A Versatile Multiple-Pass Raman System for Industrial Trace Gas Detection
Abstract
:1. Introduction
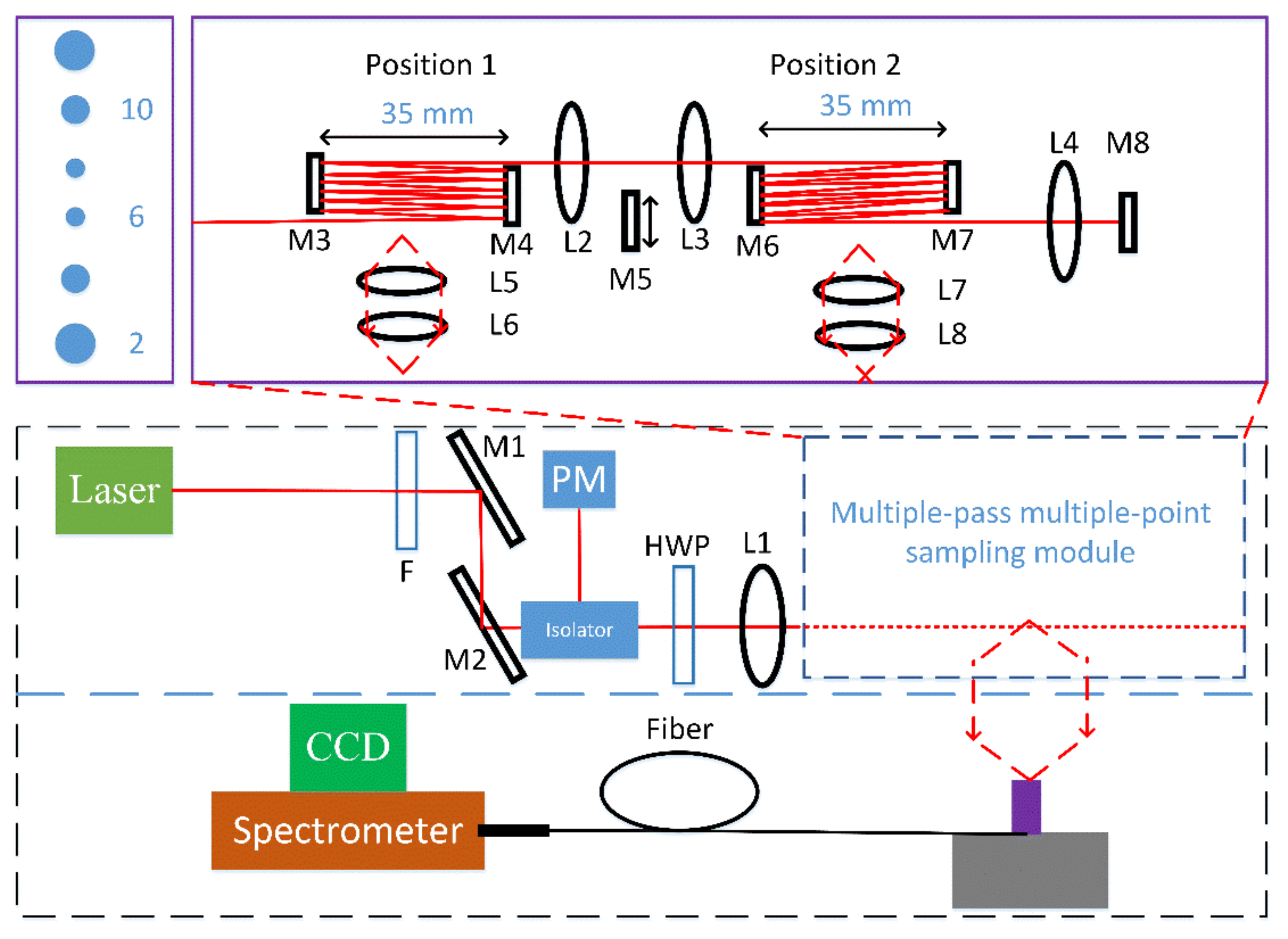
2. Materials and Methods
3. Results
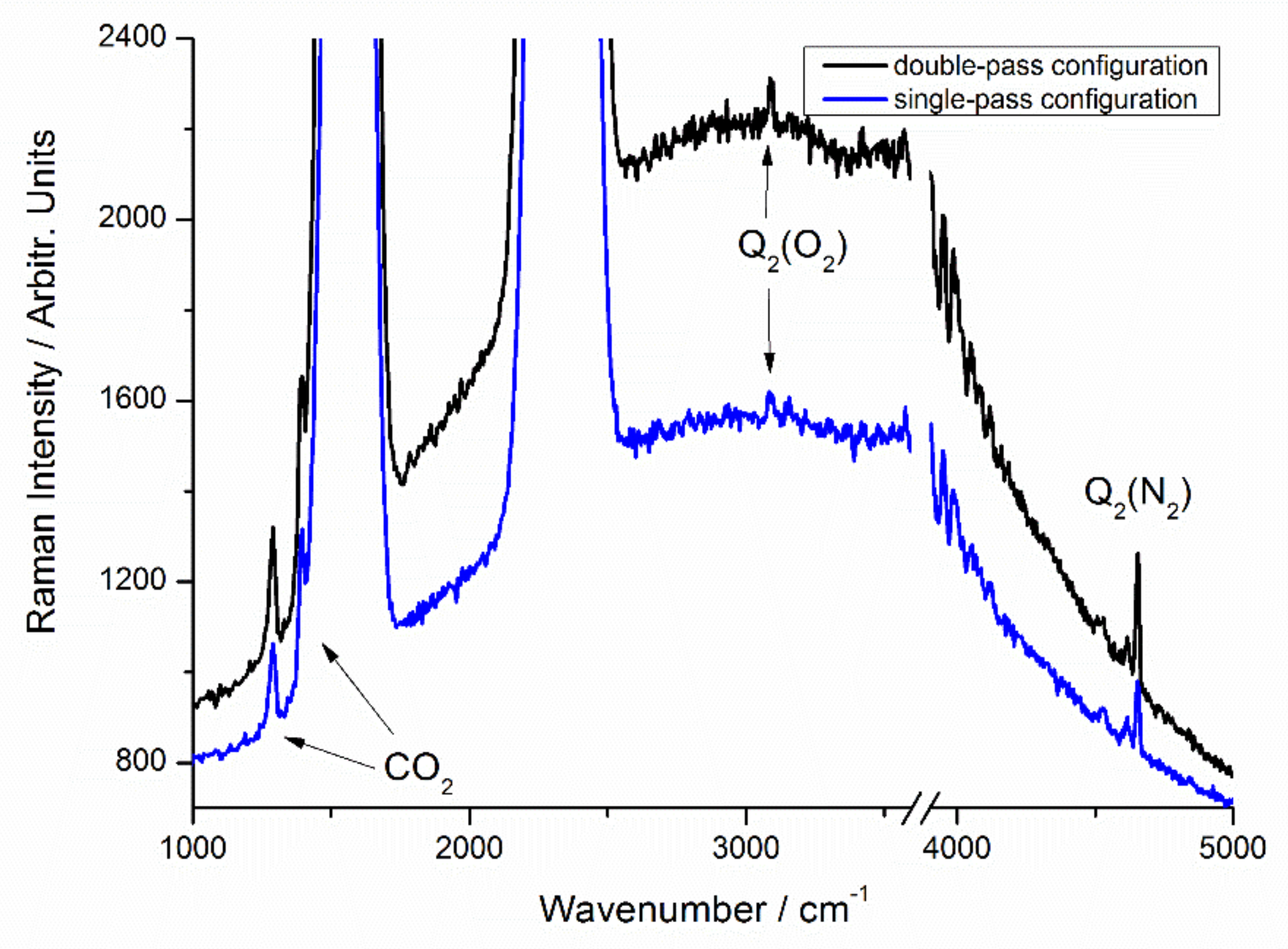
3.1. Performance of Current Multiple-Pass Raman System
3.2. Characterization of the Two-Channel Detection System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reid, J.; Labrie, D. Second-Harmonic Detection with Tunable Diode Lasers? Comparison of Experiment and Theory. Appl. Phys. A 1981, 26, 203–210. [Google Scholar] [CrossRef]
- Li, S.; Dong, L.; Wu, H.; Sampaolo, A.; Patimisco, P.; Spagnolo, V.; Tittel, F.K. Ppb-Level Quartz-Enhanced Photoacoustic Detection of Carbon Monoxide Exploiting a Surface Grooved Tuning Fork. Anal. Chem. 2019, 91, 5834–5840. [Google Scholar] [CrossRef]
- Banik, G.D.; Som, S.; Maity, A.; Pal, M.; Maithani, S.; Mandal, S.; Pradhan, M. An EC-QCL based N2O Sensor at 5.2 μm using Cavity Ring-Down Spectroscopy for Environmental Applications. Anal. Methods 2017, 9, 2315–2320. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Wang, J.; Tang, J.; Shi, Y.; Wan, F. Multigas Analysis by Cavity-Enhanced Raman Spectroscopy for Power Transformer Diagnosis. Anal. Chem. 2020, 92, 5969–5977. [Google Scholar] [CrossRef]
- Chow, K.K.; Short, M.; Lam, S.; McWilliams, A.; Zeng, H. A Raman Cell Based on Hollow Core Photonic Crystal Fiber for Human Breath Analysis. Med. Phys. 2014, 41, 092701. [Google Scholar] [CrossRef] [PubMed]
- Hanf, S.; Bögözi, T.; Keiner, R.; Frosch, T.; Popp, J. Fast and Highly Sensitive Fiber-Enhanced Raman Spectroscopic Monitoring of Molecular H2 and CH4 for Point-of-Care Diagnosis of Malabsorption Disorders in Exhaled Human Breath. Anal. Chem. 2014, 87, 982–988. [Google Scholar] [CrossRef]
- Kiefer, J.; Seeger, T.; Steuer, S.; Schorsch, S.; Weikl, M.; Leipertz, A. Design and Characterization of a Raman-Scattering-Based Sensor System for Temporally Resolved Gas Analysis and its Application in a Gas Turbine Power Plant. Meas. Sci. Technol. 2008, 19, 085408. [Google Scholar] [CrossRef]
