lim, K.M.; Lee, S.J.; Teo, L.S.L.; Wang, D.Y. Passive movement of human soft palate during respiration: A simulation of 3D fluid/structure interaction. J. Biomech. 2012, 45, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Barber, T.; Cistulli, P.A. Simulation of upper airway occlusion without and with mandibular advancement in obstructive sleep apnea using fluid-structure interaction. J. Biomech. 2013, 46, 2586–2592. [Google Scholar] [CrossRef]
- Kato, J.; Isono, S.; Tanaka, A.; Watanabe, T.; Araki, D.; Tanzawa, H.; Nishino, T. Dose-dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep-disordered breathing. Chest 2000, 117, 1065–1072. [Google Scholar] [CrossRef]
- Cen, R.L.; Chen, L.; Zeng, Q.S.; Xin-Chun, L.I.; Liang, J.X. The feasibility of MSCT during awaken and induced sleep OSAHS patients. Chin. Med. Imag. 2008, 16, 189–191. [Google Scholar]
- Mezzanotte, W.S.; Tangel, D.J.; White, D.P. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J. Clin. Investig. 1992, 89, 1571–1579. [Google Scholar] [CrossRef]
- Lambeth, C.; Wang, Z.Y.; Kairaitis, K.; Moshfegh, A.; Jabbarzadeh, A.; Amish, T. Modelling mucosal surface roughness in the human velopharynx: A computational fluid dynamics study of healthy and obstructive sleep apnea airways. J. Appl. Physiol. 2018, 125, 1821–1831. [Google Scholar] [CrossRef]
