Preparation of Eu0.075Tb0.925-Metal Organic Framework as a Fluorescent Probe and Application in the Detection of Fe3+ and Cr2O72−
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
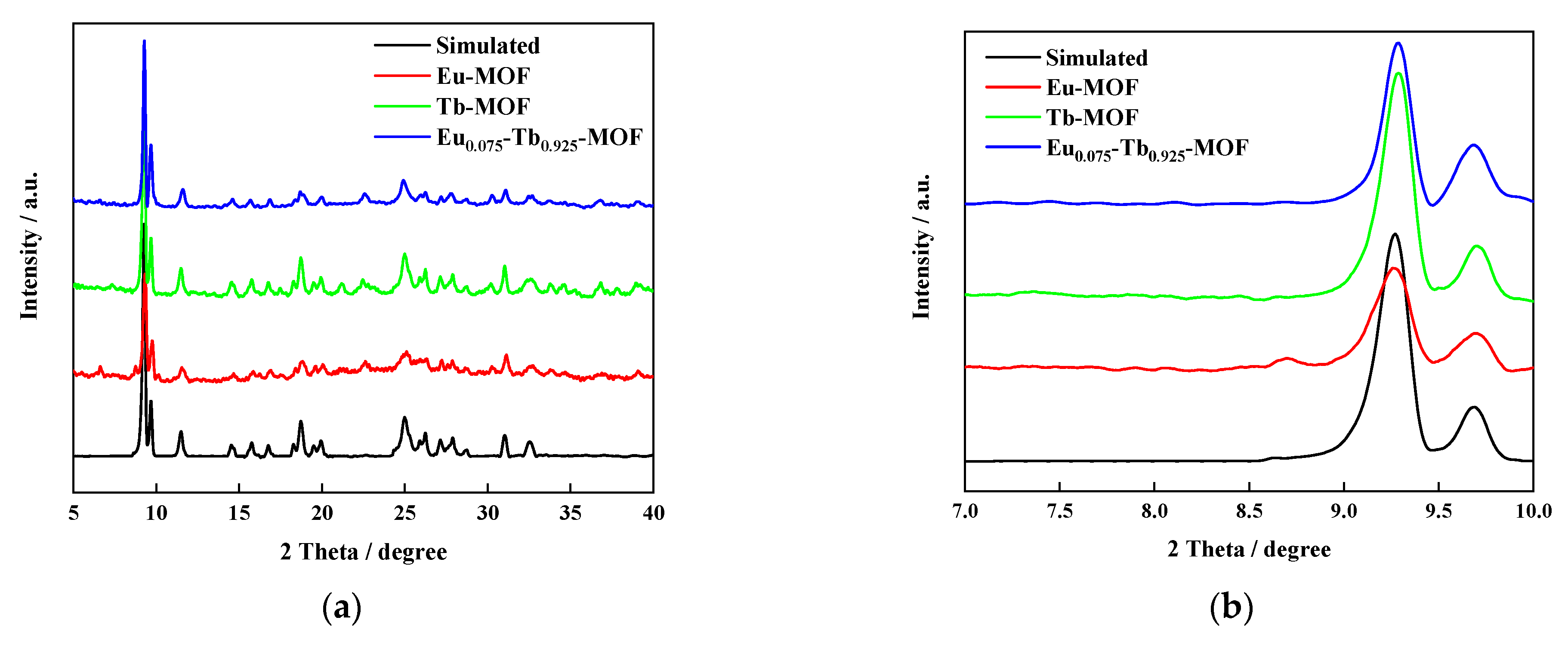
3.1. XRD Characterisation
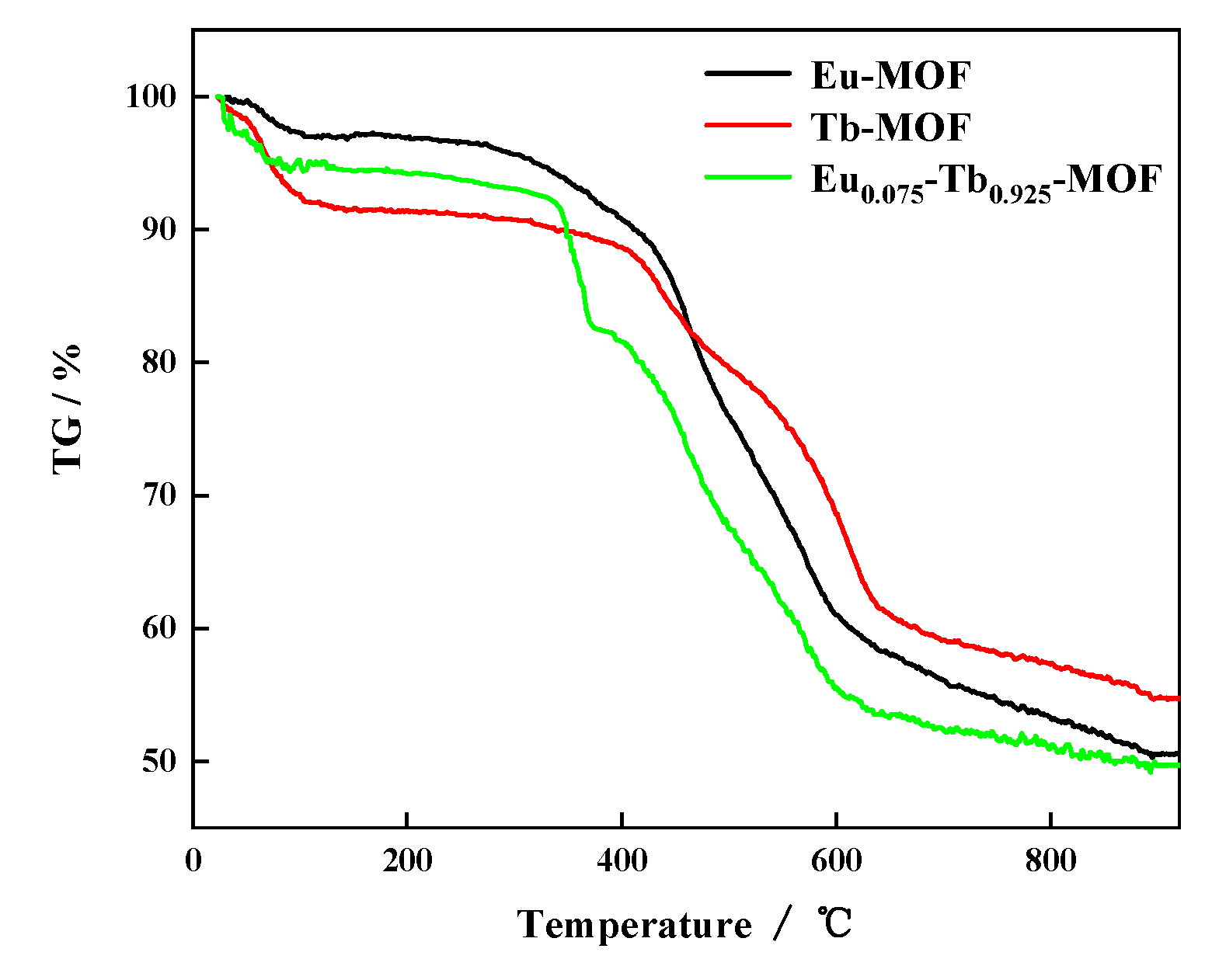
3.2. TG Analysis
3.3. FTIR Analysis
3.4. Elemental Analysis and XPS
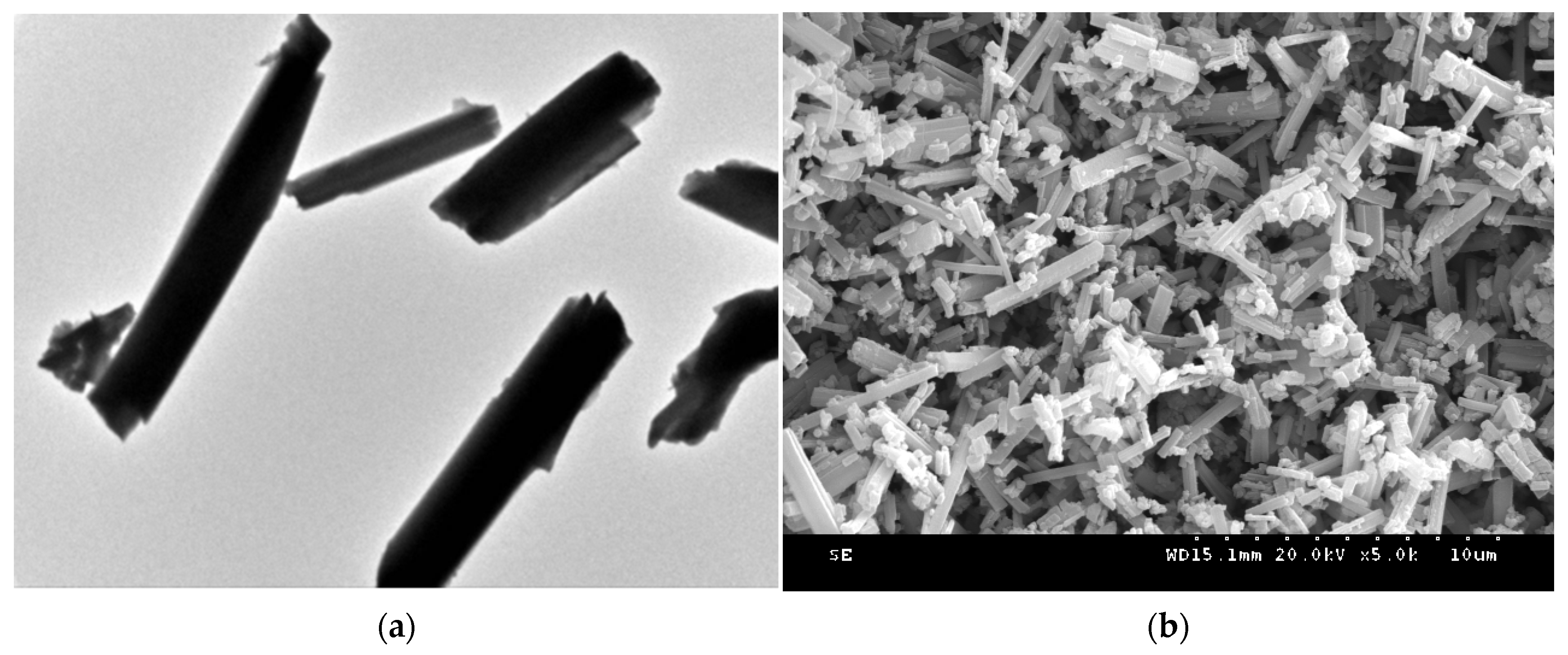
3.5. EM Characterisation
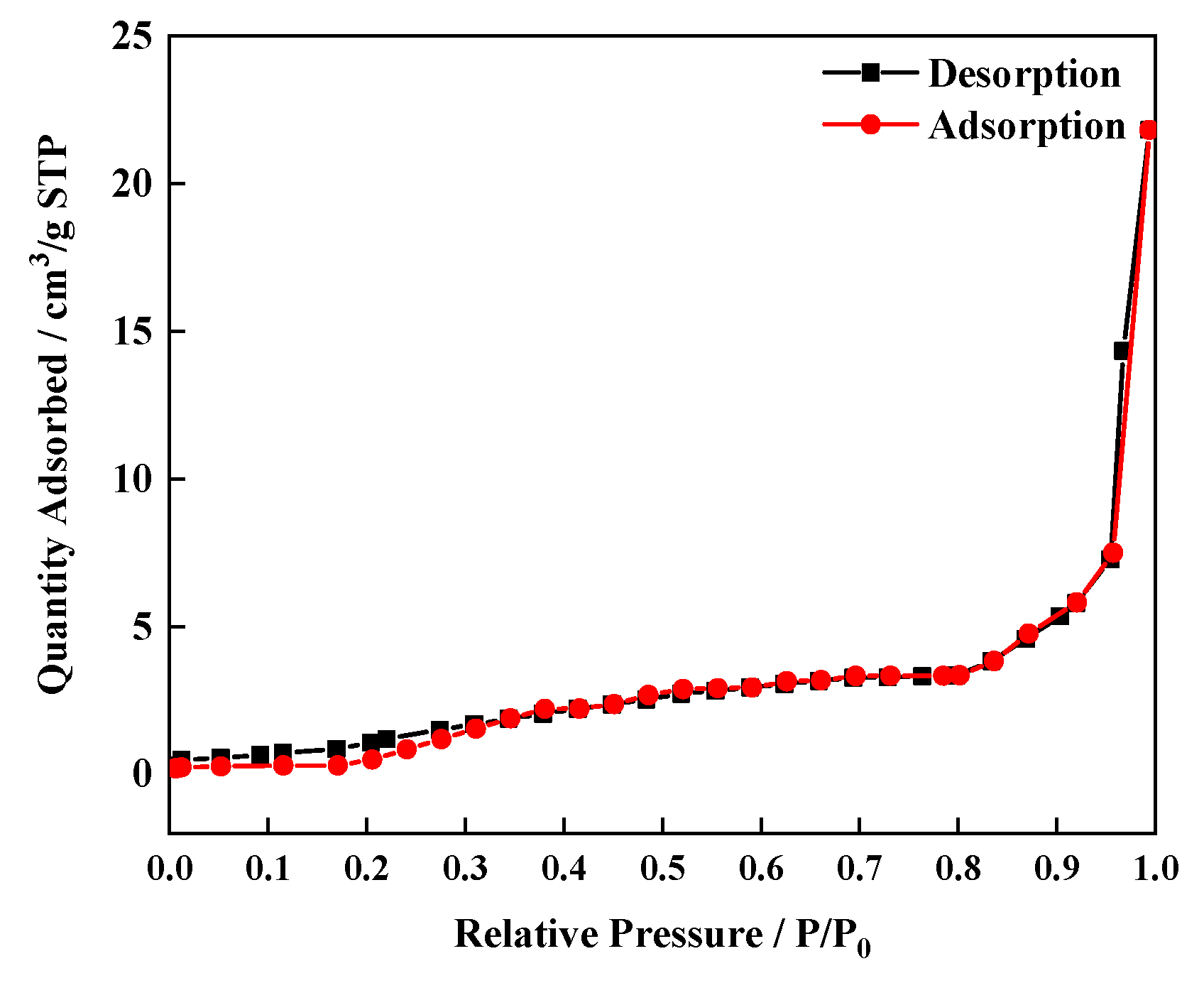
3.6. Adsorption Characteristics of Eu0.075Tb0.925-MOF
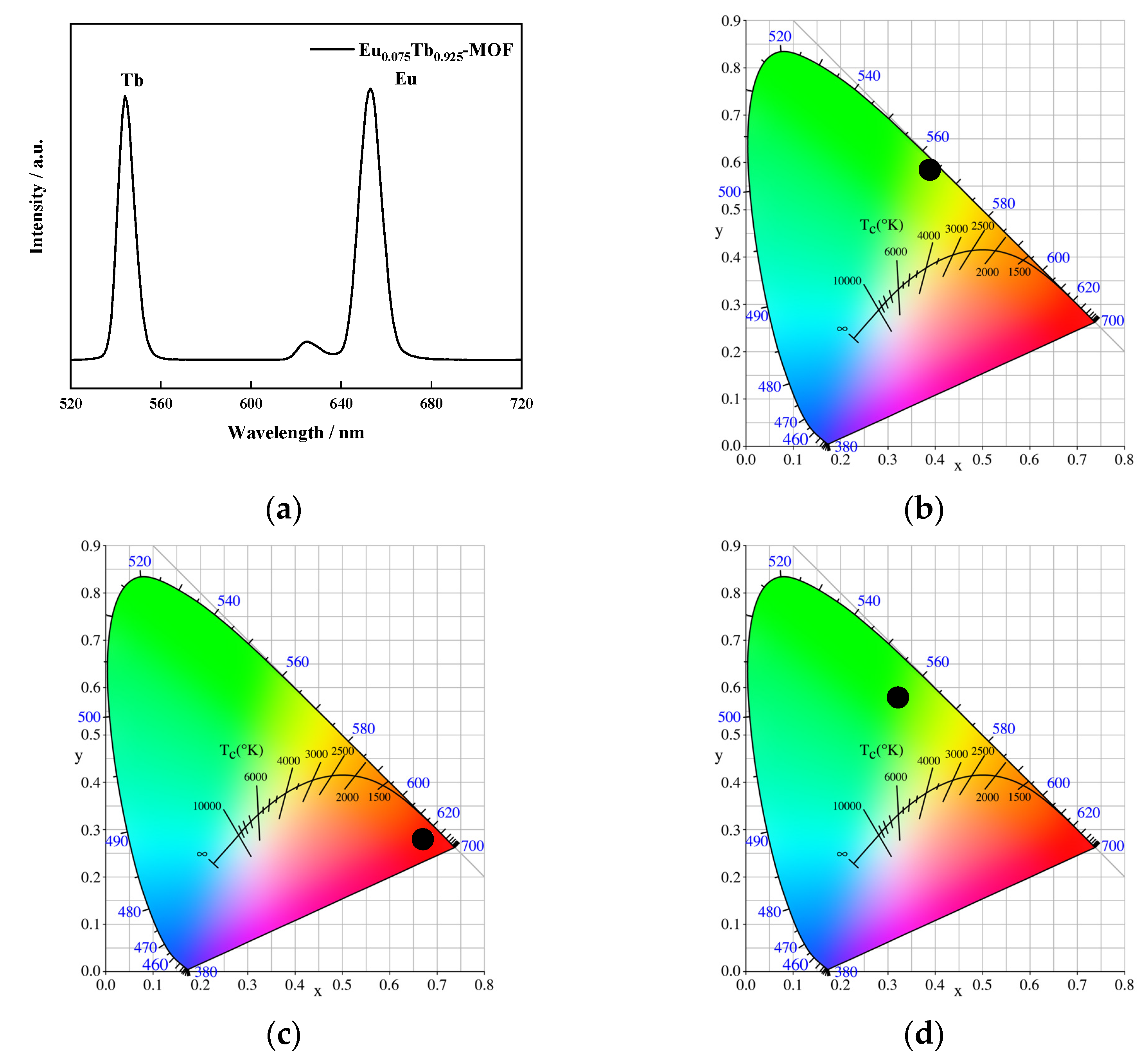
3.7. Photoluminescence Characteristics
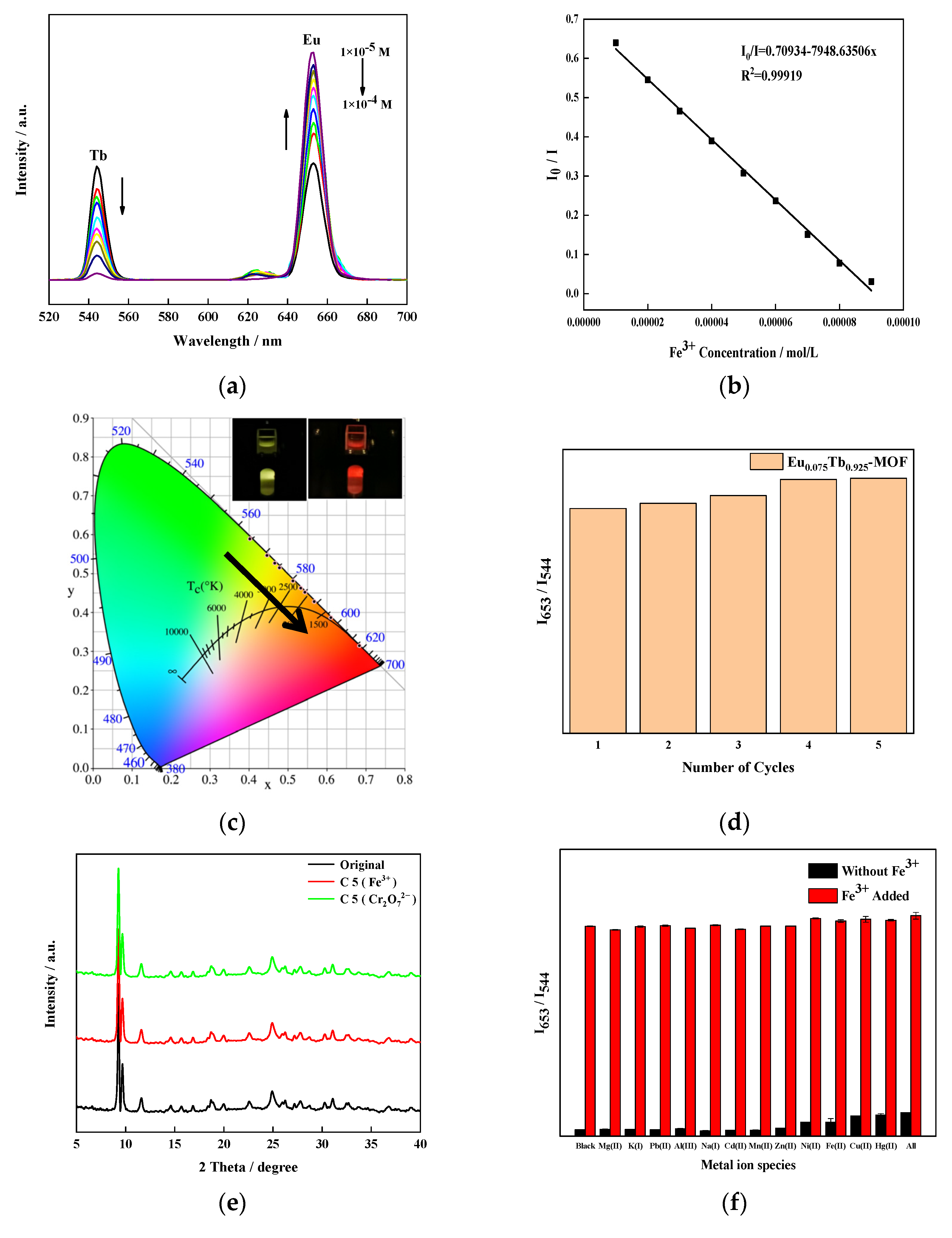
3.8. Fluorescence Sensing of Fe3+
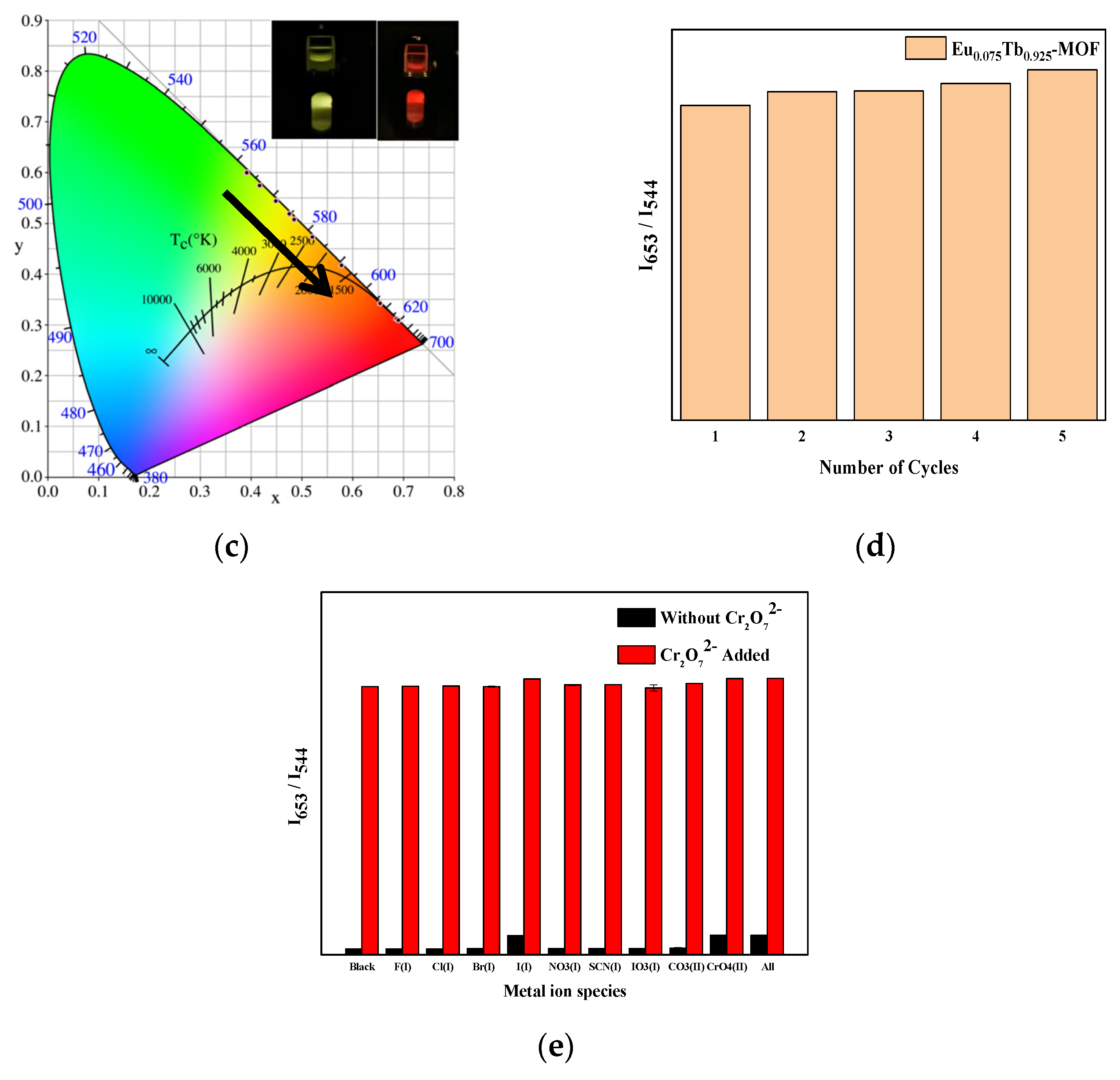
3.9. Fluorescence Sensing of Cr2O72−
3.10. Comparison with Other Sensors That Detect Fe3+ and Cr2O72− Ions
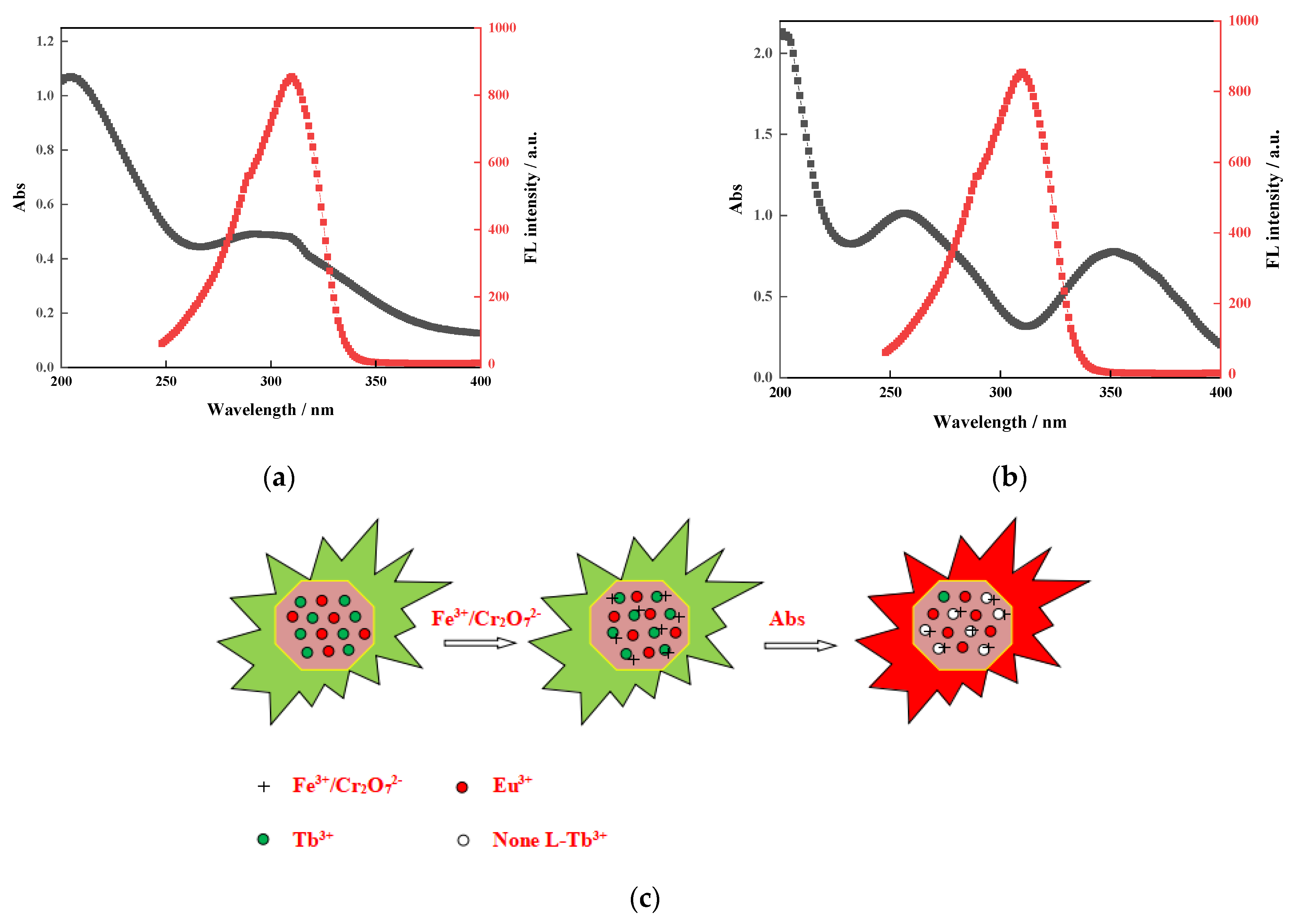
3.11. Mechanism Study
3.12. Application in Actual Water Sample Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saod, W.M.; Awad, S.S.; Mokadem, K. Assessment of some physico-chemical and microbial pollutants in the water of the Euphrates River between the cities of Hit and Fallujah in Iraq. Desalin. Water Treat. 2021, 211, 331–337. [Google Scholar] [CrossRef]
- Salonen, J.T.; Nyyssonen, K.; Korpela, H.; Tuomilehto, J.; Seppanen, R.; Salonen, R. High stored iron levels are associated with excess risk of myocardial infarction in eastern finnish men. Circulation 1992, 86, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Bijeh, N.; Hejazi, K. The effect of aerobic exercise on serum ferritin levels in untrained middle-aged women. international journal of sport studies. Int. J. Sport Stud. 2012, 2, 379–384. [Google Scholar]
- Jehn, M.L.; Guallar, E.; Clark, J.M.; Couper, D.; Duncan, B.B. A prospective study of plasma ferritin level and incident diabetes the atherosclerosis risk in communities (aric) study. Am. J. Epidemiol. 2007, 165, 1047–1054. [Google Scholar] [CrossRef] [Green Version]
- Fatih, S.; Demir, D. Investigation of reduction kinetics of Cr2O72− in FeSO4 solution. Chem. Eng. J. 2008, 143, 161–166. [Google Scholar]
