On-Chip Purification of Tetracyclines Based on Copper Ions Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Microbeads
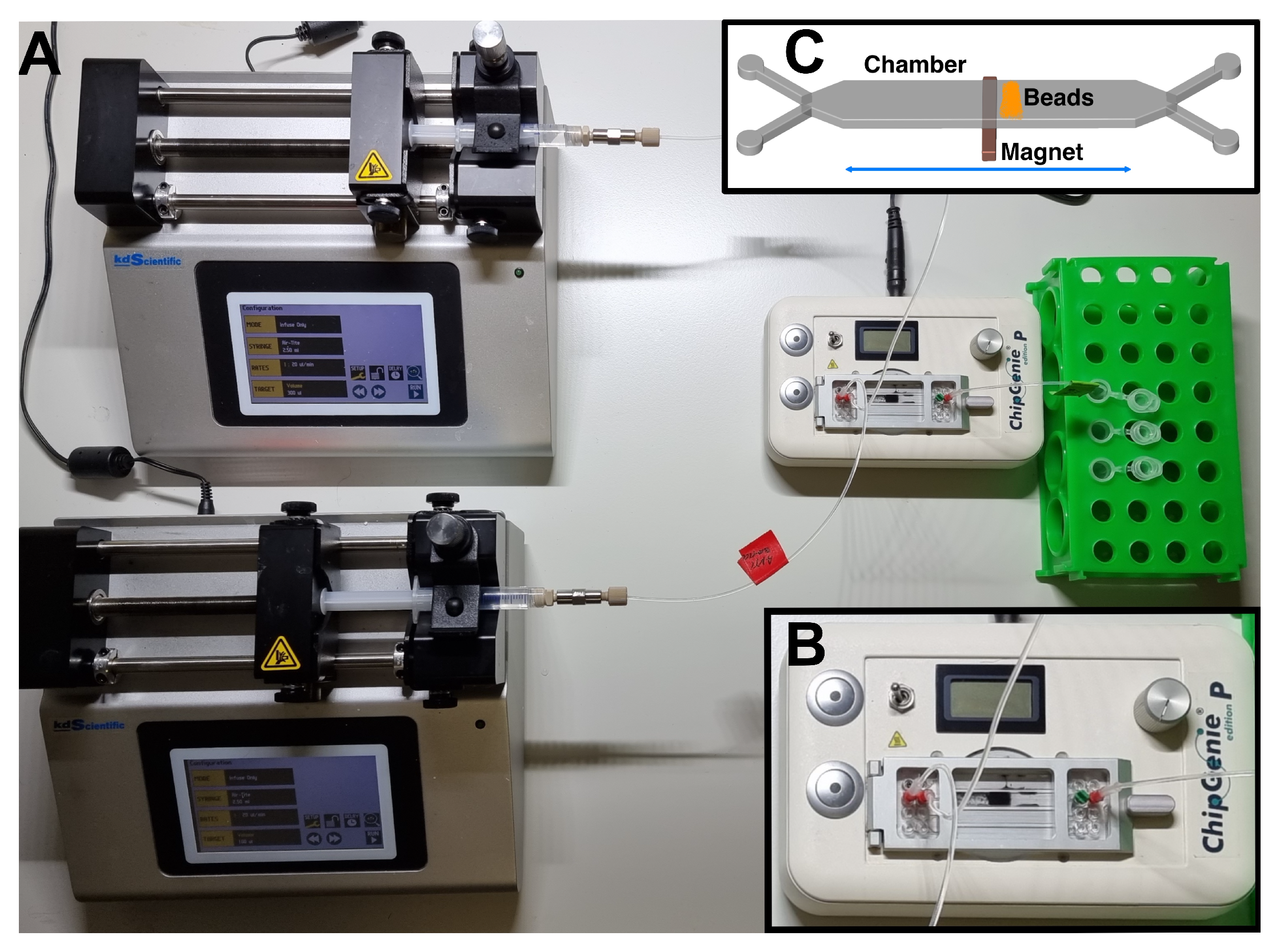
2.3. Purification System and Working Protocol
2.4. Analysis of Fractions Collected with the Purification Device
3. Results and Discussion
3.1. Characterization of Magnetic Beads
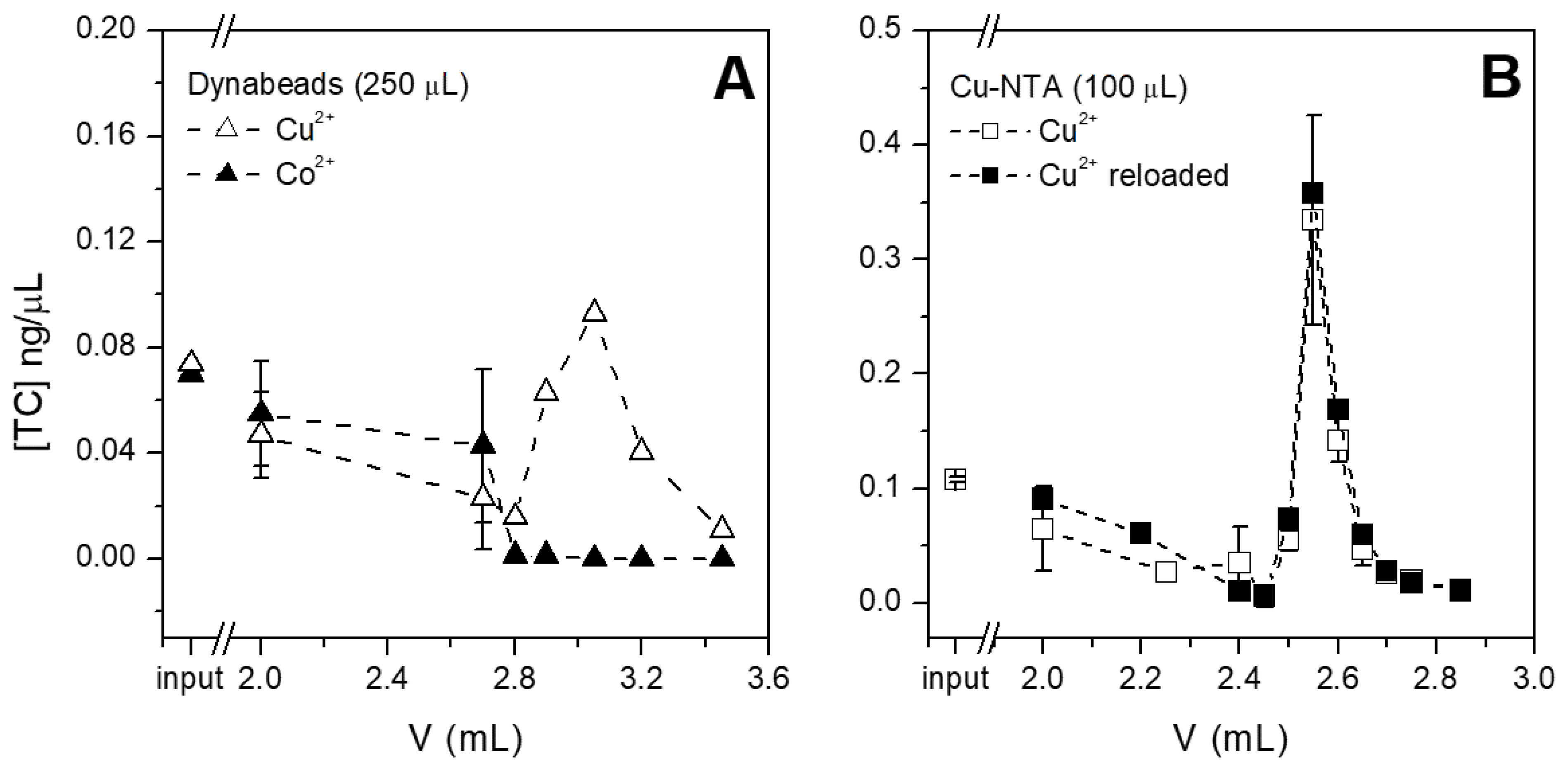
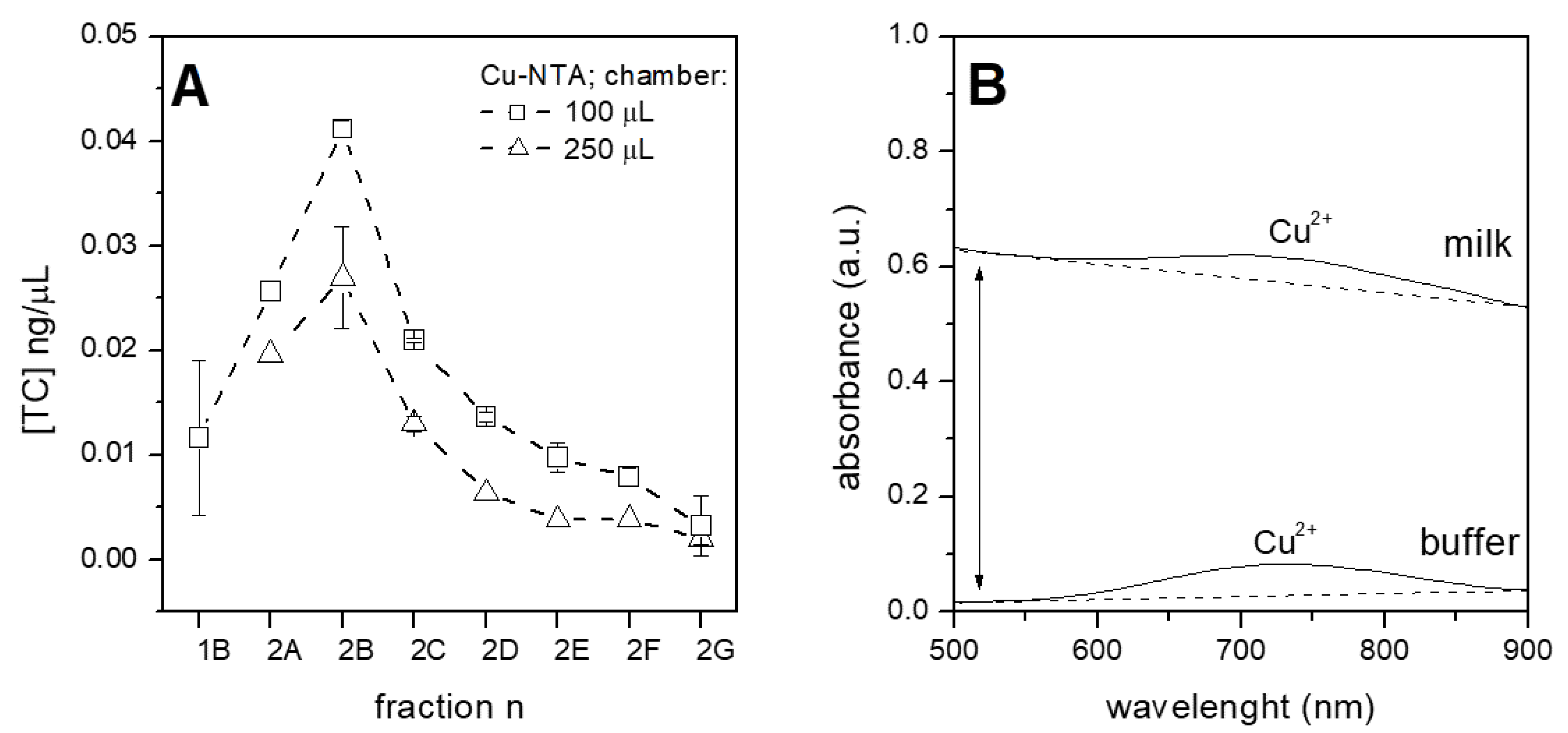
3.2. Selection of the Optimal Chip Chamber/Volume and Beads
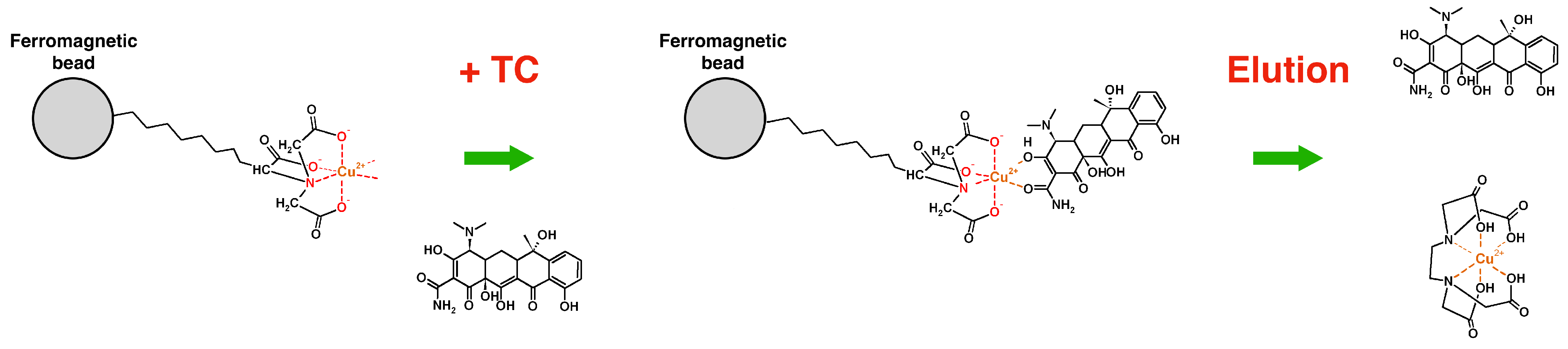
3.3. Testing the System with Different Amounts and Types of Antibiotics
3.4. Purification of Tetracycline from Raw Milk
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| TC | tetracycline |
| MRL | maximum residue limit |
| NTA | nitrilotriacetic acid |
| EDTA | ethylenediaminetetraacetic acid |
| XPS | X-ray photoelectron spectroscopy |
References
- Chen, J.; Ying, G.G.; Deng, W.J. Antibiotic residues in food: Extraction, analysis, and human health concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.J.; Wellington, M.; Shah, R.M.; Ferreira, M.J. Antibiotic Stewardship in Food-producing Animals: Challenges, Progress, and Opportunities. Clin. Ther. 2020, 42, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque Fernandes, S.A.; Magnavita, A.P.A.; Ferrao, S.P.B.; Gualberto, S.A.; Faleiro, A.S.; Figueiredo, A.J.; Matarazzo, S.V. Daily ingestion of tetracycline residue present in pasteurized milk: A public health problem. Environ. Sci. Pollut. Res. Int. 2014, 21, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Companyó, R.; Granados, M.; Guiteras, J.; Prat, M.D. Antibiotics in food: Legislation and validation of analytical methodologies. Anal. Bioanal. Chem. 2009, 395, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Léger, A.; Alban, L.; Veldhuis, A.; van Schaik, G.; Stärk, K.D.C. Comparison of international legislation and standards on veterinary drug residues in food of animal origin. J. Public Health Policy 2019, 40, 308–341. [Google Scholar] [CrossRef] [PubMed]
- Ronquillo, M.G.; Hernandez, J.C.A. Antibiotic and synthetic growth promoters in animal diets: Review of impact and analytical methods. Food Control 2017, 72, 255–267. [Google Scholar] [CrossRef]
- Moga, A.; Vergara-Barberàn, M.; Lerma-García, M.J.; Carrasco-Correa, E.J.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Determination of antibiotics in meat samples using analytical methodologies: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1681–1716. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, B.; O’Mahony, J.; Malone, E.; Moloney, M.; Cantwell, H.; Furey, A.; Danaher, M. Current trends in sample preparation for growth promoter and veterinary drug residue analysis. J. Chromatogr. A 2009, 1216, 7977–8015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; MacNeil, J.D.; Kay, J.F. Chemical Analysis of Antibiotic Residues in Food; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 38. [Google Scholar]
- Chen, T.; Cheng, G.; Ahmed, S.; Wang, Y.; Wang, X.; Hao, H.; Yuan, Z. New methodologies in screening of antibiotic residues in animal-derived foods: Biosensors. Talanta 2017, 175, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalova, E.; PNovotna; Schlegelova, J. Tetracyclines in veterinary medicine and bacterial resistance to them. Vet. Med. 2012, 49, 79–100. [Google Scholar] [CrossRef] [Green Version]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Commission Regulation (EU) No37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, L15, 1–72. [Google Scholar]
