Blood Pressure Continuous Measurement through a Wearable Device: Development and Validation of a Cuffless Method
Abstract
:1. Introduction
- Patients may perceive the cuff pressure as being unbearable, especially during sleep, in the case of very high BP and if it is required to frequently repeat the readings;
- Avoiding false elevation or reduction in BP readings and obtaining an accurate measurement require the selection of an appropriate cuff size according to the users’ upper arm circumference;
- Readings by conventional devices may be insufficient indicators for hypertension since only intermittent measurements are provided. These devices cannot record time-varying BP or capture the dynamic state of the cardiovascular system throughout the day.
1.1. Continuous Cuffless BP Measurement Techniques
Time-Delay Method
- SOMNOtouch NIBP [6], designed by SOMNOmedics, estimates blood pressure through the simultaneous recording of 3-lead ECG signal using wet electrodes and finger PPG signal. Initial calibration with a conventional BP measurement technique is required and its main application is for sleep monitoring.
- CareUp [7] is a smartwatch with an embedded algorithm for blood pressure detection using two different PPG waveforms. One PPG waveform is taken from the back sensor of the watch directly in contact with the wrist skin, and the second is acquired by positioning the index finger of the other hand on the front oximeter sensor. CareUp requires an initial calibration with a conventional BP measurement technique, and the BP estimation is performed intermittently when the subject puts their index finger on the oximeter sensor.
- SeismoWatch [8] is another smartwatch, obtaining blood pressure readings by asking the user to place the upper part of the watch onto their sternum for a short period. An accelerometer inside the watch measures the thoracic vibrations associated with the heartbeat, the seismocardiogram (SCG), to obtain the proximal timing indicating the blood ejection from the left ventricle into the aorta. An optical sensor on the watch measures the photoplethysmographic signal to obtain the distal timing associated with the arrival of the pulse wave at the wrist radial artery. SeismoWatch requires an initial calibration with a conventional BP measurement. The BP estimation is performed intermittently when the subject puts the watch on their sternum.
1.2. Aim
2. Materials and Methods
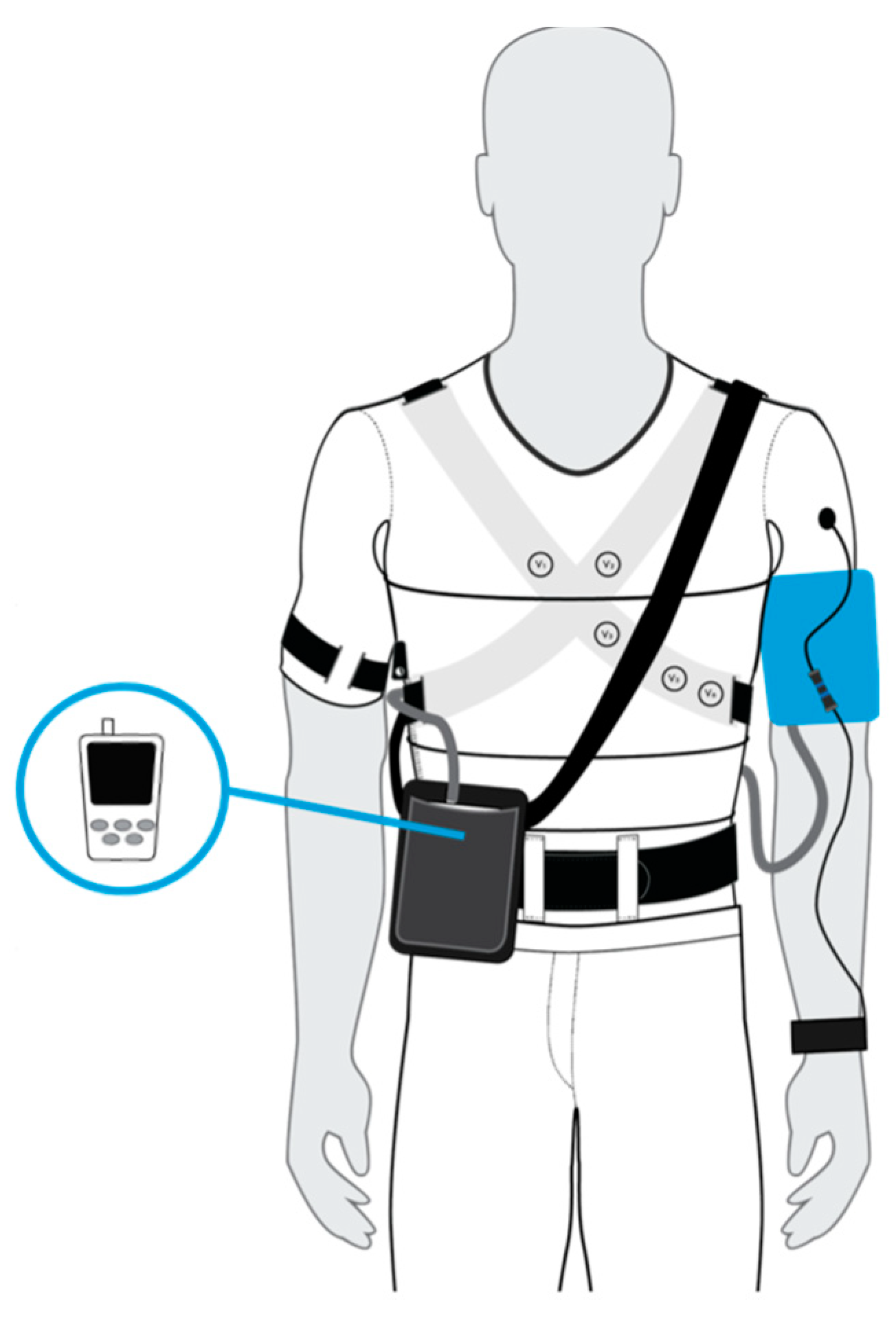
2.1. Reference and Test Devices
- A 12-lead ECG signal by using 10 ink-based dry electrodes;
- A 3-channel respiratory signal by using strain circumferential sensors positioned around the body at the thoracic level, xiphoid process, and abdominal level;
- Skin temperature by using a contact sensor near the left armpit;
- Activity level and body position by using one inertial measurement unit (IMU) on the back.
