Wearable Electronic Tongue for Non-Invasive Assessment of Human Sweat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Electrochemical Measurements
2.3. Statistical Analysis
2.4. 1H-NMR
3. Results
3.1. Characterization of Electronic Tongue in Complex Buffer and Physiological Fluids
3.2. Wearable E-Tongue for On-Body Sweat Characterization
3.3. NMR-Analysis of Sweat Samples Characterized by the Wearable E-Tongue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Ma, Y.; Li, Y.; Wu, D.; Zhang, Y.; Youn, C.H. Wearable 2.0: Enabling Human-Cloud Integration in Next Generation Healthcare Systems. IEEE Commun. Mag. 2017, 55, 54–61. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Mena-Bravo, A.; Luque de Castro, M.D. Sweat: A sample with limited present applications and promising future in metabolomics. J. Pharm. Biomed. Anal. 2014, 90, 139–147. [Google Scholar] [CrossRef]
- Heikenfeld, J. Non-invasive Analyte Access and Sensing through Eccrine Sweat: Challenges and Outlook circa 2016. Electroanalysis 2016, 28, 1242–1249. [Google Scholar] [CrossRef]
- Sonner, Z.; Wilder, E.; Heikenfeld, J.; Kasting, G.; Beyette, F.; Swaile, D.; Sherman, F.; Joyce, J.; Hagen, J.; Kelley-Loughnane, N.; et al. The microfluidics of the eccrine sweat gland, including biomarker partitioning, transport, and biosensing implications. Biomicrofluidics 2015, 9, 031301. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Ghaffari, R.; Baker, L.B.; Rogers, J.A. Skin-interfaced systems for sweat collection and analytics. Sci. Adv. 2018, 4, eaar3921. [Google Scholar] [CrossRef] [Green Version]