- Knebl, A.; Domes, R.; Wolf, S.; Domes, C.; Popp, J.; Frosch, T. Fiber-Enhanced Raman Gas Spectroscopy for the Study of Microbial Methanogenesis. Anal. Chem. 2020, 92, 12564–12571. [Google Scholar] [CrossRef] [PubMed]
- O’Hira, S.; Hayashi, T.; Nakamura, H.; Kobayashi, K.; Todokoro, T.; Nakamura, H.; Itoh, T.; Yamanishi, T.; Kawamura, Y.; Iwai, Y.; et al. Improvement of Tritium Accountancy Technology for ITER Fuel Cycle Safety Enhancement. Nucl. Fusion. 2000, 40, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Demange, D.; Alecu, C.G.; Bekris, N.; Borisevich, O.; Bornschein, B.; Fischer, S.; Gramlich, N.; Köllö, Z.; Le, T.L.; Michling, R.; et al. Overview of R&D at TLK for Process and Analytical Issues on Tritium Management in Breeder Blankets of ITER and DEMO. Fusion Eng. Des. 2012, 87, 1206–1213. [Google Scholar]
- Salter, R.; Chu, J.; Hippler, M. Cavity-Enhanced Raman Spectroscopy with Optical Feedback Cw Diode Lasers for Gas Phase Analysis and Spectroscopy. Analyst 2012, 137, 4669–4676. [Google Scholar] [CrossRef] [PubMed]
- Hippler, M. Cavity-Enhanced Raman Spectroscopy of Natural Gas with Optical Feedback cw-Diode Lasers. Anal. Chem. 2015, 87, 7803–7809. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J.; Glugla, M.; Penzhorn, R.-D. Enhanced Raman Sensitivity Using an Actively Stabilized External Resonator. Rev. Sci. Instrum. 2001, 72, 1970–1976. [Google Scholar] [CrossRef]
- Sieburg, A.; Schneider, S.; Yan, D.; Popp, J.; Frosch, T. Monitoring of Gas Composition in a Laboratory Biogas Plant Using Cavity Enhanced Raman Spectroscopy. Analyst 2018, 143, 1358–1366. [Google Scholar] [CrossRef]
- Frosch, T.; Keiner, R.; Michalzik, B.; Fischer, B.; Popp, J. Investigation of Gas Exchange Processes in Peat Bog Ecosystems by Means of Innovative Raman Gas Spectroscopy. Anal. Chem. 2013, 85, 1295–1299. [Google Scholar] [CrossRef]
- Pearman, W.F.; Carter, J.C.; Angel, S.M.; Chan, J.W.-J. Multipass Capillary Cell for Enhanced Raman Measurements of Gases. Appl. Spectrosc. 2008, 62, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Buric, M.P.; Chen, K.P.; Falk, J.; Woodruff, S.D. Multimode Metal-Lined Capillaries for Raman Collection and Sensing. J. Opt. Soc. Am. B 2010, 27, 2612–2619. [Google Scholar] [CrossRef] [Green Version]