2.2. Population
2.3. Data Acquisition Protocol
- HANDGRIP PROTOCOLIn order to assess the reliability of the test device against minor blood pressure variations, subjects were called to perform an isometric handgrip (IHG) exercise with the right arm [14]. Three reference blood pressure measurements were performed: one during the rest, one immediately after the exercise, and the last after the recovery. The exercise was repeated on two different days to assess the intrasubject variability.The subject had to be seated in rest position for 5 min, and then a blood pressure value was measured with the reference device. The exercise consisted of four 1.5 min contractions of an IHG exercise at 40% maximum effort, with 30 s of recovery periods. For the maximum contraction value determination, the subject was called to perform three grips at the maximum intensity with 10 s rest periods to avoid fatigue; then, the maximum contraction value was given by the average. At the end of the four trials, while the test device was still recording both PPG and ECG signals, the blood pressure was being measured with the reference device. Two minutes after the end of the exercise, with the subject still in rest position, blood pressure was measured with the reference device.
- RUNNING PROTOCOLIn order to assess the reliability of the test device to more significant blood pressure variations, the subjects were asked to perform running repetitions alternated with walking recovery periods [15] for a total of 10 reference blood pressure measurements immediately after the end of each specific phase. Even in this case, the exercise was repeated on two different days to assess the intrasubject variability.The subject had to be seated in rest position for 5 min, and then a blood pressure value was measured with the reference device. After that, the exercise started: 5 min of warm-up walking; 2 min of running at maximum speed; additional 2 min of running at maximum speed; 4 min of recovery walking; 3 min of sprint running; 6 min of recovery walking; 1 min of skipping; 10 min of recovery walking. After each of the aforementioned steps, a BP measurement was performed with the reference device with the subject in a standing position. The subject had to be seated again in the rest position immediately after the recovery walking, and their blood pressure was measured with the reference device. After 2 min in the same position, we proceeded with another measurement.