- Heikenfeld, J.; Jajack, A.; Feldman, B.; Granger, S.W.; Gaitonde, S.; Begtrup, G.; Katchman, B.A. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 2019, 37, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Lara, B.; Gallo-Salazar, C.; Puente, C.; Areces, F.; Salinero, J.J.; Del Coso, J. Interindividual variability in sweat electrolyte concentration in marathoners. J. Int. Soc. Sports Nutr. 2016, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Heeley, M.E.; Woolf, D.A.; Heeley, A.F. Indirect measurements of sweat electrolyte concentration in the laboratory diagnosis of cystic fibrosis. Arch. Dis. Child. 2000, 82, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matzeu, G.; O’Quigley, C.; McNamara, E.; Zuliani, C.; Fay, C.; Glennon, T.; Diamond, D. An integrated sensing and wireless communications platform for sensing sodium in sweat. Anal. Methods 2016, 8, 64–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Kim, S.B.; Wu, Y.; Ostojich, D.; Park, S.H.; Wang, X.; Weng, Z.; Li, R.; Bandodkar, A.J.; et al. Passive sweat collection and colorimetric analysis of biomarkers relevant to kidney disorders using a soft microfluidic system. Lab Chip 2019, 19, 1545–1555. [Google Scholar] [CrossRef]
- Tai, L.-C.; Gao, W.; Chao, M.; Bariya, M.; Ngo, Q.P.; Shahpar, Z.; Nyein, H.Y.Y.; Park, H.; Sun, J.; Jung, Y.; et al. Methylxanthine Drug Monitoring with Wearable Sweat Sensors. Adv. Mater. 2018, 30, 1707442. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids: (IUPAC technical report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Legin, A.V.; Rudnitskaya, A.M.; Vlasov, Y.G.; Di Natale, C.; D’Amico, A. The features of the electronic tongue in comparison with the characteristics of the discrete ion-selective sensors. Sens. Actuators B Chem. 1999, 58, 464–468. [Google Scholar] [CrossRef]
- Ciosek, P.; Augustyniak, E.; Wroblewski, W. Polymeric membrane ion-selective and cross-sensitive electrode-based electronic tongue for qualitative analysis of beverages. Analyst 2004, 129, 639–644. [Google Scholar] [CrossRef]