- Mansi, G.; Desikan, R.; Annamalai, S.K. In situ electro-organic synthesis of hydroquinone using anisole on MWCNT/Nafion modified electrode surface and its heterogeneous electrocatalytic reduction of toxic Cr(VI) species. RSC Adv. 2021, 11, 4062–4076. [Google Scholar]
- Costa, M. Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit. Rev. Toxicol. 1997, 27, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Zhao, F.; Liu, J.J.; Liu, Z.; Wang, Y.Q. An ultrastable zinc–organic framework as a recyclable multi-responsive luminescent sensor for Cr3+, Cr6+ and 4-nitrophenol in the aqueous phase with high selectivity and sensitivity. J. Mater. Chem. A 2017, 5, 20035–20043. [Google Scholar] [CrossRef]
- Wang, B.G.; Lin, Y.; Tan, H.; Luo, M.; Dai, S.S.; Lu, H.S. One-pot synthesis of n-doped carbon dots by pyrolyzing the gel composed of ethanolamine and 1-carboxyethyl-3-methylimidazolium chloride and their selective fluorescence sensing for Cr6+ ions. Analyst 2018, 143, 1906–1915. [Google Scholar] [CrossRef]
- Hyman, L.M.; Franz, K.J. Probing oxidative stress: Small molecule fluorescent sensors of metal ions, reactive oxygen species, and thiols. Coord. Chem. Rev. 2012, 256, 2333–2356. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Li, X.; Li, Y.; Zhu, R.; Pang, H. Applications of metal-organic-framework-derived carbon materials. Adv. Mater. 2019, 31, 1804740. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.Y.; Das, P.; White, D.L. Luminescence “turn-on” detection of gossy polusing Ln3+-basedmetal-organic frameworks and Lnsalts. J. Am. Chem. Soc. 2020, 142, 2897–2904. [Google Scholar] [CrossRef]
- Ye, J.; Bogale, R.F.; Shi, Y.; Chen, Y.; Liu, X.; Zhang, S.; Yang, Y.; Zhao, J.; Ning, G. A water-stable dual-channel luminescence sensor for UO22+ ions based on an anionic terbium(III) metal-organic framework. Chem. A Eur. J. 2017, 23, 7657–7662. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Y.; Bai, Z.; Li, Y.; Wang, Y.; Chen, L.; Xu, L.; Chai, Z.; Wang, S. A hydrolytically stable luminescent cationic metal-organic framework for highly sensitive and selective sensing of chromate anion in natural water systems. ACS Appl. Mater. Interfaces 2017, 9, 16448–16457. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.S.; Dou, W.; Kirillov, A.M.; Liu, W.S.; Xu, C.; Xu, C.L.; Fang, R.; Yang, L.Z. Novel double layer lanthanide metal–organic networks for sensing applications. Dalton Trans. 2018, 47, 465–474. [Google Scholar] [CrossRef]
- Liu, Q.; Tan, J.Y.; Zhang, J.Y.; Zhang, N.; Deng, W. R-substituents induced structural diversity, synergistic effect and highly selective luminescence sensing for Fe3+ detection by postsynthetically modified Cd-MOF. CrystEngComm 2020, 22, 3871–3883. [Google Scholar] [CrossRef]
- Chen, J.; Chen, T.; Xiang, S.; Zhang, J.; Zhang, Z. Triazine based MOFs with abundant n sites for selective nitrobenzene detection. Z. Anorg. Allg. Chem. 2021, 647, 1301–1304. [Google Scholar] [CrossRef]
- Li, Y.W.; Li, J.; Wan, X.Y.; Sheng, D.F.; Sun, D. Nanocage-based n-rich metal–organic framework for luminescence sensing toward Fe3+ and Cu2+ ions. Inorg. Chem. 2021, 60, 671–681. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, W.; Wang, L.; Li, Z.; Song, Z.; Chen, J.; Zhang, Z.; Xiang, S.; Chen, B. Straightforward loading of imidazole molecules into metal-organic framework for high proton conduction. J. Am. Chem. Soc. 2017, 139, 15604–15607. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Samanta, P.; Desai, A.V.; Ghosh, S.K. Guest-responsive metal organic frameworks as scaffolds for sand sensing Applications. Acc. Chem. Res. 2017, 50, 2457–2469. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.J.; Song, B. A stimuli-responsive smart lanthanide nanocomposite for multidimensional optical recording and encryption. Angew. Chem. Int. Ed. 2017, 56, 2689–2693. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Y.; Yang, S.L.; Zhang, C.; Wang, Q.L. A new europium metal–organic framework with both high proton conductivity and highly sensitive detection of ascorbic acid. CrystEngComm 2018, 20, 6989–6994. [Google Scholar] [CrossRef]
