1. Introduction
With the recent increase in the use of smart phones and wearable devices, we can record and access a plethora of raw data and information from built-in inexpensive sensors. Human activity recognition refers to analyzing these data to extract meaningful information about human daily habits and physical activity patterns [
1]. Breakthroughs in HAR research have led to various applications in health care and rehabilitation, elderly fall detection, fitness trackers, assisted living and smart homes [
2]. One of the most frequently used sources of data for activity recognition purposes is the inertial sensor [
3,
4,
5]. Inertial measurement unit (IMU) contains a triaxial accelerometer (3D-ACC), gyroscope, and magnetometer to measure velocity and acceleration, rotation, and strength of a magnetic field, respectively. Based on previous studies, the 3D-ACC sensor outperforms gyroscope and magnetometer, however, combining 3D-ACC with gyroscope yields better performance in classifying activities [
6]. This suggests sensor combination has the potential to offer better classification power for a HAR system.
HAR research leverages multiple approaches to advance the accuracy and performance of systems, such as different sensor positioning [
1], varying feature extraction approaches [
7] and exploring several classification methods [
8,
9] to extract more informative knowledge from a dataset and enhance the HAR system performance. Bayat et al. evaluated the effectiveness of different 3D-ACC sensor placements in recognizing human activities [
10]. Participants were instructed to perform tasks first using the smartphone in their hands, then perform the same tasks with the phone in their pockets. Based on the reported result, smart phone positioning in the hand and in the pocket yield similar results in the HAR models. Casale et al. used data acquired from only one 3D-ACC sensor and proposed a new set of feature extraction methods to be used with a random forest classifier in recognizing five different daily activities [
11]. They observed that the new feature set is more informative compared to the commonly used feature sets; thus, they reported model performance enhancements after using the new feature set. In many cases, researchers evaluate the significance of one signal individually or IMU signals in combination, thus, the combined contribution of other types of signals (e.g., bio-signals) requires more exploration [
12,
13].
To overcome the limitations of using a single sensor, researchers have explored the idea of applying fusion methods to enhance a HAR system [
4]. Fusion methods refer to any integration of information from different sources, at the sensor, feature or classifier levels [
4]; thus, they can be categorized in early fusion and late fusion methods [
14]. To elaborate, early fusion refers to combining raw data or extracted features, and then feed the new combined dataset or feature set to a classifier [
15]. It is relevant to mention that combining features extracted with different approaches, i.e., time domain and frequency domain features, is also considered as early fusion [
16]. Another method of combining for a HAR system is the late fusion, or decision-level fusion. As the name implies, in this method, the models are trained separately on each signal, but their predictions are combined into an ensemble model (e.g., voting system), that predicts the final classifications [
14]. In this study, we apply the early fusion method, thus, in the following, we elaborate more on previous works in which researchers applied the early fusion method.
1.1. Early Fusion with IMU
Early fusion methods fuse extracted features from different signal sources into a combined dataset, which serves as input for a Human-Activity classifier. Several works have applied this technique to improve the performance of classifier models. Chung et al. applied the sensor fusion approach by placing eight IMU sensors on different body parts of five right-handed individuals [
1]. They trained a Long Short-Term Memory (LSTM) network model to classify nine activities. Based on their results, to get a reasonable classification performance, one sensor should be placed on the upper half of the body and one on the lower half; particularly, on the right wrist and right ankle. Regarding signal fusion, the authors stated that a 3D-ACC sensor combined with a gyroscope performed better (with accuracy
) than its combination with a magnetometer. In another study, Shoaib et al. followed the same data-level fusion approach and generated their own dataset by placing one smart phone in a subject’s pocket and another one on his dominant wrist and recording 3D-ACC, gyroscope and linear acceleration signals [
13]. The authors tried different scenarios, such as combination of 3D-ACC and gyroscope signals, which they claim leads to more accurate results, particularly for “stairs” and “walking” activities. Moreover, they claim that the combination of signals captured from both the pocket and wrist improves the performance, especially for complex activities.