- Winquist, F.; Wide, P.; Lundström, I. An electronic tongue based on voltammetry. Anal. Chim. Acta 1997, 357, 21–31. [Google Scholar] [CrossRef]
- Winquist, F.; Krantz-Rülcker, C.; Wide, P.; Lundström, I. Monitoring of freshness of milk by an electronic tongue on the basis of voltammetry. Meas. Sci. Technol. 1998, 9, 1937. [Google Scholar] [CrossRef]
- Winquist, F.; Holmin, S.; Krantz-Rülcker, C.; Wide, P.; Lundström, I. A hybrid electronic tongue. Anal. Chim. Acta 2000, 406, 147–157. [Google Scholar] [CrossRef]
- Ivarsson, P.; Holmin, S.; Höjer, N.-E.; Krantz-Rülcker, C.; Winquist, F. Discrimination of tea by means of a voltammetric electronic tongue and different applied waveforms. Sens. Actuators B Chem. 2001, 76, 449–454. [Google Scholar] [CrossRef]
- Söderström, C.; Borén, H.; Krantz-Rülcker, C. Use of an electronic tongue and HPLC with electrochemical detection to differentiate molds in culture media. Int. J. Food Microbiol. 2005, 97, 247–257. [Google Scholar] [CrossRef]
- Söderström, C.; Winquist, F.; Krantz-Rülcker, C. Recognition of six microbial species with an electronic tongue. Sens. Actuators B Chem. 2003, 89, 248–255. [Google Scholar] [CrossRef]
- Krantz-Rülcker, C.; Stenberg, M.; Winquist, F.; Lundström, I. Electronic tongues for environmental monitoring based on sensor arrays and pattern recognition: A review. Anal. Chim. Acta 2001, 426, 217–226. [Google Scholar] [CrossRef]
- Nery, E.W.; Kubota, L.T. Integrated, paper-based potentiometric electronic tongue for the analysis of beer and wine. Anal. Chim. Acta 2016, 918, 60–68. [Google Scholar] [CrossRef]