- DAILY PROTOCOLThe reference device occlusion occurred automatically every 15 min. At the beginning of each reference device occlusion and for its duration, subjects had to stop and maintain their position to reduce measurement artefacts.As an extension of the daily protocol, both devices could also be worn during the night to assess the reliability of the test device against the detection of blood pressure variations during sleep. In this case, the reference device occlusion occurred automatically every 15 min.Data from the daily protocol were valuable to understand the reliability of the test device in a real scenario, continuously monitoring blood pressure during daily activities. For this reason, and for the uncontrolled conditions of the protocol, these data were used only for model test. In addition, real use-case scenario data trends were implemented.
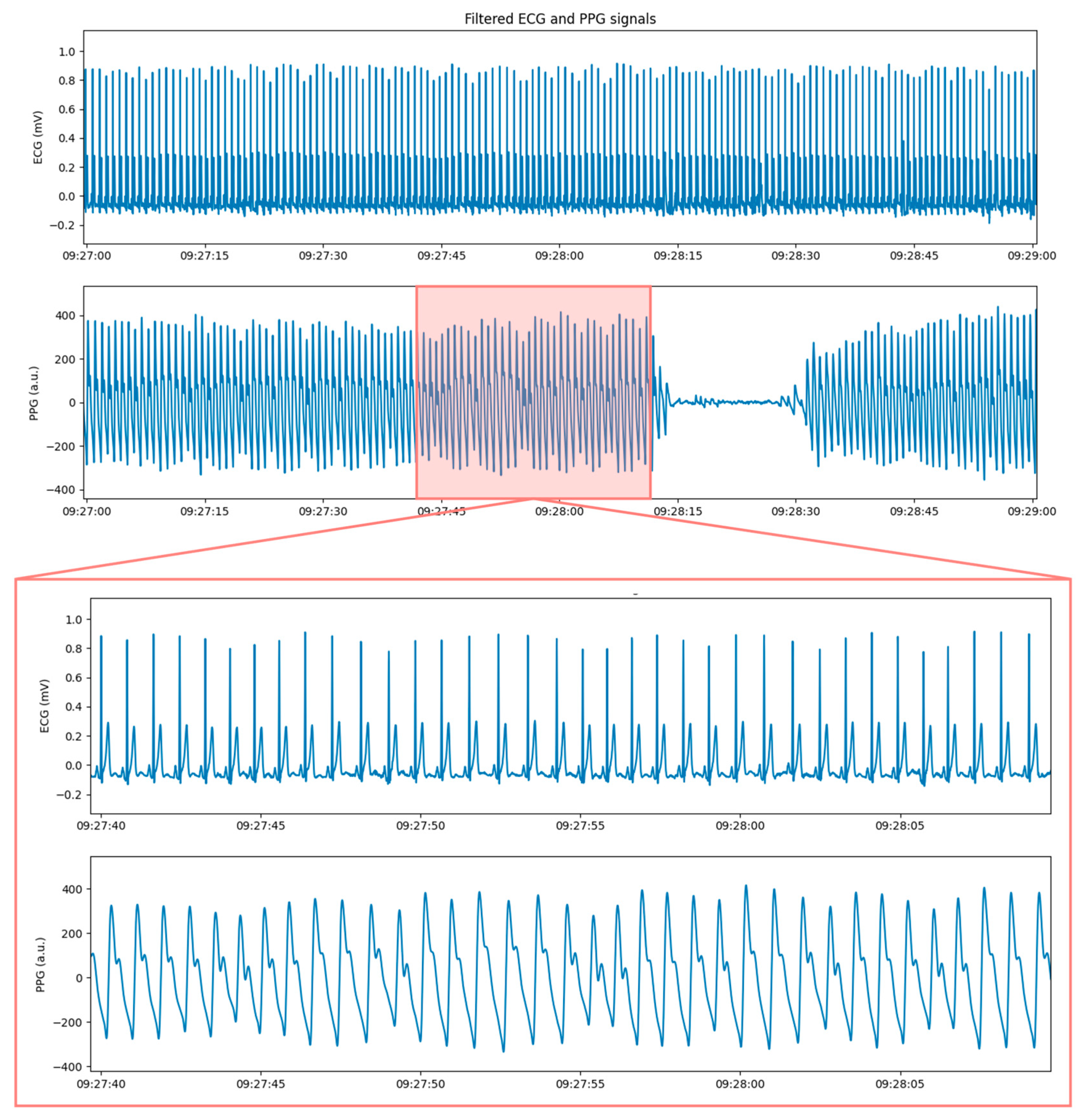
2.4. PPG Features
- Maximum of the waveform’s first derivative (Md).
- Maximum of the waveform, representing the systolic peak (P).
- Minimum of the waveform, representing the diastolic valley (V).
- Pulse arrival time at the maximum of the waveform’s first derivative (PATMd).
- Pulse arrival time at the systolic peak (PATP).
- Pulse arrival time at the diastolic valley (PATV); it is important to highlight that, for this feature, it is necessary to consider the minimum within the following RR period.
- Systolic time (𝑆𝑌𝑆𝑇𝐼𝑀𝐸): representing the time occurring from the wave start (identified with the previous diastolic valley) to the systolic peak;
- Diastolic time (𝐷𝐼𝐴𝑇𝐼𝑀𝐸): representing the time from the systolic peak to the diastolic valley;
- Duty cycle (DC): representing the ratio of the systolic time and the total PPG wave duration, given by the sum of the systolic and diastolic times ().
2.5. Features Extraction Algorithm
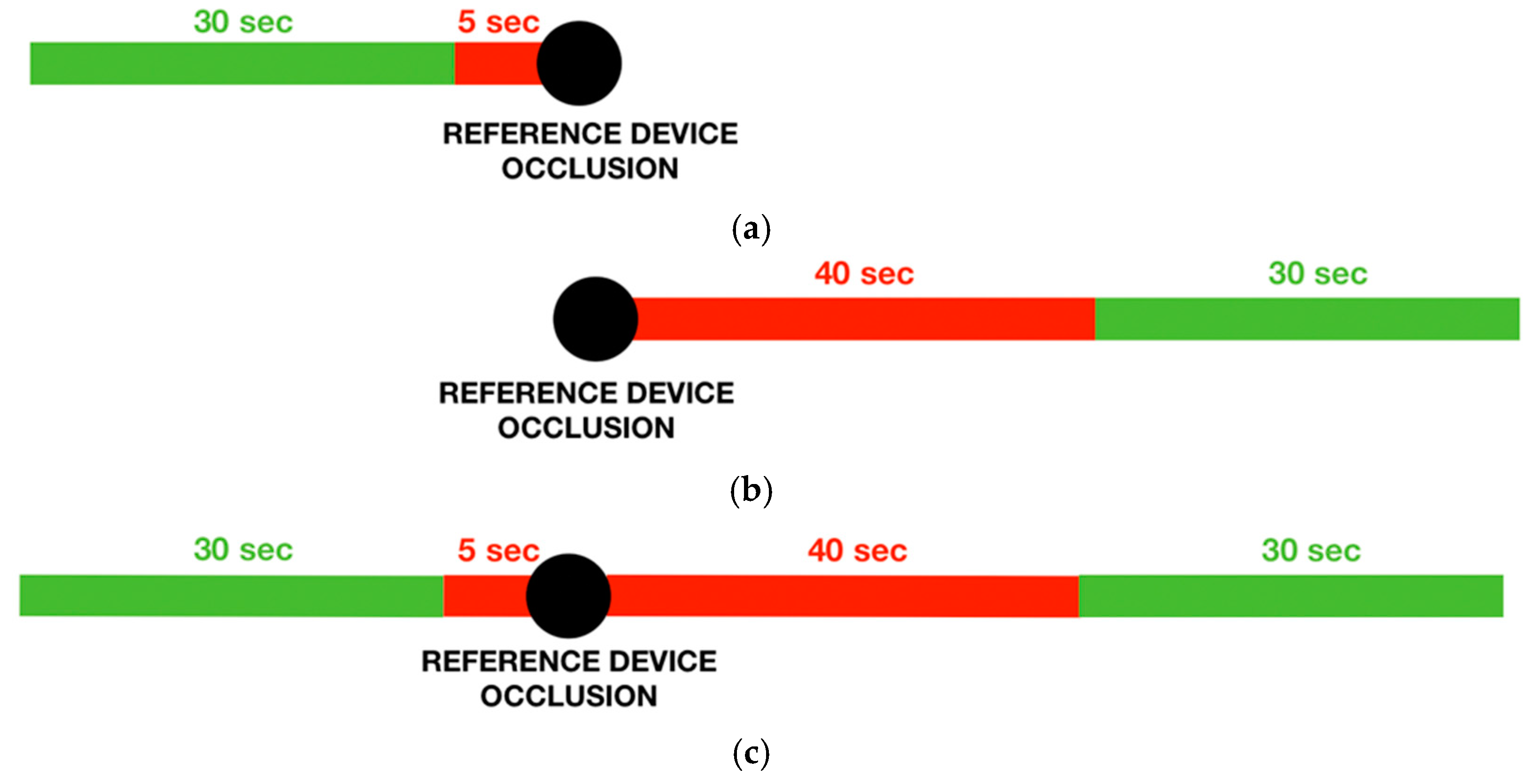
- PREVIOUS LOGICThe previous logic selects a 30 s window before the occlusion starting time, removing the first 5 s to avoid possible artefacts in the PPG wave due to the occlusion. This logic was used for the handgrip protocol, in which the subject was in the rest position throughout the protocol, and the stimulus entity was small. Following this logic, it was possible to consider the test device signal during the stimulus occurrence.