1.2. Early Fusion with Bio-Signals
Bio-signal refers to any signal generated by a living creature that can be recorded continuously [
17]. Given that the heart rate is sensitive to physically demanding activities [
18], can we rely on bio-signals to complement 3D-ACC sensors in recognizing certain types of activities? Bio-signals sensors have been shown to be quite accurate in capturing the bio-signals [
19], but they have not yet been extensively explored in the context of HAR systems. Park et al. performed an experiment to extract Heart-Rate Variability (HRV) parameters from recorded electrocardiogram (ECG) data and combined it with a 3D-ACC signal for HAR researches [
20]. They employed a feature-level fusion approach by fusing features extracted from HRV and 3D-ACC signals and classified five activities by examining three different scenarios. First, using only four features extracted from 3D-ACC (
); second, considering 31 more features extracted from the ECG signal along with the ones used in the first scenario (
). Finally, using features extracted from 3D-ACC and only some selected features from ECG signal, this combination outperformed previous scenarios by achieving a
accuracy. Park et al. conclude that the ECG signal is a good complementary source of information along with 3D-ACC for HAR researches. Tapia et al. applied early fusion by recording an acceleration signal obtained from five 3D-ACC in addition to heart rate (HR) information [
21]. The authors applied a C4.5 decision tree and Naïve Bayes classifier to classify 30 gymnastic activities with different levels of intensity. They claim that adding HR to 3D-ACC can improve the model performance by
and
for subject-dependent and subject-independent approaches, respectively. Based on [
21], for the subject-independent approach, different fitness level and variation in heartbeat rate during non-resting activities are potential reasons for this minor recognition improvement.
As for photoplethysmogram (PPG) signal fusion, Biagetti et al. investigated the level of contribution of a PPG signal in addition to a 3D-ACC signal toward accurately detecting human activities [
22]. The authors proposed a feature extraction technique based on singular value decomposition (SVD) in addition to Karhunen–Loeve transform (KLT) method for feature reduction. According to the authors, employing only a PPG signal is not enough for physical activity recognition. Thus, they compared applying only a 3D-ACC signal with a combination of PPG and 3D-ACC, consequently, they conclude that signal fusion incremented the overall accuracy by 12.30% to 78.00%. In another study, Mehrange et al. used a single PPG-ACC wrist-worn sensor placed on the dominant wrist of 25 male subjects to evaluate fused HAR system power in classifying indoor activities with different intensity [
23]. They extracted time and frequency domain features and fed them to a random forest classifier. In terms of contribution level of PPG-based HR-related features in classifying activities, their results suggest a very slight overall improvement. Regarding the activity performance, HR addition did not help the classifier to indicate most of the activities except for intensive stationary cycling with 7% improvement in accuracy.
1.3. Our Contribution
As summarized above, studies have shown that the combination of one type of bio-signal with 3D-ACC enhanced the HAR system’s performance. Our study differs and complements the former studies in the following ways. First, thanks to the dataset that we used, we have 3D-ACC, ECG and PPG signals all recorded simultaneously and related to same group of subjects, thus, beside evaluating the added value of bio-signals to 3D-ACC, we can also compare the significance of each of the mentioned bio-signals. Moreover, we investigate the impact of bio-signal, not only on the overall performance of the HAR models, but also per single activity, to assess the impact of bio-signals on each set of activities.
To compare the performance of different signals, we analyze the data acquired from 3D-ACC, ECG and PPG sensors individually. Moreover, we use fusion methods to combine data from mentioned signals to examine their contribution level in the HAR system’s output. To analyze the signal’s contribution in HAR systems, we segment the signals, using a sliding window method to extract time and frequency domain features. Finally, we train random forest classifier models for subject-dependent and subject-independent setups. We evaluate the bio-signals significance in HAR using two types of models: subject-specific and cross-subject models. Both models are commonly used in HAR systems and research, and more importantly, each has its advantages and disadvantages [
24,
25]. Subject-specific models are personalized models, trained and evaluated using the data of a single user. Hence, subject-specific are usually more accurate than cross-subject models, at a cost of requiring training data from the target user.
A cross-subject model, on the other hand, is trained on multiple users and attempts to recognize the activity of a previously untrained user. This model tends to be more generic and is commonly used in practice, since cross-subject models are cheaper to train and easier to deploy [
26,
27].
Therefore, we formulate our research questions to cover both subject-specific and cross-subject models. Thus, in our study, we focus on answering two research questions. First, we investigate the contribution level of each source of 3D-ACC, ECG and PPG signals in subject-specific HAR systems (RQ1), and then, in cross-subject systems (RQ2). Moreover, we have the advantage of having the three signals from the same individuals performing the same activities. Hence, we can investigate: (1) the contribution level of the bio-signals when added to the 3D-ACC signal, (2) and compare the performance of ECG and PPG with each other. To summarize, we provide an overview of our contributions:
To the best of our knowledge, this is the first study to compare the combined performance of the 3D-ACC, ECG and PPG signals recorded simultaneously from subjects performing the same set of activities. We use hand-crafted features to evaluate the performance of classifiers for HAR;
We investigate the significance of bio-signals and compare the usefulness of ECG and PPG signals in HAR;
We investigate the impact of combining a 3D-ACC signal with an ECG signal in recognizing some specific activities in detail. For instance, the importance of the ECG signal in distinguishing walking activity from ascending/descending stairs.