- Podrażka, M.; Bączyńska, E.; Kundys, M.; Jeleń, P.S.; Witkowska Nery, E. Electronic Tongue-A Tool for All Tastes? Biosensors 2017, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruch, P.; Hu, R.; Capua, L.; Temiz, Y.; Paredes, S.; Lopez, A.; Barroso, J.; Cox, A.; Nakamura, E.; Matsumoto, K. A portable potentiometric electronic tongue leveraging smartphone and cloud platforms. In Proceedings of the 2019 IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; pp. 1–3. [Google Scholar] [CrossRef] [Green Version]
- Yaroshenko, I.; Kirsanov, D.; Kartsova, L.; Sidorova, A.; Borisova, I.; Legin, A. Determination of urine ionic composition with potentiometric multisensor system. Talanta 2015, 131, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, M.; Alegret, S.; del Valle, M. Potentiometric bioelectronic tongue for the analysis of urea and alkaline ions in clinical samples. Biosens. Bioelectron. 2007, 22, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, P.; Grabowska, I.; Brzózka, Z.; Wróblewski, W. Analysis of dialysate fluids with the use of a potentiometric electronic tongue. Microchim. Acta 2008, 163, 139. [Google Scholar] [CrossRef]
- Winquist, F.; Krantz-Rülcker, C.; Lundström, I. A Miniaturized Voltammetric Electronic Tongue. Anal. Lett. 2008, 41, 917–924. [Google Scholar] [CrossRef]
- Lynch, A.; Diamond, D.; Leader, M. Point-of-need diagnosis of cystic fibrosis using a potentiometric ion-selective electrode array. Analyst 2000, 125, 2264–2267. [Google Scholar] [CrossRef]
- Søpstad, S.; Johannessen, E.A.; Imenes, K. Analytical errors in biosensors employing combined counter/pseudo-reference electrodes. Results Chem. 2020, 2, 100028. [Google Scholar] [CrossRef]
- Holmin, S.; Björefors, F.; Eriksson, M.; Krantz-Rülcker, C.; Winquist, F. Investigation of Electrode Materials as Sensors in a Voltammetric Electronic Tongue. Electroanalysis 2002, 14, 839–847. [Google Scholar] [CrossRef]