- Guerra, W.; Silva-Caldeira, P.P.; Terenzi, H.; Pereira-Maia, E.C. Impact of metal coordination on the antibiotic and non-antibiotic activities of tetracycline-based drugs. Coord. Chem. Rev. 2016, 327–328, 188–199. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Speranza, G.; Canteri, R. RxpsG a new open project for Photoelectron and Electron Spectroscopy data processing. SoftwareX 2019, 10, 100282. [Google Scholar] [CrossRef]
- Pulicharla, R.; Hegde, K.; Brar, S.K.; Surampalli, R.Y. Tetracyclines metal complexation: Significance and fate of mutual existence in the environment. Environ. Pollut. 2017, 221, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.J. The measurement and significance of ionic calcium in milk—A review. Int. J. Dairy Technol. 2011, 64, 1–13. [Google Scholar] [CrossRef]




| Dynabeads | Co 2p (%) | Cu 2p (%) | N 1s (%) | O 1s (%) | C 1s (%) |
| new | 1.3 | - | 5.9 | 24.4 | 68.5 |
| dis-charged | - | - | 5.1 | 24.7 | 70.2 |
| re-loaded | - | 0.7 | 5.6 | 24.9 | 68.8 |
| Cu-NTA | Co 2p (%) | Cu 2p (%) | N 1s (%) | O 1s (%) | C 1s (%) |
| new | - | 0.5 | 1.4 | 49.3 | 34.9 |
| dis-charged | - | - | 2.8 | 46.6 | 38.2 |
| re-loaded | - | 0.4 | 1.5 | 49.2 | 35.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunelli, L.; Germanis, M.; Vanzetti, L.; Tatti, R.; Potrich, C.; Pederzolli, C. On-Chip Purification of Tetracyclines Based on Copper Ions Interaction. Sensors 2021, 21, 7236. https://doi.org/10.3390/s21217236
Lunelli L, Germanis M, Vanzetti L, Tatti R, Potrich C, Pederzolli C. On-Chip Purification of Tetracyclines Based on Copper Ions Interaction. Sensors. 2021; 21(21):7236. https://doi.org/10.3390/s21217236
Chicago/Turabian StyleLunelli, Lorenzo, Martina Germanis, Lia Vanzetti, Roberta Tatti, Cristina Potrich, and Cecilia Pederzolli. 2021. "On-Chip Purification of Tetracyclines Based on Copper Ions Interaction" Sensors 21, no. 21: 7236. https://doi.org/10.3390/s21217236
APA StyleLunelli, L., Germanis, M., Vanzetti, L., Tatti, R., Potrich, C., & Pederzolli, C. (2021). On-Chip Purification of Tetracyclines Based on Copper Ions Interaction. Sensors, 21(21), 7236. https://doi.org/10.3390/s21217236