- Yin, H.Q.; Wang, X.Y.; Yin, X.B. Rotation restricted emission and antenna effect in single metal-organic frameworks. J. Am. Chem. Soc. 2019, 141, 15166–15173. [Google Scholar] [CrossRef]
- Li, H.; Cao, X.; Fei, X.; Zhang, S.; Xian, Y. Nanoscaled luminescent terbium metal–organic frameworks for measuring and scavenging reactive oxygen species in living cells. J. Mater. Chem. B 2019, 7, 3027–3033. [Google Scholar] [CrossRef]
- Liu, S.; Liu, M.; Guo, M.; Wang, Z.; Tian, Z. Development of Eu-based metal-organic frameworks (MOFs) for luminescence sensing and entrapping of arsenate ion. J. Lumin. 2021, 236, 118102. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. and Sharmoukh, W. Intrinsic catalase-mimicking mofzyme for sensitive detection of hydrogen peroxide and ferric ions. Microchem. J. 2021, 163, 105873. [Google Scholar] [CrossRef]
- Gai, Y.; Guo, Q.; Zhao, X.; Yan, C.; Xiong, K.C. Extremely stable europium-organic framework for luminescent sensing of Cr2O72− and Fe3+ in aqueous systems. Dalton Trans. 2018, 47, 12051–12055. [Google Scholar] [CrossRef]
- Cui, Y.J.; Xu, H.; Yue, Y.F. A luminescent mixed-lanthanide metal-organic framework thermometer. J. Am. Chem. Soc. 2012, 134, 3979–3982. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.T.; Song, T.; Gao, J.K.; Cui, Y.; Qian, G. A highly sensitive mixed lanthanide metal-organic framework self-calibrated luminescent thermometer. J. Am. Chem. Soc. 2013, 135, 15559–15564. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Wei, S.; Cheng, P.; Zaworotko, M.J. A mixed-crystal lanthanide zeolite-like metal–organic framework as a fluorescent indicator for lysophosphatidic acid, a cancer biomarker. J. Am. Chem. Soc. 2015, 137, 12203–12206. [Google Scholar] [CrossRef]
- Yan, B.; Xu, X.Y. Fabrication and application of a ratiometric and colorimetric fluorescent probe for Hg2+ based on dual-emissive metal–organic framework hybrids with carbon dots and Eu3+. J. Mater. Chem. C 2016, 4, 1543–1549. [Google Scholar]
- Wu, J.X.; Yan, B. A dual-emission probe to detect moisture and water in organic solvents based on green-Tb3+ post-coordinated metal–organic frameworks with red carbon dots. Dalton Trans. 2017, 46, 7098–7105. [Google Scholar] [CrossRef]