- Patterson, M.J.; Galloway, S.D.R.; Nimmo, M.A. Variations in regional sweat composition in normal human males. Exp. Physiol. 2001, 85, 869–875. [Google Scholar] [CrossRef]
- Chiappin, S.; Antonelli, G.; Gatti, R.; De Palo, E.F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [Green Version]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation Between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Csősz, É.; Emri, G.; Kalló, G.; Tsaprailis, G.; Tőzsér, J. Highly abundant defense proteins in human sweat as revealed by targeted proteomics and label-free quantification mass spectrometry. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2024–2031. [Google Scholar] [CrossRef]
- Tang, Y.-M.; Wang, D.-G.; Li, J.; Li, X.-H.; Wang, Q.; Liu, N.; Liu, W.-T.; Li, Y.-X. Relationships between micronutrient losses in sweat and blood pressure among heat-exposed steelworkers. Ind. Health 2016, 54, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugg, J.W.H.; Ellis, F.P. Some Water-soluble Vitamins in the Sweat of Tropically Acclimatized European Men. Br. J. Nutr. 2007, 8, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Kunzelmann, K.; Schreiber, R.; Hadorn, H.B. Bicarbonate in cystic fibrosis. J. Cyst. Fibros. 2017, 16, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Derbyshire, P.J.; Barr, H.; Davis, F.; Higson, S.P.J. Lactate in human sweat: A critical review of research to the present day. J. Physiol. Sci. 2012, 62, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Wolfe, A.S. Physiological mechanisms determining eccrine sweat composition. Eur. J. Appl. Physiol. 