The rest of this article is organized as follows; in
Section 2, we elaborate on the characteristics of the sensors and signals we evaluate and the used dataset. Next, in
Section 3, we explain our methodology and workflow, from data pre-processing to feature extraction and selection. We allocate
Section 4 to classification and evaluation methods.
Section 5 describes the results and findings of our study. We discuss in details the impact of ECG signal in HAR system’s performance in
Section 6. Finally, we conclude our study in
Section 7.
3. Feature Extraction and Selection
In this section, we describe the methodology used in our study to evaluate the importance of the three different signals in HAR.
Figure 2 presents an overview of our methodology used to recognize human activity. We start by pre-processing the dataset to normalize data from different signals (and frequencies) and prepare the data for subsequent analysis. Next, we apply signal segmentation method, traditionally employed in HAR pipelines [
36]. As our third step, we extract time and frequency domain features from each segment of the previous step. Then, we standardize the extracted features, so that all the features have zero mean and unit variance. Afterwards, we identify and remove highly correlated features and we train machine learning models with the remaining features. In the following, we explain the purpose and detailed procedure of each of the mentioned steps.
3.1. Data Pre-Processing
As described in
Section 2, the dataset we use in this study contains signals with different sampling rates, as 3D-ACC was captured at 32 Hz while PPG was recorded at 64 Hz. To analyze the 3D-ACC and PPG signals captured from the wrist worn device, we up-sample the 3D-ACC signal from 32 to 64 samples per seconds. We decided not to down-sample the PPG signal, as this would mean eliminating half of the PPG dataset which was not appropriate. Thus, we up-sample the 3D-ACC signal by interpolating two-consecutive datapoints with their average value. We do not modify the original chest-worn ECG signal.
3.2. Windowing
Before we start extracting features from the data, we segment the entire signal into small sequences of fixed size (same number of datapoints). This approach is known as sliding window. The main intuition for the windowing technique is to retrieve meaningful information from the time series. Each single datapoint in the time series is not representative of any specific activity, however, a group of consecutive datapoints (a slice in the time series) is capable of providing insightful information about the human activity.
The size of the window is an important parameter in sliding window techniques. Each window must be wide enough to capture enough information for further signal processing and analyzing. However, the window size should not be too large, since larger windows may delay the real-time signal processing and the eventual activity recognition. The reason being that the model has to wait for the entire duration of the window to be able to start recognizing the next activity. Thus, there is a trade-off between capturing the appropriate amount of information and the speed of recognition. There is no standard fixed window size that researchers can utilize, as the appropriate window size depends highly on the characteristics of the signal. For instance, if we have a periodic signal, an adequate window size may be the one that is wide enough to cover at least one period of the signal in each segment.
Researchers have attempted different strategies to select an appropriate window size. One strategy is to use adaptive window sizes in which a feedback system is employed to calculate the likeliness of a signal belonging to an activity, then, the desired window size is selected based on the probabilities [
37]. In most cases, however, researchers opt for fixed window sizes, Banos et. al. studied the impact of different window sizes on HAR accuracy [
36]. They observed that many researchers have applied varying fixed window sizes from 0.1 to 12.8 s, although, nearly 50% of the considered studies have used 0.1 to 3 s window sizes.
In this study, we examine different fixed window sizes from 0.5 to 15 s on all three sources signals, and we select a window size of seven seconds. As depicted in
Figure 3, larger window sizes provide only a slight improvement in the performance of the 3D-ACC signal. This means that smaller window sizes are still capable of capturing enough information out of the 3D-ACC signal and at the same time preserve a reasonable speed in recognizing an activity. Contrasting with 3D-ACC signals, larger window sizes are more informative for the bio-signals (ECG and PPG). Because having a larger window means capturing more than one period of cardiac activity in one window, thus, the heartbeat rate can also be taken into account.
Given that we aim to compare the performance of 3D-ACC and bio-signals, we must select equal window sizes, in terms of time duration, to have a reasonable comparison. Thus, we need to keep the balance between selecting smaller window sizes for 3D-ACC and larger ones for PPG and ECG. We opt to select a window size of seven seconds, which offered a good balance across all signals.
Since each window of the segmented signal is not completely independent and identical from its neighboring windows, we applied non-overlapping sliding windows. Based on the results obtained from Dehghani et. al., such signals are not independent and identically distributed (i.i.d.), so that overlapping would lead to classification model over-fitting [
38].
3.3. Feature Extraction
After segmenting the signals in windows of seven seconds, we extract two types of features from each window: hand-crafted time and frequency domain features. In the following, we provide more detailed information about these two categories of features.