- Xin, F.; Rui, L.; Jian, S.; Hui, L.; Xin, L. A dual-emission nano-rod MOF equipped with carbon dots for visual detection of doxycycline and sensitive sensing of MnO4. RSC Adv. 2018, 8, 4766–4772. [Google Scholar]
- Jintana, O.; Boonmak, J.; Promarak, V. Sonochemical synthesis of carbon dots/lanthanoid MOFs hybrids for white light-emitting diodes with high color rendering. ACS Appl. Mater. Interfaces 2019, 11, 44421–44429. [Google Scholar]
- Gao, J.P.; Yao, R.X.; Chen, X.H.; Li, H.H.; Zhang, X.M. Blue luminescent N,S-doped carbon dots encapsulated in red emissive Eu-MOF to form dually emissive composite for reversible anti-counterfeit ink. Dalton Trans. 2020, 50, 1690–1696. [Google Scholar] [CrossRef]
- Silva, M.A.; Campos, N.R.; Ferreira, L.A.; Flores, L.S.; Júnior, J.A.; Santos, G.L.; Corrêa, C.C.; Santos, T.C.; Ronconi, C.M.; Colaço, M.V.; et al. A new photoluminescent terbium(III) coordination network constructed from 1,2,4,5-benzenetetracarboxylic acid: Synthesis, structural characterization and application as a potential marker for gunshot residues. Inorg. Chim. Acta 2019, 495, 118967. [Google Scholar] [CrossRef]
- Hu, X.F.; Wang, Z.Y.; Su, Y.M.; Chen, P.C.; Chen, J.W.; Zhang, C.K.; Wang, C. Nanoscale metal−organic frameworks and metal−organic layers with two-photon-excited fluorescence. Inorg. Chem. 2020, 59, 4181–4185. [Google Scholar] [CrossRef]
- Hao, J.N.; Yan, B. A dual-emitting 4d–4f nanocrystalline metal–organic framework as a self-calibrating luminescent sensor for indoor formaldehyde pollution. Nanoscale 2016, 8, 12047–12053. [Google Scholar] [CrossRef]
- Zhou, L.M.; Gao, L.J.; Fang, S.M.; Sun, G.H.; Hu, M.; Guo, L.Q.; Han, C.; Zhang, L.C. Preparation and characterization of novel transparent Eu(AA)3-polyurethane acrylate copolymeric materials. J. Appl. Polym. Sci. 2012, 125, 690–696. [Google Scholar] [CrossRef]