2020, 120, 719–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sempionatto, J.R.; Khorshed, A.A.; Ahmed, A.; De Loyola e Silva, A.N.; Barfidokht, A.; Yin, L.; Goud, K.Y.; Mohamed, M.A.; Bailey, E.; May, J.; et al. Epidermal Enzymatic Biosensors for Sweat Vitamin C: Toward Personalized Nutrition. ACS Sens. 2020, 5, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Ament, W.; Huizenga, J.R.; Mook, G.A.; Gips, C.H.; Verkerke, C.J. Lactate and Ammonia Concentration in Blood and Sweat During Incremental Cycle Ergometer Exercise. Int. J. Sports Med. 1997, 18, 35–39. [Google Scholar] [CrossRef]
- Delgado-Povedano, M.M.; Calderón-Santiago, M.; Luque de Castro, M.D.; Priego-Capote, F. Metabolomics analysis of human sweat collected after moderate exercise. Talanta 2018, 177, 47–65. [Google Scholar] [CrossRef]
- Harker, M.; Coulson, H.; Fairweather, I.; Taylor, D.; Daykin, C.A. Study of metabolite composition of eccrine sweat from healthy male and female human subjects by 1H NMR spectroscopy. Metabolomics 2006, 2, 105–112. [Google Scholar] [CrossRef]
- Dutkiewicz, E.P.; Lin, J.-D.; Tseng, T.-W.; Wang, Y.-S.; Urban, P.L. Hydrogel Micropatches for Sampling and Profiling Skin Metabolites. Anal. Chem. 2014, 86, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Santiago, M.; Priego-Capote, F.; Jurado-Gámez, B.; Luque de Castro, M.D. Optimization study for metabolomics analysis of human sweat by liquid chromatography-tandem mass spectrometry in high resolution mode. J. Chromatogr. A 2014, 1333, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kutyshenko, V.P.; Molchanov, M.; Beskaravayny, P.; Uversky, V.N.; Timchenko, M.A. Analyzing and Mapping Sweat Metabolomics by High-Resolution NMR Spectroscopy. PLoS ONE 2011, 6, e28824. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Li, J.; Li, J.; Wu, Y.; Xiao, Y.; Hu, C. D-Excess-LaA Production Directly from Biomass by Trivalent Yttrium Species. iScience 2019, 12, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Mudalige, A.; Pemberton, J.E. Raman spectroscopy of glycerol/D2O solutions. Vib. Spectrosc. 2007, 45, 27–35. [Google Scholar] [CrossRef]