- Okita, Y.; Katagiri, T.; Matsuura, Y. A Raman Cell Based on Hollow Optical Fibers for Breath Analysis. Proc. SPIE 2010, 7559, 755908. [Google Scholar] [CrossRef]
- James, T.M.; Rupp, S.; Telle, H.H. Trace Gas and Dynamic Process Monitoring by Raman Spectroscopy in Metal-Coated Hollow Glass Fibres. Anal. Methods 2015, 7, 2568–2576. [Google Scholar] [CrossRef]
- Sieburg, A.; Knebl, A.; Jacob, J.M.; Frosch, T. Characterization of Fuel Gases with Fiber-Enhanced Raman Spectroscopy. Anal. Bioanal. Chem. 2019, 411, 7399–7408. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Frosch, T.; Popp, J.; Pletz, M.W.; Frosch, T. Highly Sensitive Detection of the Antibiotic Ciprofloxacin by Means of Fiber Enhanced Raman Spectroscopy. Molecules 2019, 24, 4512. [Google Scholar] [CrossRef] [Green Version]
- Petrak, B.; Djeu, N.; Muller, A. Purcell-Enhanced Raman Scattering from Atmospheric Gases in a High-Finesse Microcavity. Phys. Rev. A 2014, 89, 023811. [Google Scholar] [CrossRef]
- Petrak, B.; Cooper, J.; Konthasinghe, K.; Peiris, M.; Djeu, N.; Hopkins, A.J.; Muller, A. Isotopic Gas Analysis through Purcell Cavity Enhanced Raman Scattering. Appl. Phys. Lett. 2016, 108, 091107. [Google Scholar] [CrossRef]
- Borysow, J.; Fink, M. NIR Raman Spectrometer for Monitoring Protonation Reactions in Gaseous Hydrogen. J. Nucl. Mater. 2005, 341, 224–230. [Google Scholar] [CrossRef]
- Schlüter, S.; Krischke, F.; Popovska-Leipertz, N.; Seeger, T.; Breuer, G.; Jeleazcov, C.; Schüttler, J.; Leipertz, A. Demonstration of a Signal Enhanced Fast Raman Sensor for Multi-Species Gas Analyses at a Low Pressure Range for Anesthesia Monitoring. J. Raman Spectrosc. 2015, 46, 708–715. [Google Scholar] [CrossRef]
- Petrov, D.V. Multipass Optical System for a Raman Gas Spectrometer. Appl. Opt. 2016, 55, 9521–9525. [Google Scholar] [CrossRef] [PubMed]
- Velez, J.G.; Muller, A. Trace Gas Sensing Using Diode-Pumped Collinearly Detected Spontaneous Raman Scattering Enhanced by a Multipass Cell. Opt. Lett. 2020, 45, 133–136. [Google Scholar] [CrossRef]