- Yang, C.; Liu, S.; Xu, J.; Li, Y.; Shang, M.; Lei, L.; Wang, G.; He, J.; Wang, X.; Lu, M. Efficient red emission from poly(vinyl butyral) films doped with a novel europium complex based terpyridyl as ancillary ligand: Synthesis, structural elucidation by Sparkle/RM1 calculation, and photophysical properties. Polym. Chem. 2016, 7, 1147–1157. [Google Scholar] [CrossRef]
- Wang, Y.M.; Tian, X.T.; Zhang, H.; Yang, Z.R.; Yin, X.B. Anticounterfeiting quick response code with emission color of invisible metal-organic frameworks as encoding information. Acs Appl. Mater. Interfaces 2018, 10, 22445–22452. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lin, Y.; Liu, J.; Shi, W.; Yang, G.; Cheng, P. Rapid detection of the biomarkers for carcinoid tumors by a water stable luminescent lanthanide metal-organic framework sensor. Adv. Funct. Mater. 2018, 28, 1707169. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, D.; Jia, P.; Gao, W.; Li, Y.; He, J.; Wang, C.; Zheng, X.; Yang, Q.; Yang, C. Bonded-luminescent foam based on europium complexes as a reversible copper (II) ions sensor in pure water. Eur. Polym. J. 2019, 112, 461–465. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, D.; Jia, P.; Gao, W.; Li, Y.; Bai, Z.; Liu, X.; Deng, Q.; Xu, J.; Yang, C. Highly selective and sensitive long fluorescence lifetime polyurethane foam sensor based on Tb-complex as chromophore for the detection of H2PO4− in water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 217, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, K.A.; Sokolovskii, G.S.; Khomylev, M.; Livshits, D.A.; Rafailov, E.U. Efficient yellow-green light generation at 561nm by frequency-doubling of a QD-FBG laser diode in a PPLN waveguide. Opt. Lett. 2014, 39, 6672–6674. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Feng, L.; Zhang, B.Y.; Zhang, X.M.; Liu, J.P.; Gao, E.Q. 2D carboxylate-bridged LnIII coordination polymers: Displaying slow magnetic relaxation and luminescence properties in the detection of Fe3+, Cr2O72− and nitrobenzene. Dalton Trans. 2017, 46, 13878–13887. [Google Scholar] [CrossRef]