- Zhao, F.J.; Bonmarin, M.; Chen, Z.C.; Larson, M.; Fay, D.; Runnoe, D.; Heikenfeld, J. Ultra-simple wearable local sweat volume monitoring patch based on swellable hydrogels. Lab Chip 2020, 20, 168–174. [Google Scholar] [CrossRef]
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal Microfluidic Electrochemical Detection System: Enhanced Sweat Sampling and Metabolite Detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.J.; Cordas, C.M.; Moura, J.J.G.; Noronha, J.P.; Branco, L.C. Screening of Potential Stress Biomarkers in Sweat Associated with Sports Training. Sports Med. Open 2021, 7, 8. [Google Scholar] [CrossRef]

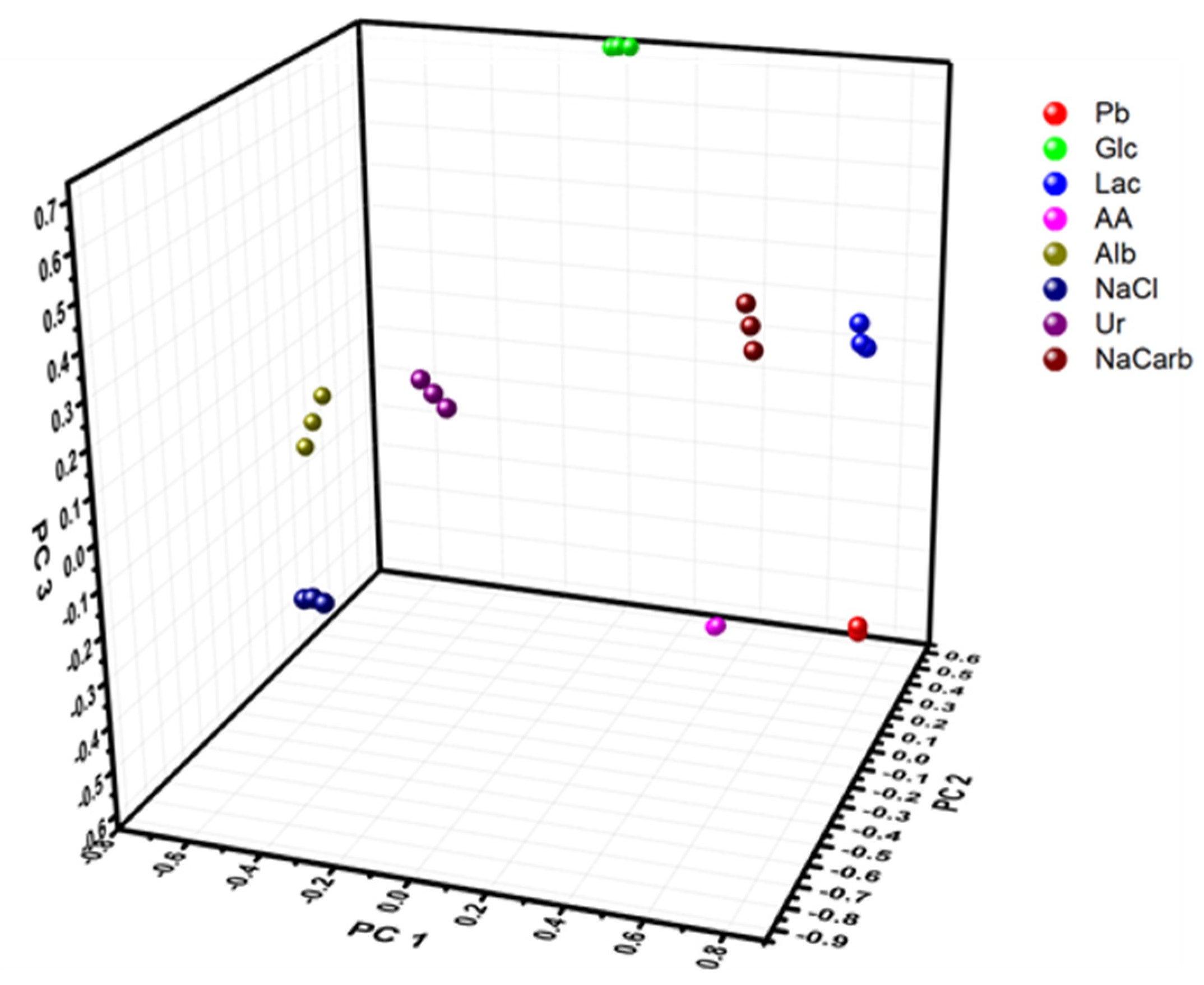
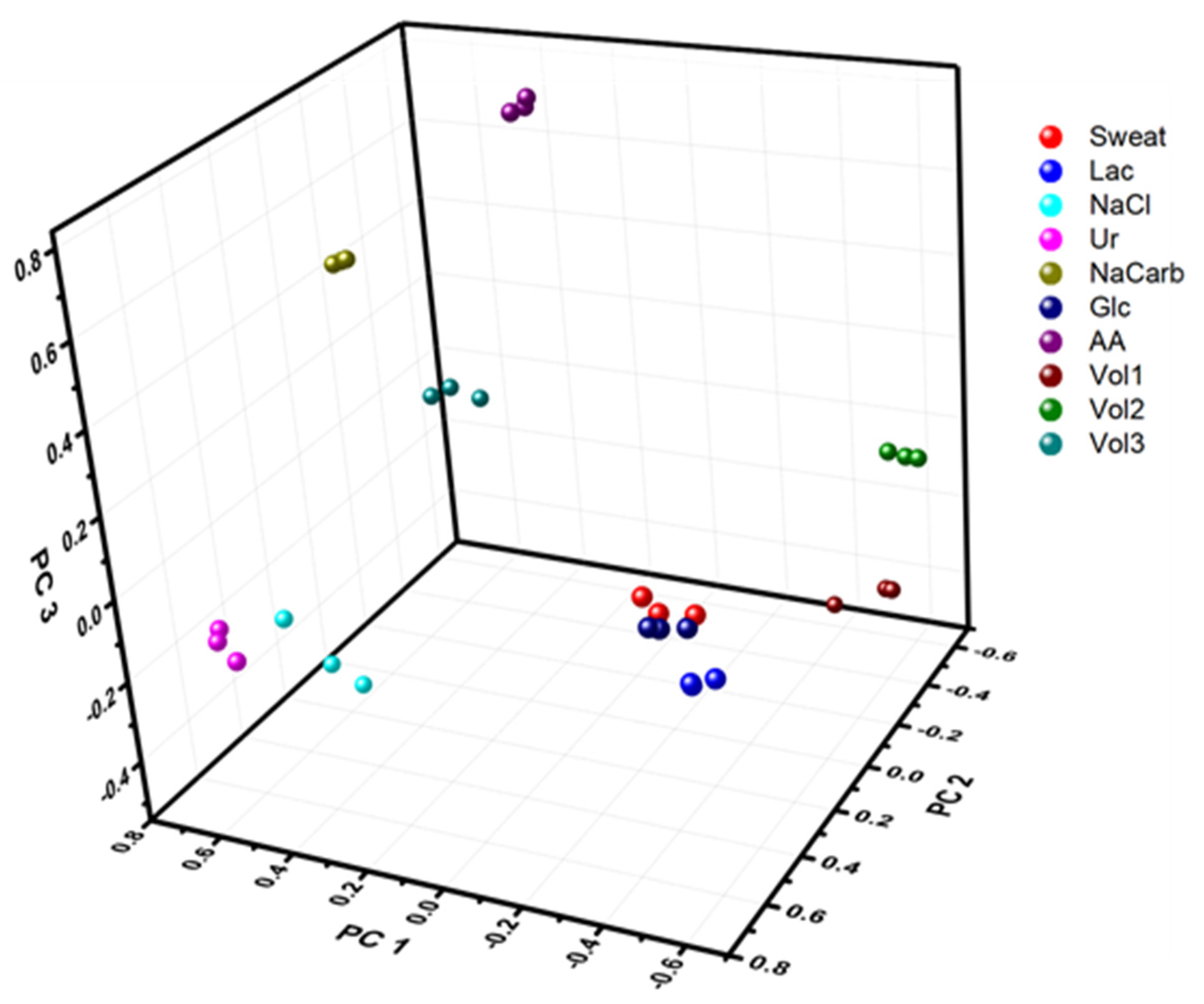

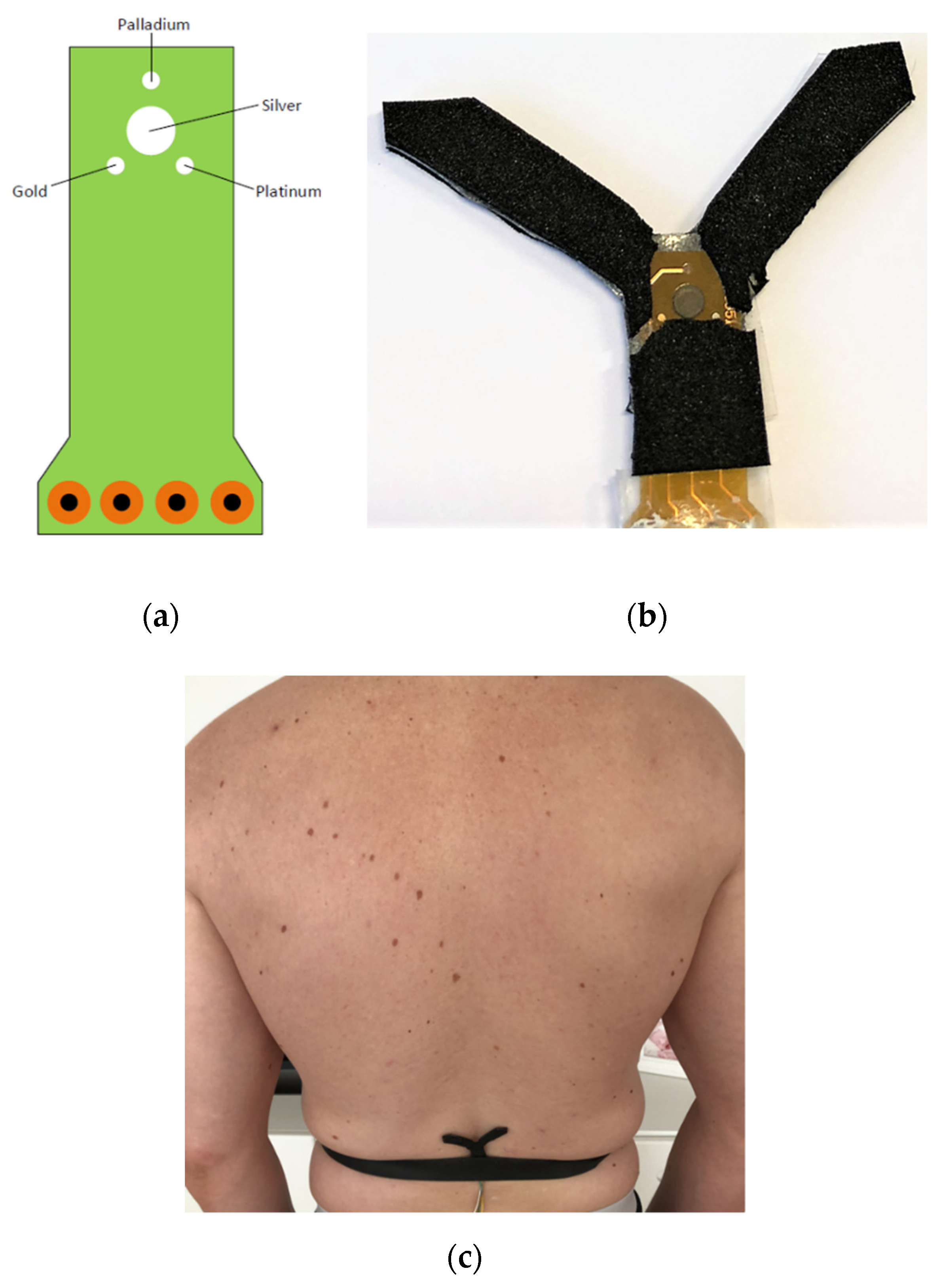
| Compound | Concentration (mM) |
|---|---|
| Lactate | 5–40 (Ref [45]) |
| Glucose | 0.1–2 (Ref [38,39]) |
| Sodium carbonate | 0.5–100 (Ref [36,43]) |
| Na+, Cl− | below 10, above 100 (Ref [9,10]) |
| Urea | 5– above 100 (Ref [14,45]) |
| Ascorbic acid | 0.01–0.5 (Ref [45,46]) |
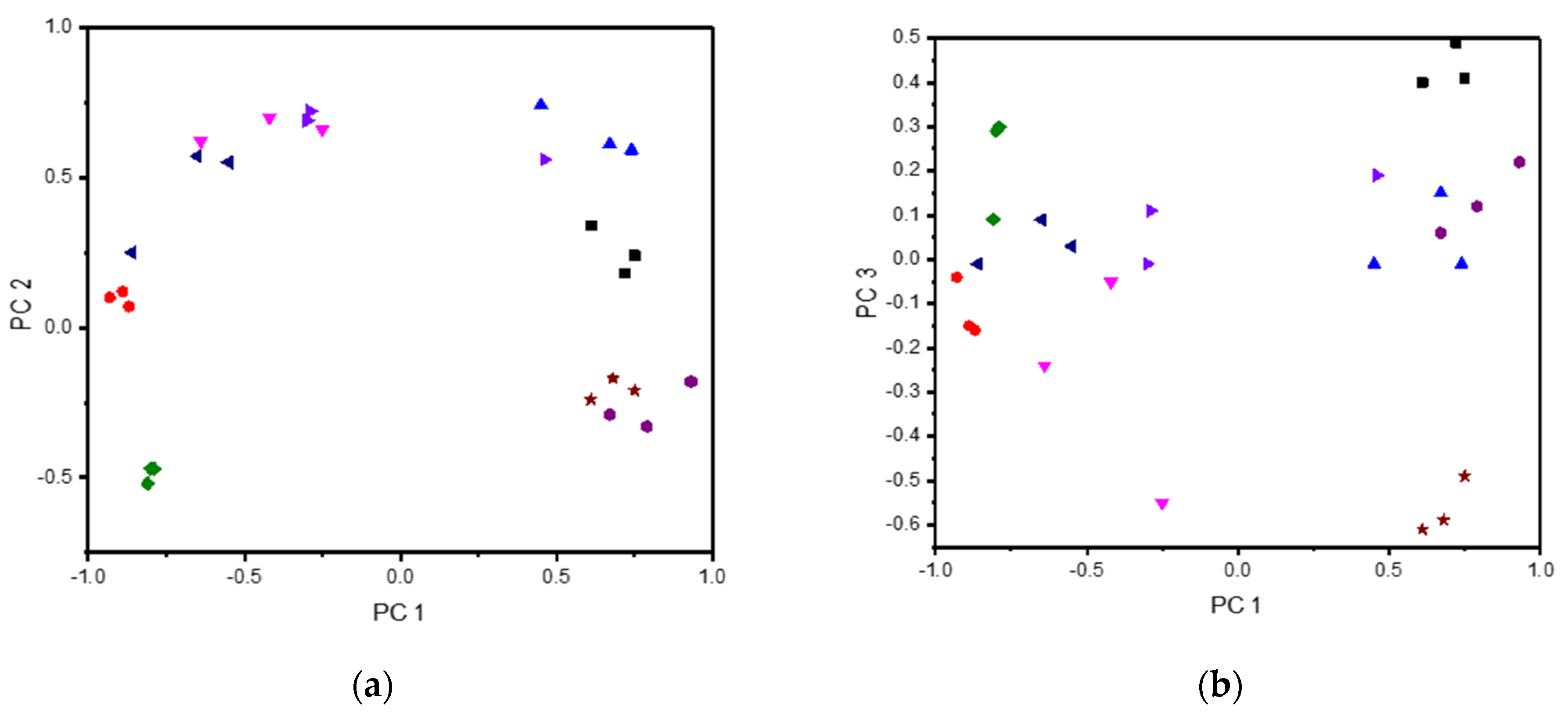
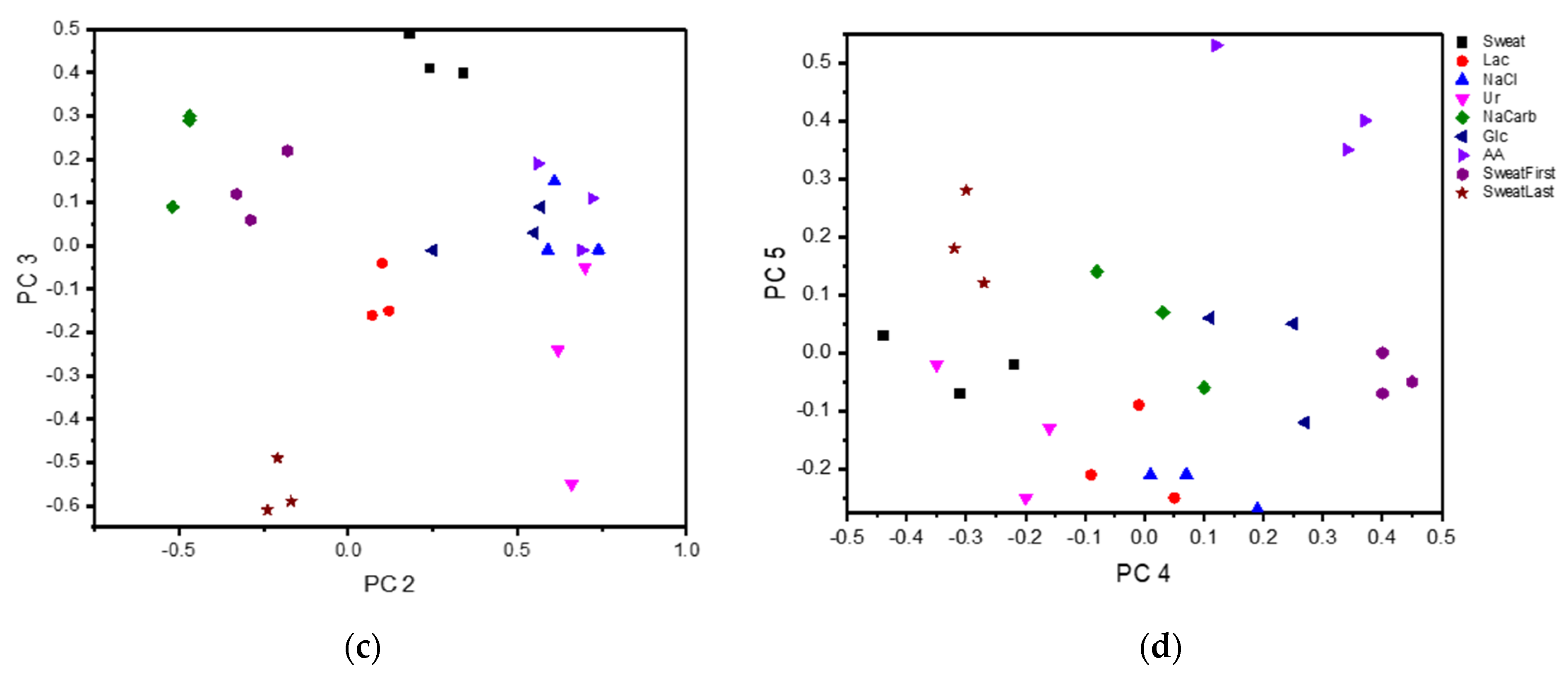
| Compound | PC 1 | PC 2 | PC 3 | PC 4 | PC 5 |
|---|---|---|---|---|---|
| Sweat | 0.61 | 0.34 | 0.40 | −0.44 | 0.03 |
| Lac | −0.89 | 0.12 | −0.15 | −0.01 | −0.09 |
| NaCarb | −0.81 | −0.52 | 0.09 | 0.10 | −0.06 |
| Glc | −0.86 | 0.25 | −0.01 | 0.11 | 0.06 |
| Ur | −0.42 | 0.70 | −0.05 | −0.35 | −0.02 |
| AA | −0.30 | 0.69 | −0.01 | 0.37 | 0.40 |
| NaCl | 0.45 | 0.74 | −0.01 | 0.19 | −0.27 |
| SweatFirst | 0.93 | −0.18 | 0.22 | 0.4 | −0.07 |