- Mohammad Rasani, M. Numerical Modelling of Fluid-Structure Interactions for Fluid-Induced Instability in the Upper Airway. Ph.D. Thesis, RMIT University, Melbourne, Victoria, Australia, 2012. Available online: https://researchrepository.rmit.edu.au/esploro/outputs/doctoral/Numerical-modelling-of-fluid-structure-interactions-for/9921861281301341#files_and_links (accessed on 16 August 2021).
- Remmers, J.E.; Degroot, W.J.; Sauerland, E.K.; Anch, A.M. Pathogenesis of upper airway occlusion during sleep. J. Appl. Physiol. 1978, 44, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.R.; Schwartz, A.R. The pharyngeal critical pressure: The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest 1996, 110, 1077–1088. [Google Scholar] [CrossRef]
- Yucel, A. Evaluation of the upper airway cross-sectional area changes in different degrees of severity of obstructive sleep apnea syndrome: Cephalometric and dynamic CT study. Am. J. Neuroradiol. 2005, 26, 2624–2629. Available online: http://www.ajnr.org/content/26/10/2624 (accessed on 25 August 2021).
- Morrell, M.J.; Arabi, Y.; Zahn, B.; Badr, M.S. Progressive retropalatal narrowing preceding obstructive apnea. Am. J. Respir. Crit. Care Med. 1998, 158, 1974–1981. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Verbraecken, J.A.; Wa, D.B. Upper airway mechanics. Respiration 2009, 78, 121–133. [Google Scholar] [CrossRef]
- Subramaniam, D.R.; Mylavarapu, G.; McConnell, K.; Fleck, R.J.; Shott, S.R.; Amin, R.S.; Gutmark, E.J. Upper airway elasticity estimation in pediatric down syndrome sleep apnea patients using collapsible tube theory. Ann. Biomed. Eng. 2016, 44, 1538–1552. [Google Scholar] [CrossRef] [PubMed]
- Aurégan, Y.; Depollier, C. Snoring: Linear stability analysis andin-vitroexperiments. J. Sound Vibrat. 1995, 188, 39–54. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Prot, V.E.; Skallerud, B.H. Soft palate muscle activation: A modeling approach for improved understanding of obstructive sleep apnea. Biomech. Modeling Mechanobiol. 2019, 18, 531–546. [Google Scholar] [CrossRef]
- Berry, D.A.; Moon, J.B.; Kuehn, D.P. A finite element model of the soft palate. Cleft Palate Craniofac. J. 1999, 36, 217–223. [Google Scholar] [CrossRef]
- Huang, R.; Li, X.; Rong, Q. Control mechanism for the upper airway collapse in patients with obstructive sleep apnea syndrome: A finite element study. Sci. Chi. Life Sci. 2013, 56, 366–372. [Google Scholar] [CrossRef]