- Ma, J.J.; Liu, W.S. Effective luminescence sensing of Fe3+, Cr2O72−, MnO4− and 4-nitrophenol by lanthanide metal–organic frameworks with a new topology type. Dalton Trans. 2019, 48, 12287–12295. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, J.P.; Zhou, Z.; Wang, R.; Zang, S.Q. Robust lanthanide metal–organic frameworks with “all-in-one” multifunction: Efficient gas adsorption and separation, tunable light emission and luminescent sensing. J. Mater. Chem. C 2021, 9, 3429–3439. [Google Scholar] [CrossRef]
- Xu, H.; Hu, H.C.; Cao, C.S.; Zhao, B. Lanthanide organic framework as a regenerable luminescent probe for Fe3+. Inorg. Chem. 2015, 54, 4585–4587. [Google Scholar] [CrossRef]
- Kang, Y.; Zheng, X.J.; Jin, L.P. A microscale multi-functional metal-organic framework as a fluorescence chemosensor for Fe3+, Al3+ and 2-hydroxy-1-naphthaldehyde. J. Colloid Interface 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Gu, J.Z.; Cai, Y.; Liu, Y.; Liang, X.X.; Kirillov, A.M. New lanthanide 2d coordination polymers constructed from a flexible ether-bridged tricarboxylate block: Synthesis, structures and luminescence sensing. Inorg. Chim. Acta 2018, 1, 98–104. [Google Scholar] [CrossRef]
- Du, Y.; Yang, H.Y.; Liu, R.J.; Shao, C.Y.; Yang, L.R. A multi-responsive chemosensor for highly sensitive and selective detection of Fe3+, Cu2+, Cr2O72− and nitrobenzene based on a luminescent lanthanide metal–organic framework. Dalton Trans. 2020, 49, 13003–13016. [Google Scholar] [CrossRef] [PubMed]



| Ln-MOFs | C | H | N | O | Eu/Tb |
|---|---|---|---|---|---|
| Eu-MOF | 30.37% | 1.87% | 1.87% | 26.64% | 39.25% |
| Tb-MOF | 22.01% | 1.84% | 1.13% | 26.11% | 48.91% |
| Eu0.075Tb0.925-MOF | 23.53% | 1.85% | 1.56% | 26.85% | 46.21% |
| Ln-MOFs | Detect Ion | LOD (M) | Ratio Fluorescent Probe | Linear Range | References |
|---|---|---|---|---|---|
| Eu0.075Tb0.925-MOF | Fe3+ | 2.71 × 10−7 | Dual emission | 10–100 μM (R2 = 0.99919, R2 = 0.99937) | This work |
| Cr2O72− | 8.72 × 10−7 | ||||
| Eu-MOF; Tb-MOF [Eu/Tb, 4,4′-(((5- carboxy-1,3-phenylene)bis(azanediyl))bis(carbonyl)) dibenzoic acid] | Fe3+ | 1 × 10−5 | Single emission | 0–1.0 mM (R2 = 0.9021, R2 = 0.9752) | [47] |