| SweatLast | 0.61 | −0.24 | −0.61 | -0.3 | 0.28 |
| Sample | Sweat | Lac | NaCarb | Glc | Ur | AA | NaCl | SweatFirst | SweatLast |
|---|---|---|---|---|---|---|---|---|---|
| Sweat | 0 | 2.823 | 3.163 | 2.643 | 1.409 | 1.901 | 0.846 | 0.632 | 1.602 |
| Lac | 0 | 0.492 | 0.393 | 0.687 | 1.184 | 2.278 | 3.533 | 2.728 | |
| NaCarb | 0 | 1.242 | 1.862 | 2.003 | 3.230 | 3.142 | 2.680 | ||
| Glc | 0 | 0.614 | 0.684 | 2.070 | 3.430 | 2.805 | |||
| Ur | 0 | 0.702 | 1.117 | 2.792 | 2.457 | ||||
| AA | 0 | 1.039 | 2.611 | 2.183 | |||||
| NaCl | 0 | 1.176 | 1.673 | ||||||
| SweatFirst | 0 | 0.902 | |||||||
| SweatLast | 0 |
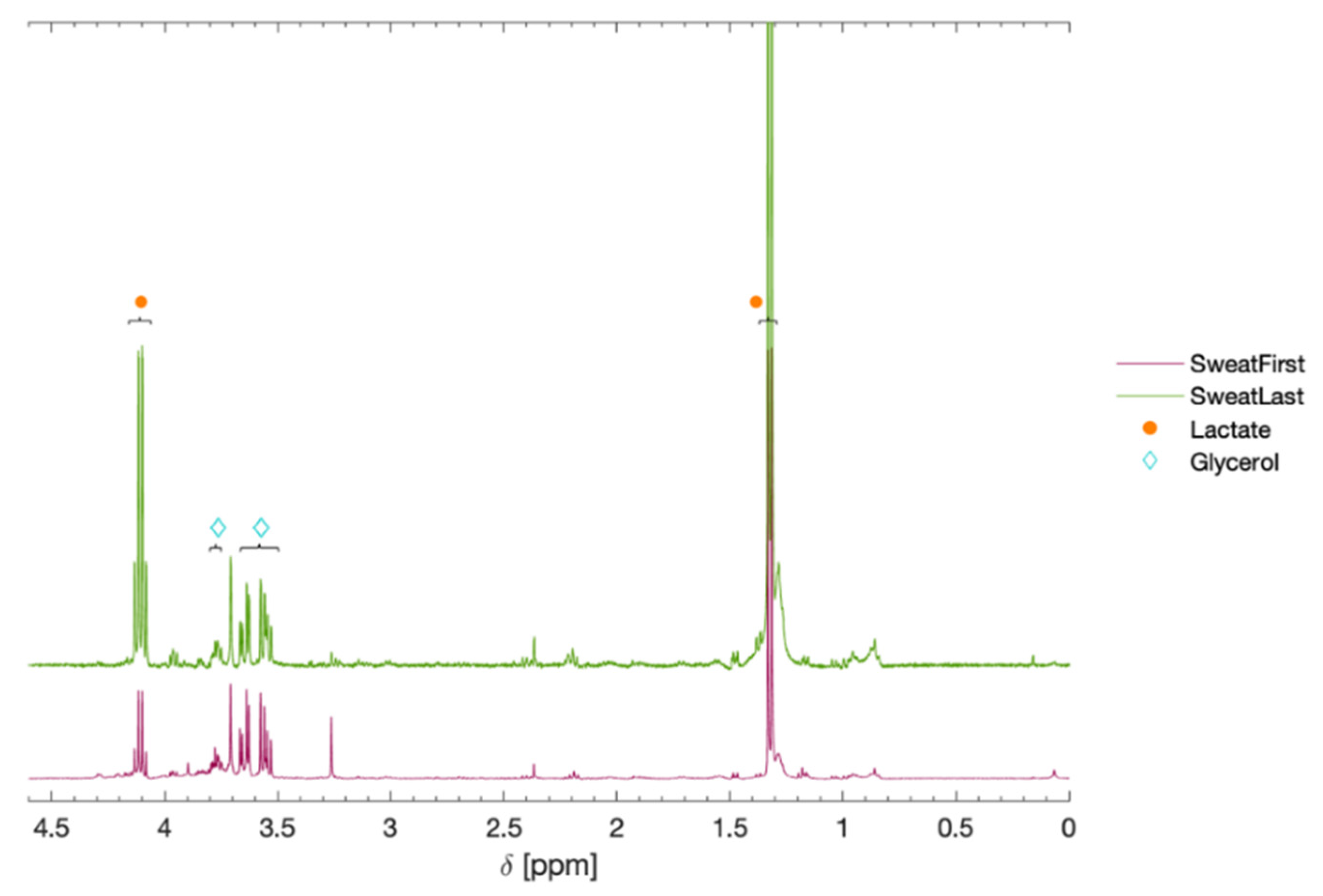
| Metabolite | Chemical Shift (δ) | Area, First | Area, Last |
|---|---|---|---|
| Lactate | 4.11 (q), 1.32 (d) | 1.0 | 5.9 |
| Serine | 3.96 (m), 3.85 (m) | trace | trace |
| Alanine | 3.79 (q), 1.48 (d) | trace | trace |
| Glycerol | 3.77 (m), 3.65 (m), 3.55 (m) | 0.5 | 0.6 |
| Pyruvate | 2.37 (s) | trace | trace |
| Lipids (-CH2-) | 1.28 (broad) | trace | trace |
| Lipids/Protein (CH3-) | 1.15 (broad) | trace | trace |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falk, M.; Nilsson, E.J.; Cirovic, S.; Tudosoiu, B.; Shleev, S. Wearable Electronic Tongue for Non-Invasive Assessment of Human Sweat. Sensors 2021, 21, 7311. https://doi.org/10.3390/s21217311
Falk M, Nilsson EJ, Cirovic S, Tudosoiu B, Shleev S. Wearable Electronic Tongue for Non-Invasive Assessment of Human Sweat. Sensors. 2021; 21(21):7311. https://doi.org/10.3390/s21217311
Chicago/Turabian StyleFalk, Magnus, Emelie J. Nilsson, Stefan Cirovic, Bogdan Tudosoiu, and Sergey Shleev. 2021. "Wearable Electronic Tongue for Non-Invasive Assessment of Human Sweat" Sensors 21, no. 21: 7311. https://doi.org/10.3390/s21217311