- Trudo, F.J.; Gefter, W.B.; Welch, K.C.; Gupta, K.B.; Maislin, G.; Schwab, R.J. State-related changes in upper airway caliber and surrounding soft-tissue structures in normal subjects. Am. J. Respir. Crit. Care Med. 1998, 158, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Malhotra, A.; White, D.P. Computational simulation of human upper airway collapse using a pressure-/state-dependent model of genioglossal muscle contraction under laminar flow conditions. J. Appl. Physiol. 2005, 99, 1138–1148. [Google Scholar] [CrossRef]
- Martonen, T.B.; Quan, L.; Zhang, Z.; Musante, C. Flow simulation in the human upper respiratory tract. Cell Biochem. Biophys. 2002, 37, 27–36. [Google Scholar] [CrossRef]
- Lee, H.P.; Poh, H.J.; Chong, F.H.; Wang, D.Y. Changes of airflow pattern in inferior turbinate hypertrophy: A computational fluid dynamics model. Am. J. Rhinol. Allergy 2009, 23, 153–158. [Google Scholar] [CrossRef]
- Van, H.A.; Pelorson, X.; Lagree, P.Y. In vitro validation of some flow assumptions for the prediction of the pressure distribution during obstructive sleep apnoea. Med. Biol. Eng. Comput. 2005, 43, 162–171. [Google Scholar] [CrossRef]