- Li, X.; Xia, Y.; Zhan, L.; Huang, J. Near-Confocal Cavity-Enhanced Raman Spectroscopy for Multitrace-Gas Detection. Opt. Lett. 2008, 33, 2143–2145. [Google Scholar] [CrossRef]
- Wen, C.; Huang, X.; Shen, C. Multiple-Pass Enhanced Raman Spectroscopy for Fast Industrial Trace Gas Detection and Process Control. J. Raman Spectrosc. 2020, 51, 781–787. [Google Scholar] [CrossRef]
- Wen, C.; Huang, X.; Shen, C. Multiple-Pass Enhanced Multiple-Point Gas Raman Analyzer for Industrial Process Control Applications. J. Raman Spectrosc. 2020, 51, 2046. [Google Scholar] [CrossRef]
- Wen, C.; Huang, X.; Wang, W.; Shen, C.; Li, H. Multiple-Pass Enhanced Raman Spectroscopy for Long-Term Monitoring of Hydrogen Isotoplogues. J. Raman Spectrosc. 2019, 50, 1555. [Google Scholar] [CrossRef]
- Godot, A.; Coindet, G.; Hubinois, J.C. Analysis of Gases by Raman Spectroscopy: Determination of Isotopic Composition of Hydrogen Mixtures (H2, D2 and T2). Fusion Sci. Technol. 2011, 60, 998–1001. [Google Scholar] [CrossRef]
- Hanf, S.; Keiner, R.; Yan, D.; Popp, J.; Frosch, T. Fiber-Enhanced Raman Multigas Spectroscopy: A Versatile Tool for Environmental Gas Sensing and Breath Analysis. Anal. Chem. 2014, 86, 5278–5285. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Wen, C.; Huang, X.; Long, X. A Versatile Multiple-Pass Raman System for Industrial Trace Gas Detection. Sensors 2021, 21, 7173. https://doi.org/10.3390/s21217173
Shen C, Wen C, Huang X, Long X. A Versatile Multiple-Pass Raman System for Industrial Trace Gas Detection. Sensors. 2021; 21(21):7173. https://doi.org/10.3390/s21217173
Chicago/Turabian StyleShen, Chunlei, Chengwei Wen, Xin Huang, and Xinggui Long. 2021. "A Versatile Multiple-Pass Raman System for Industrial Trace Gas Detection" Sensors 21, no. 21: 7173. https://doi.org/10.3390/s21217173
APA StyleShen, C., Wen, C., Huang, X., & Long, X. (2021). A Versatile Multiple-Pass Raman System for Industrial Trace Gas Detection. Sensors, 21(21), 7173. https://doi.org/10.3390/s21217173