| Cr2O72− | 8.94 × 10−5 | ||||
| Eu-MOF [Eu, 5-(2′,5′-dicarboxylphenyl) picolinic acid ligand] | Fe3+ | 5.7 × 10−7 | Single emission | 0–50 μM (R2 = 0.9948, R2 = 0.9979) | [48] |
| Cr2O72− | 4.2 × 10−7 | ||||
| Tb-MOF [Tb,H3BTB] | Fe3+ | 1 × 10−5 | Single emission | - | [49] |
| Eu-MOF [Eu, 2-aminoterephthalic acid 1,10-phenanthroline] | Fe3+ | 4.5 × 10−5 | Single emission | 0–0.25 mM (R2 = 0.992) | [50] |
| Tb-MOF [Tb, 2-(2-carboxyphenoxy)terephthalic acid] | Fe3+ | 2.0 × 10−4 | Single emission | 10−4–10−3 M (R2 = 0.978) | [51] |
| Eu-MOF [Eu, 2-(3′,4′-dicarboxylphenoxy)isophthalic acid, 4,4′-bis(imidazolyl) biphenyl | Fe3+ | 1.32 × 10−5 | Single emission | 0–10−5 M (R2 = 0.9885, R2 = 0.9927) | [52] |
| Cr2O72− | 1.01 × 10−5 |
| Sample | Spiked (nM) | Found (nM) | Recovery (%) |
|---|---|---|---|
| Tap water (Fe3+) | 20.0 | 22.1 | 110.5 |
| 40.0 | 45.7 | 114.3 | |
| 60.0 | 61.6 | 102.7 | |
| 800 | 88.7 | 110.9 | |
| Tap water (Cr2O72−) | 20.0 | 20.9 | 104.5 |
| 40.0 | 41.3 | 103.3 | |
| 60.0 | 60.9 | 101.5 | |
| 80.0 | 80.8 | 101.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Chu, H.; Qin, S.; Qi, H.; Hu, M. Preparation of Eu0.075Tb0.925-Metal Organic Framework as a Fluorescent Probe and Application in the Detection of Fe3+ and Cr2O72−. Sensors 2021, 21, 7355. https://doi.org/10.3390/s21217355
Yin J, Chu H, Qin S, Qi H, Hu M. Preparation of Eu0.075Tb0.925-Metal Organic Framework as a Fluorescent Probe and Application in the Detection of Fe3+ and Cr2O72−. Sensors. 2021; 21(21):7355. https://doi.org/10.3390/s21217355
Chicago/Turabian StyleYin, Jie, Hongtao Chu, Shili Qin, Haiyan Qi, and Minggang Hu. 2021. "Preparation of Eu0.075Tb0.925-Metal Organic Framework as a Fluorescent Probe and Application in the Detection of Fe3+ and Cr2O72−" Sensors 21, no. 21: 7355. https://doi.org/10.3390/s21217355
APA StyleYin, J., Chu, H., Qin, S., Qi, H., & Hu, M. (2021). Preparation of Eu0.075Tb0.925-Metal Organic Framework as a Fluorescent Probe and Application in the Detection of Fe3+ and Cr2O72−. Sensors, 21(21), 7355. https://doi.org/10.3390/s21217355