- Jeong, S.J.; Kim, W.S.; Sung, S.J. Numerical investigation on the flow characteristics and aerodynamic force of the upper airway of patient with obstructive sleep apnea using computational fluid dynamics. Med. Eng. Phys. 2007, 29, 637–651. [Google Scholar] [CrossRef]
- Sung, S.J.; Jeong, S.J.; Yu, Y.S.; Hwang, C.J.; Pae, E.K. Customized three-dimensional computational fluid dynamics simulation of the upper airway of obstructive sleep apnea. Angle Orthod. 2006, 76, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Beni, H.M.; Hassani, K.; Khorramymehr, S. In silico investigation of sneezing in a full real human upper airway using computational fluid dynamics method. Comput. Meth. Programs Biomed. 2019, 177, 203–209. [Google Scholar] [CrossRef]




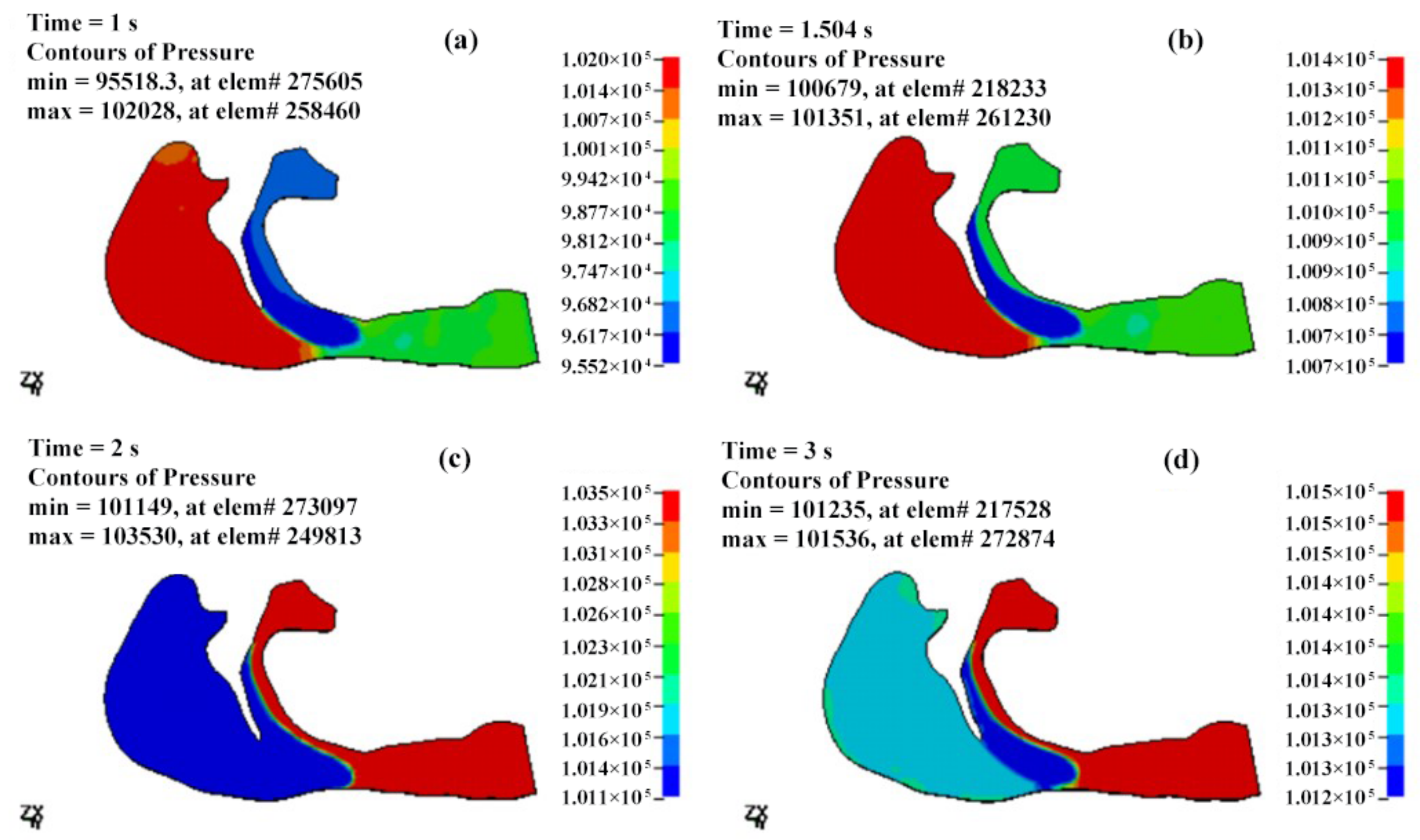
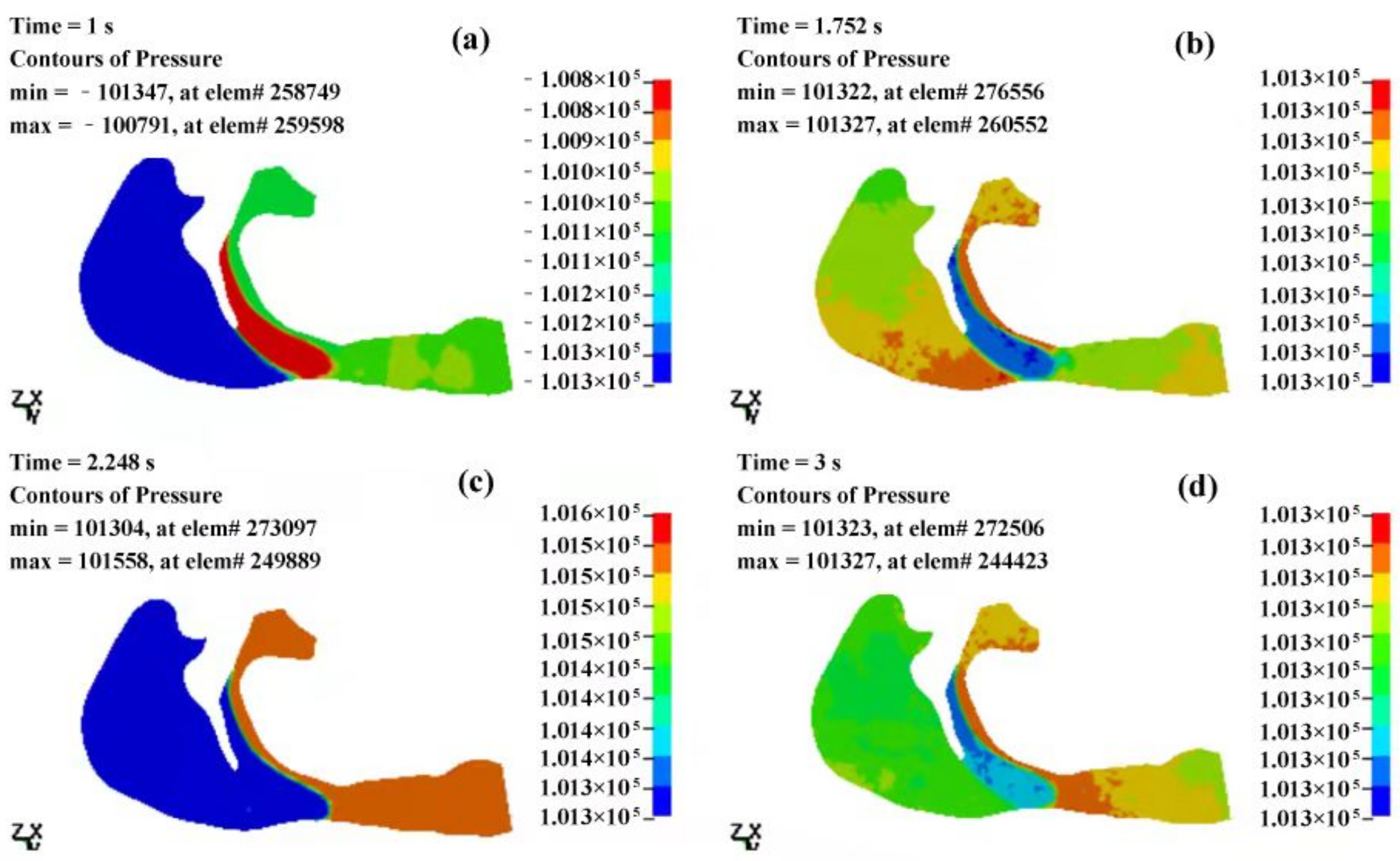
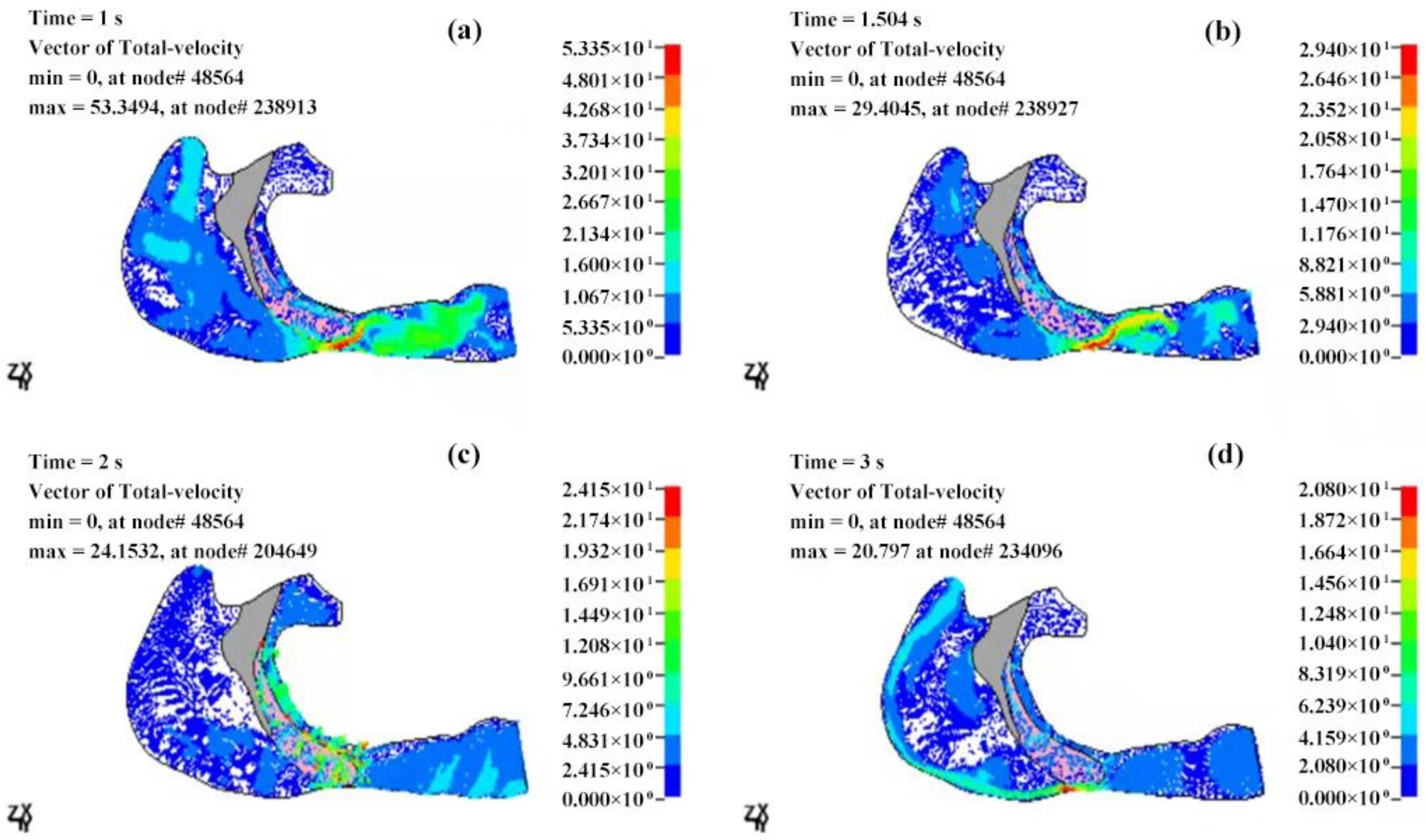
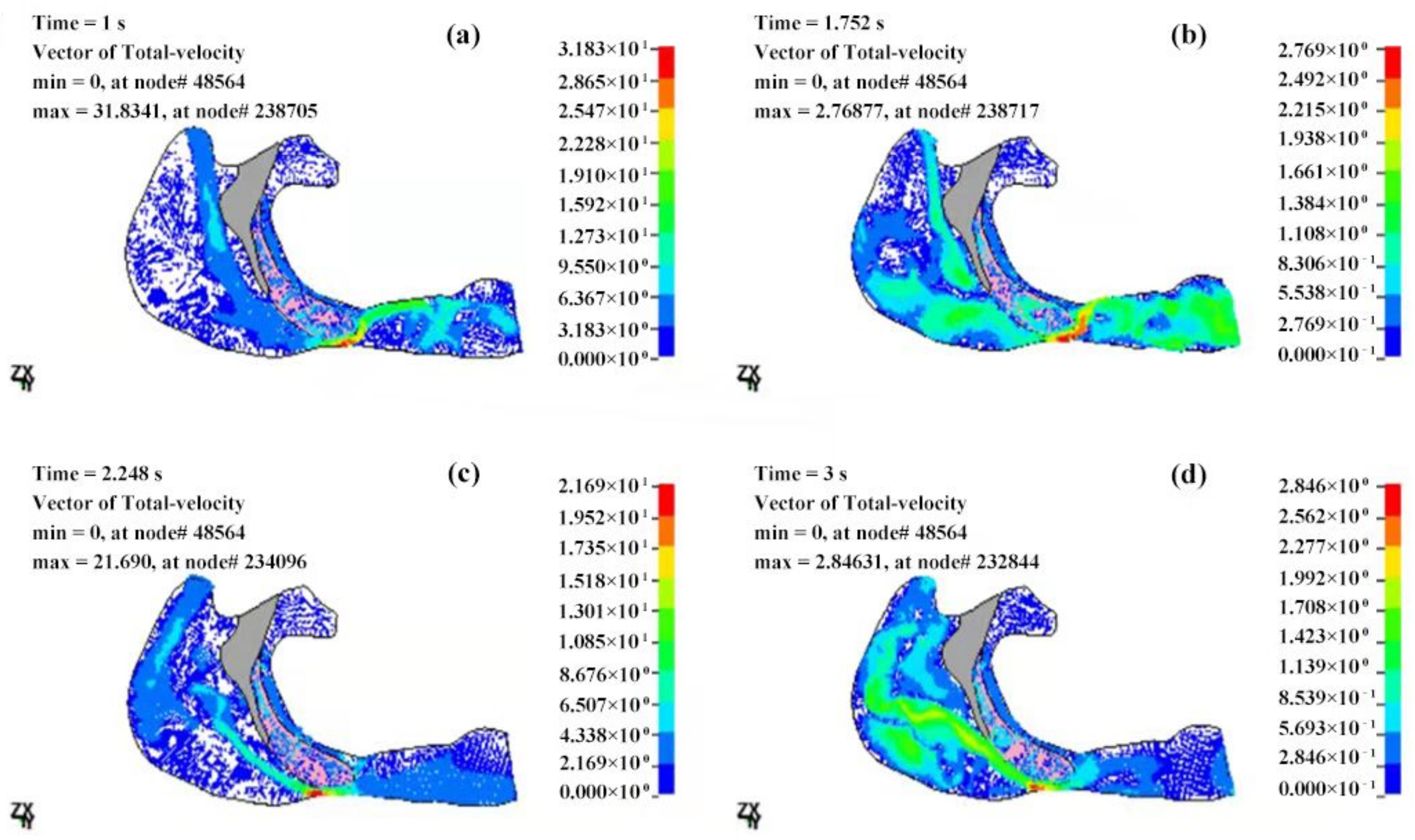
| T (s) | Pmax (Pa) | Pmin (Pa) | ΔP (Pa) | Pressure Drop (Pa) |
|---|---|---|---|---|
| 0.5 | 101,408 | 98,943 | 2465 | −1301 |
| 1 | 102,028 | 95,518 | 6510 | −2495 |
| 1.25 | 101,306 | 98,671 | 2635 | −1712 |
| 1.5 | 101,351 | 100,679 | 672 | −321 |
| 1.75 | 102,387 | 101,189 | 1198 | 1010 |
| 2 | 103,530 | 101,149 | 2381 | 2099 |
| 2.5 | 103,034 | 101,184 | 1850 | 1591 |
| T (s) | Pmax (Pa) | Pmin (Pa) | ΔP (Pa) | Pressure Drop (Pa) |