- FOLLOWING LOGICThe following logic selects a 30-s window after the occlusion starting time, removing the first 45 s to wait for a normal PPG signal after the end of the occlusion. This logic was used for the running protocol, in which subjects needed to stop the activity before the reference device measurement. Because of the larger entity of the stimulus, also a signal window immediately after the end of the activity could be appropriate.
- PREVIOUS AND FOLLOWING LOGICThe previous and following logic selects two 30 s windows, one following the previous logic and the other one, the following logic. This logic was used for the daily protocol, in which the subjects’ activity immediately before and after the occlusion could be considered equivalent.
3. Results
3.1. Enrolled Population
- HANDGRIP PROTOCOLFor the handgrip protocol, 26 subjects (15 men and 11 women) with an average age of 35 (±11 std) years were enrolled. All the enrolled subjects successfully completed the protocol on the first day. Four subjects did not repeat the protocol on the second day because they were unavailable during the second acquisition period. The quality of one subject’s signals was insufficient for the ECG and PPG signals quality check block of the features extraction algorithm that was not able to extract good quality windows close to the reference device measurement; therefore, this subject was subsequently removed from the dataset. The low quality, in particular of the PPG signal, can result due to an unsuitable sensor’s position or exerted pressure. The handgrip protocol dataset consisted of 25 subjects for the first day and 21 subjects for the second day. The dataset dimension is compliant with the validation study sample size of 20 subjects required by the Institute of Electrical and Electronics Engineers (IEEE) standard [20] for the first phase validation.
- RUNNING PROTOCOLFor the running protocol, three subjects (one man and two women) with an average age of 30 (±6 std) years were enrolled. All the three enrolled subjects successfully completed the protocol on both days, and all the data were included in the dataset.
- DAILY PROTOCOLFor the daily protocol, 20 subjects (12 men and eight women) with an average age of 36 (±11 std) years were enrolled. A total of 12 subjects (six men and six women) successfully completed the protocol, with an acquisition period higher than the required five hours. The other eight subjects had lower acquisition periods due to technical problems during the acquisitions. A 24-year-old woman assessed the reliability of the device also during the night.
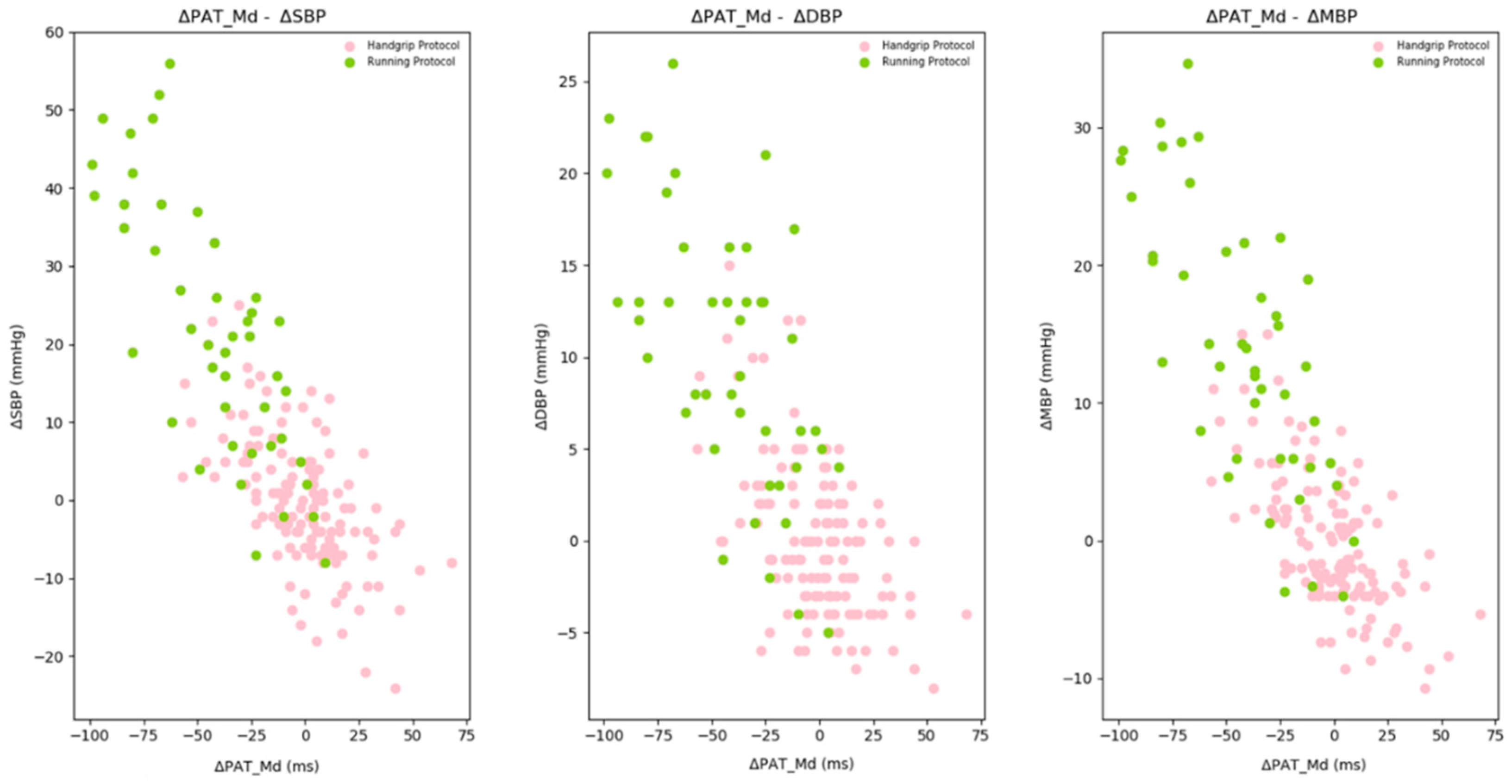
3.2. Statistical Handgrip Protocol Data Analysis
- The difference between effort and rest values, called ΔEffort: a blood pressure increase and a corresponding PATMd decrease are expected.
- The difference between recovery and effort values, called ΔRecovery: a blood pressure decrease and a corresponding PATMd increase are expected.
- The difference between recovery and rest values, called ΔRest: these two values are expected to be very similar, so the difference needs to be around zero.
3.3. Statistical Training Dataset Analysis
3.4. Linear Regression Model for Blood Pressure Estimation
3.5. Models’ Performances Assessment
- SBP MODELFor the SBP model, 84.0% of the errors was under 10 mmHg (Table 6), and the MAE was 6 mmHg. From the corresponding Bland–Altman plot (Figure 10a), the model error did not seem dependent on a specific mean value. No significant bias was present: the average of the differences between the paired data was −0.62 mmHg. In addition, more than 94% of the data were within both the 15 mmHg and the 1.96 s ranges.
- DBP MODELFor the DBP model, 96.5% of the errors were less than 10 mmHg (Table 6), and the MAE was 4 mmHg. The 1.96s ranges corresponded with the 10 mmHg ones, and over 96% of the data were within them. The corresponding Bland–Altman plot is shown in Figure 10b.No significant bias was present: the average of the differences between the paired data was −0.67 mmHg. Even in this case, no relationship seems to be present between the differences and mean values. However, if high differences were recorded, these were negative and correlated with significant and positive blood pressure variations.
- MBP MODELFor the MBP model, 95.4% of the errors were less than 10 mmHg (Table 6), and the MAE was 4 mmHg. The 1.96 s ranges corresponded with the 10 mmHg ones, and over 93% of the data were within them. No significant bias was present: the average of the differences between the paired data was −0.67 mmHg. The corresponding Bland–Altman plot is shown in Figure 10c. Even in this case, no relationship seems to be present between differences and mean values.