|---|---|---|---|---|
| 0.5 | 101,332 | 100,945 | 387 | −201 |
| 1 | 101,337 | 100,709 | 628 | −302 |
| 1.25 | 101,333 | 100,960 | 373 | −201 |
| 1.5 | 101,329 | 101,127 | 202 | −98 |
| 1.75 | 101,327 | 101,321 | 6 | 1 |
| 2 | 101,429 | 101,258 | 171 | 99 |
| 2.5 | 101,533 | 101,225 | 308 | 198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Xiao, T.; Ng, C.T. The Biomechanical Mechanism of Upper Airway Collapse in OSAHS Patients Using Clinical Monitoring Data during Natural Sleep. Sensors 2021, 21, 7457. https://doi.org/10.3390/s21227457
Chen L, Xiao T, Ng CT. The Biomechanical Mechanism of Upper Airway Collapse in OSAHS Patients Using Clinical Monitoring Data during Natural Sleep. Sensors. 2021; 21(22):7457. https://doi.org/10.3390/s21227457
Chicago/Turabian StyleChen, Liujie, Tan Xiao, and Ching Tai Ng. 2021. "The Biomechanical Mechanism of Upper Airway Collapse in OSAHS Patients Using Clinical Monitoring Data during Natural Sleep" Sensors 21, no. 22: 7457. https://doi.org/10.3390/s21227457
APA StyleChen, L., Xiao, T., & Ng, C. T. (2021). The Biomechanical Mechanism of Upper Airway Collapse in OSAHS Patients Using Clinical Monitoring Data during Natural Sleep. Sensors, 21(22), 7457. https://doi.org/10.3390/s21227457