3.6. Models’ Test
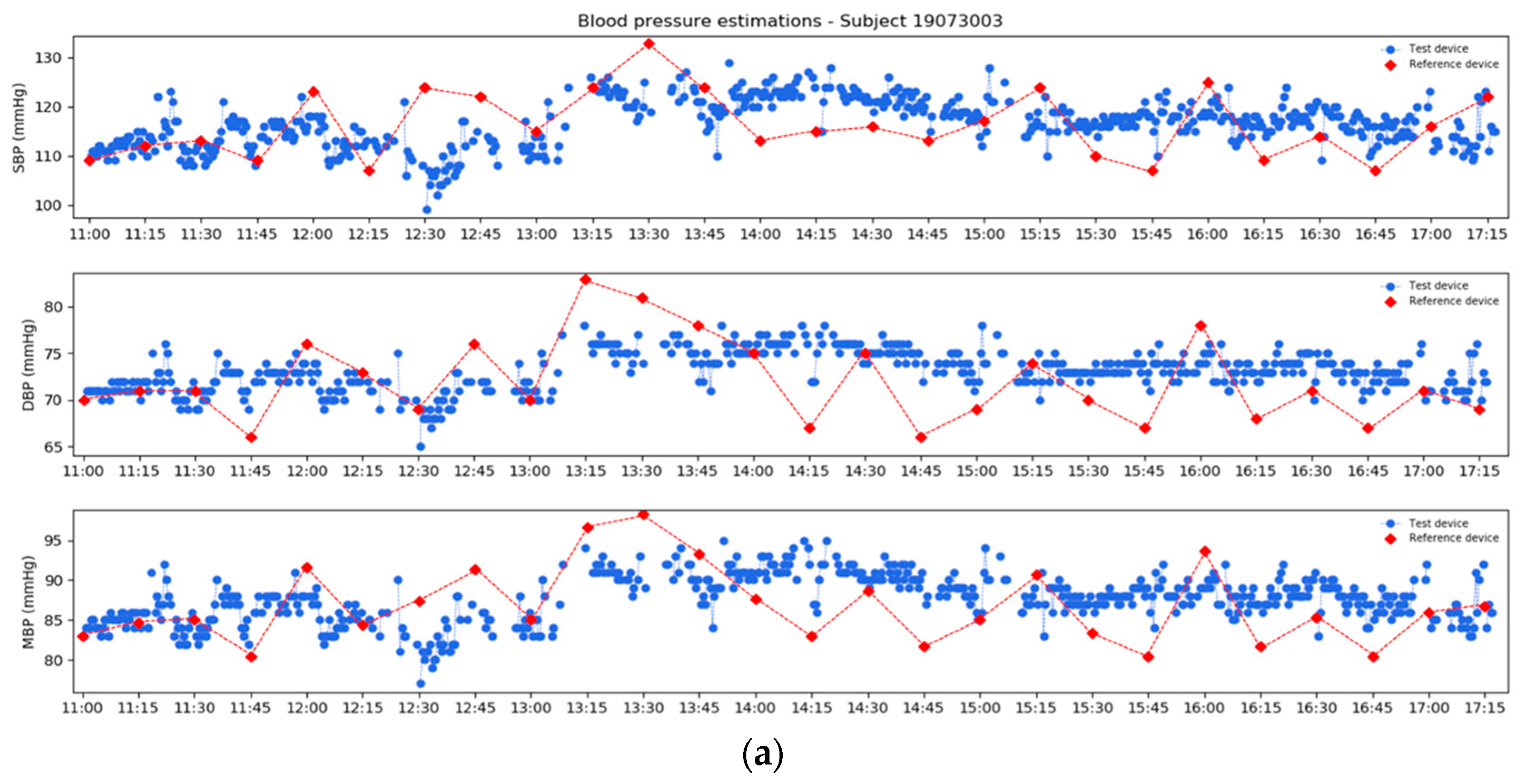
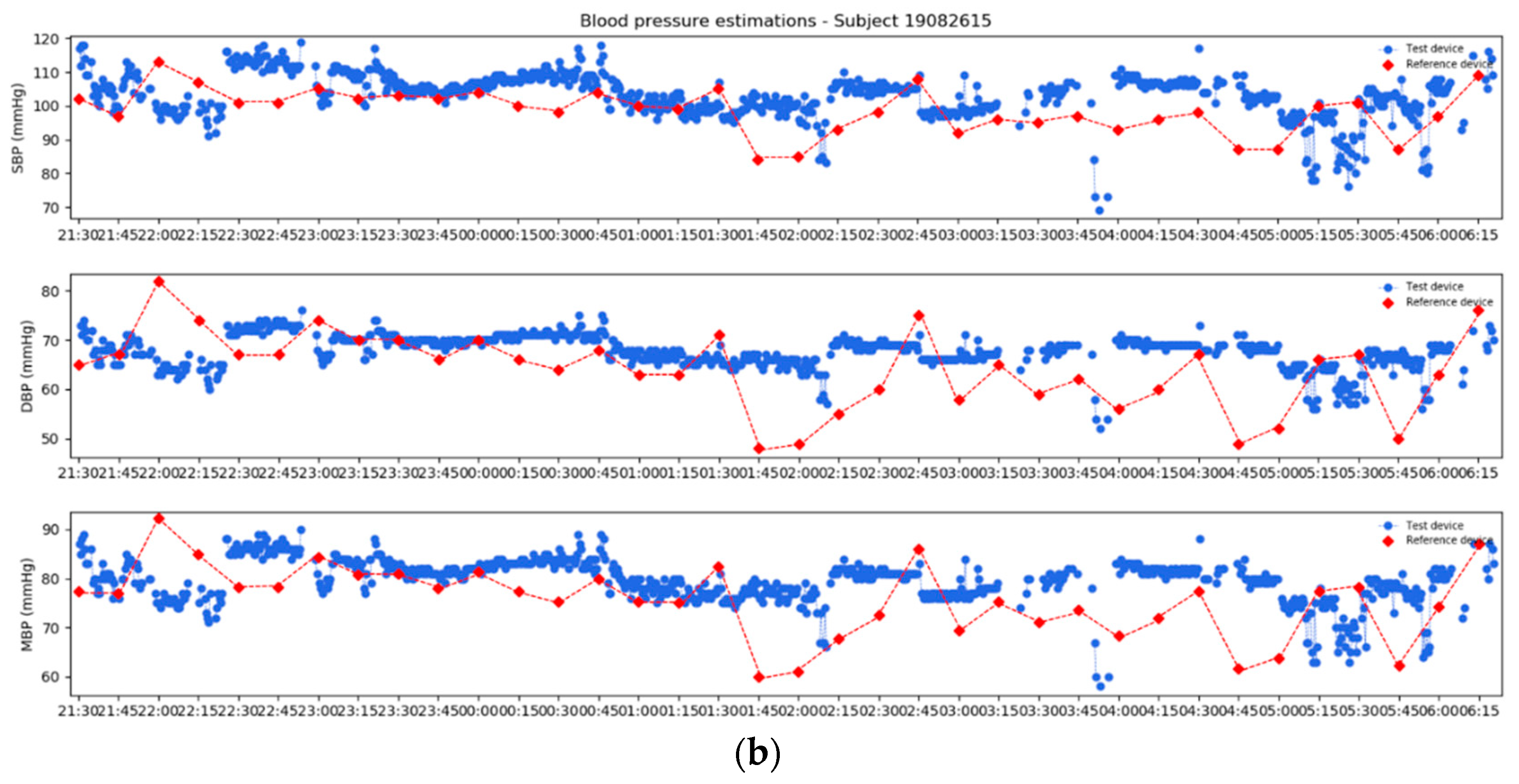
3.7. Real Use Case Scenario
4. Discussion
4.1. Limits
4.2. Strengths
4.3. Future Developments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grossman, E. Ambulatory Blood Pressure Monitoring in the Diagnosis and Management of Hypertension. Diabetes Care 2013, 36, S307–S311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattivelli, F.S.; Garudadri, H. Noninvasive Cuffless Estimation of Blood Pressure from Pulse Arrival Time and Heart Rate with Adaptive Calibration. In 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks; IEEE: Piscataway, NJ, USA, 2009; pp. 114–119. [Google Scholar] [CrossRef]
- Sharma, M.; Barbosa, K.; Ho, V.; Griggs, D.; Ghirmai, T.; Krishnan, S.K.; Hsiai, T.K.; Chiao, J.-C.; Cao, H. Cuff-Less and Continuous Blood Pressure Monitoring: A Methodological Review. Technologies 2017, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Elgendi, M.; Fletcher, R.; Liang, Y.; Howard, N.; Lovell, N.H.; Abbott, D.; Lim, K.; Ward, R. The use of photoplethysmography for assessing hypertension. NPJ Digit. Med. 2019, 2, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanfranchi, A.; Pépin, J.; Somers, V. Chapter 14—Cardiovascular Physiology: Autonomic Control in Health and in Sleep Disorders. In Principles and Practice of Sleep Medicine (Sixth Edition); Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Bilo, G.; Zorzi, C.; Munera, J.E.O.; Torlasco, C.; Giuli, V.; Parati, G. Validation of the Somnotouch-NIBP noninvasive continuous blood pressure monitor according to the European Society of Hypertension International Protocol revision 2010. Blood Press. Monit. 2015, 20, 291–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazazzera, R.; Belhaj, Y.; Carrault, G. A New Wearable Device for Blood Pressure Estimation Using Photoplethysmogram. Sensors 2019, 19, 2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carek, A.M.; Conant, J.; Joshi, A.; Kang, H.; Inan, O.T. SeismoWatch: Wearable Cuffless Blood Pressure Monitoring Using Pulse Transit Time. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2017, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- GIMA ABPM50—User Manual. Available online: https://www.gimaitaly.com/DocumentiGIMA/Manuali/EN/M35110EN.pdf (accessed on 5 October 2021).
- GIMA ABPM50—Declaration of Conformity. Available online: https://www.gimaitaly.com/DocumentiGIMA/DC/d33676.pdf (accessed on 5 October 2021).
- Sarmento, A.; Vignati, C.; Paolillo, S.; Lombardi, C.; Scoccia, A.; Nicoli, F.; Mapelli, M.; Leonardi, A.; Ossola, D.; Rigoni, R.; et al. Qualitative and quantitative evaluation of a new wearable device for ECG and respiratory Holter monitoring. Int. J. Cardiol. 2018, 272, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Contini, M.; Sarmento, A.; Gugliandolo, P.; Leonardi, A.; Longinotti-Buitoni, G.; Minella, C.; Vignati, C.; Mapelli, M.; Aliverti, A.; Agostoni, P. Validation of a new wearable device for type 3 sleep test without flowmeter. PLoS ONE 2021, 16, e0249470. [Google Scholar] [CrossRef] [PubMed]
- Maxim Integrated MAX30102—Datasheet. Available online: https://datasheets.maximintegrated.com/en/ds/MAX30102.pdf (accessed on 5 October 2021).
- Garg, R.; Malhotra, V.; Kumar, A.; Dhar, U.; Tripathi, Y. Effect of Isometric Handgrip Exercise Training on Resting Blood Pressure in Normal Healthy Adults. J. Clin. Diagn. Res. 2014, 8, BC08–BC10. [Google Scholar] [CrossRef]
- Sharman, J.; La Gerche, A. Exercise blood pressure: Clinical relevance and correct measurement. J. Hum. Hypertens. 2015, 29, 351–358. [Google Scholar] [CrossRef]
- Elgendi, M. On the Analysis of Fingertip Photoplethysmogram Signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Orphanidou, C. Quality Assessment for the Electrocardiogram (ECG). In Signal Quality Assessment in Physiological Monitoring; Springer Briefs in Bioengineering; Springer: Cham, Switzerland, 2018; pp. 15–40. [Google Scholar]
- Elgendi, M. Optimal Signal Quality Index for Photoplethysmogram Signals. Bioengineering 2016, 3, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, E.; Vergara, J.M.; Laguna, P. Detection of decreases in the amplitude fluctuation of pulse photoplethysmography signal as indication of obstructive sleep apnea syndrome in children. Biomed. Signal Process. Control. 2008, 3, 267–277. [Google Scholar] [CrossRef]
- IEEE Standard Association. IEEE Standard for Wearable Cuffless Blood Pressure Measuring Devices; IEEE Standard Association: Piscataway, NJ, USA, 2014; pp. 1708–2014. [Google Scholar] [CrossRef]







| All (26 Subjects) | Men (15 Subjects) | Women (11 Subjects) | |
|---|---|---|---|
| Mean ± Std | Mean ± Std | Mean ± Std | |
| Age (years) | 35 ± 11 | 38 ± 12 | 34 ± 9 |
| Height (cm) | 173 ± 7.26 | 177 ± 6.40 | 168 ± 3.45 |
| Weight (kg) | 67 ± 8.99 | 72 ± 6.10 | 61 ± 8.40 |
| BMI (kg/m2) | 22 ± 2.30 | 23 ± 1.76 | 22 ± 2.80 |
| Smoking | 3 out of 26 | 2 out of 15 | 1 out of 11 |
| Regular physical activity | 19 out of 26 | 10 out of 15 | 9 out of 11 |
| r | p-Value | |
|---|---|---|
| ΔSBP and ΔPATMd | −0.61 | 1.98 × 10−14 |
| ΔDBP and ΔPATMd | −0.53 | 8.82 × 10−11 |
| ΔMBP and ΔPATMd | −0.65 | 3.67 × 10−17 |
| ΔPAT (ms) | ΔSBP (mmHg) | ΔDBP (mmHg) | ΔMBP (mmHg) | |
|---|---|---|---|---|
| Effort–Rest | −18.0 22.5 | 8.0 9.00 | 3.0 5.00 | 4.0 5.33 |
| Recovery–Effort | 16 24.50 | −7.0 7.50 | −3.0 4.00 | −3.67 4.16 |
| Recovery–Rest | 0.0 17.50 | 0.0 5.50 | −1.0 4.50 | −1.0 4.33 |
| Variables | r | p-Value |
|---|---|---|
| ΔSBP and ΔPATMd | −0.80 | 4.26 × 10−40 |
| ΔDBP and ΔPATMd | −0.73 | 1.52 × 10−30 |
| ΔMBP and ΔPATMd | −0.81 | 3.21 × 10−41 |
| SBP | DBP | MBP | |
|---|---|---|---|
| r (p-Value) | r (p-Value) | r (p-Value) | |
| PATMd | −0.80 (<0.001) | −0.73 (<0.001) | −0.79 (<0.001) |
| PATP | −0.46 (<0.001) | −0.50 (<0.001) | −0.55 (<0.001) |
| PATV | −0.78 (<0.001) | −0.72 (<0.001) | −0.82 (<0.001) |
| SYSTIME | −0.07 (=0.33) | −0.13 (=0.10) | −0.16 (=0.03) |
| DIATIME | −0.75 (<0.001) | −0.65 (<0.001) | −0.75 (<0.001) |
| DC | 0.45 (<0.001) | 0.35 (<0.001) | 0.39 (<0.001) |
| SBP Model | DBP Model | MBP Model | |
|---|---|---|---|
| # of Values (%) | # of Values (%) | # of Values (%) | |
| 5 mmHg | 99 out of 174 (56.9%) | 135 out of 174 (77.6%) | 135 out of 174 (77.6%) |
| 10 mmHg | 146 out of 174 (84.0%) | 168 out of 174 (96.5%) | 166 out of 174 (95.4%) |
| 15 mmHg | 164 out of 174 (94.25%) | 174 out of 174 (100%) | 173 out of 174 (99.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Marchi, B.; Frigerio, M.; De Nadai, S.; Longinotti-Buitoni, G.; Aliverti, A. Blood Pressure Continuous Measurement through a Wearable Device: Development and Validation of a Cuffless Method. Sensors 2021, 21, 7334. https://doi.org/10.3390/s21217334
De Marchi B, Frigerio M, De Nadai S, Longinotti-Buitoni G, Aliverti A. Blood Pressure Continuous Measurement through a Wearable Device: Development and Validation of a Cuffless Method. Sensors. 2021; 21(21):7334. https://doi.org/10.3390/s21217334
Chicago/Turabian StyleDe Marchi, Beatrice, Mattia Frigerio, Silvia De Nadai, Gianluigi Longinotti-Buitoni, and Andrea Aliverti. 2021. "Blood Pressure Continuous Measurement through a Wearable Device: Development and Validation of a Cuffless Method" Sensors 21, no. 21: 7334. https://doi.org/10.3390/s21217334
APA StyleDe Marchi, B., Frigerio, M., De Nadai, S., Longinotti-Buitoni, G., & Aliverti, A. (2021). Blood Pressure Continuous Measurement through a Wearable Device: Development and Validation of a Cuffless Method. Sensors, 21(21), 7334. https://doi.org/10.3390/s21217334