3.3.1. Time-Domain Features
Time-domain features are the statistical measurements calculated and extracted from each window in a time series. As formerly described, we segmented five raw signals 3D-ACC, PPG and ECG with a sampling rate of 64, 64 and 700 Hz, respectively. In total, we extract seven statistical features from each of these windows.
Table 2 presents the type of the features and their respective description. Features that we mention in the following table are easy to understand and are not computationally expensive, moreover, are capable of providing relevant information for HAR systems. Therefore, these features are frequently used in the field of HAR [
13,
39,
40].
3.3.2. Frequency-Domain Features
Transferring time-domain signals to the frequency domain provides insights from a new perspective of the signal. This approach is widely used in signal processing research as well as HAR field [
39,
40,
41].
In the first step to extract frequency-domain features, we segment the raw time-domain signals into fixed window sizes. Then, we transfer each segmented signal into the frequency domain using the Fast Fourier Transform (FFT) method [
42]. It is important to perform these two steps in the aforementioned order, otherwise, each window would not contain all the frequency information. That is, low-frequency information would appear in the early windows and, then, the high-frequency components would be placed in the last windows. By contrast, the correct way is that each window must have all the frequency components. After obtaining frequency components from each window, we extract eight statistical and frequency-related features.
Table 3 presents different extracted features and a brief description for each of them.
From the frequency-domain features presented in
Table 3, the DC component and dominant frequency are the less intuitive ones. Thus, next we explain these two features in more detail. The “DC component” is the frequency-domain amplitude value which occurs at zero frequency. In other words, DC component is the average value of signal in time-domain over one period. Regarding a 3D-ACC signal, its DC component corresponds to gravitational accelerations [
43]. To be more precise, in the absence of device acceleration, the accelerometer output is equivalent to device rotation along axes [
44]. This explains the reason why its DC-component value is relatively larger than the rest of the frequency coefficients in the same window [
45]. As for bio-signals, however, the DC component is not highly greater than other frequency coefficients for which the reason is that bio-signals such as PPG and ECG are dynamic signals.
Figure 4 represents PPG and X-axis of ACC signals related to a specific time span of seven seconds of cycling activity in the frequency domain. The difference between DC-component values between these two signals is clear.
Regarding a 3D-ACC signal, since the DC component is also the maximum amplitude, we decide to introduce another feature, namely, “second-max” to avoid feature redundancy and differentiate between mentioned features. However, for bio-signals, we only consider the maximum amplitude.
Dominant frequency is the frequency at which the highest amplitude occurs [
46]. Based on this definition, again for a 3D-ACC signal, we disregard the amplitude corresponding to the zero frequency (DC component); instead, we consider the frequency corresponding to the second largest amplitude value (second max) as the “dominant frequency”. However, for the bio-signals, namely ECG and PPG signals, “dominant frequency” is based on the first maximum amplitude value.
3.4. Feature Standardization
Since we are examining different sources of signals with different characteristics, feature values will inevitably have different ranges, thus, we need to standardize the features before the model classification. For instance, the scale difference between X-axis of the ACC and the PPG signals have clearly distinct standard deviation of
and
, respectively. This shows a huge difference in the amplitude of these signals which must be addressed. To standardize the extracted features, we calculate the standard score for each feature using Formula
1. This approach is used in some previous HAR studies [
20,
47].
where for a given feature vector of size
m,
represents the
ith element in the feature vector,
and
are the mean and standard deviation for the same vector, respectively. The resulting value,
, is the scaled version of the original feature value,
. Using this method, we reinforce each feature vector to have zero mean and unit variance. However, the mentioned transformation retains the original distribution of the feature vector. Note that we split the dataset into train and test set before the standardization step. It is necessary to standardize the train set and the test set separately; because we do not want the test set data to influence the
and
of the training set, which would create an undesired dependency between the sets [
48].
3.5. Feature Selection
In total, we extract 77 features out of all sources of signals. Following the standardization phase, we remove the features which were not sufficiently informative. Omitting redundant features helps reducing the feature table dimensionality, hence, decreasing the computational complexity and training time. To perform feature selection, we apply the Correlation-based Feature Selection (CFS) method and calculate the pairwise Spearman rank correlation coefficient for all features [
49]. Correlation coefficient has a value in the
interval, for which zero indicates having no correlation,
or
refer to a situation in which two features are strongly correlated in a direct and inverse manner, respectively. In this study, we set the correlation coefficient threshold to
, moreover, among two recognized correlated features, we omit the one which was less correlated to the target vector. Finally, we select 45 features from all signals.
4. Classifier Models and Experiment Setup
In the following sections we explain the applied classifiers and detailed configuration for the preferred classifier. Next, we describe the model evaluation approaches, namely, subject-specific and cross-subject setups.
4.1. Classification
In our study, we examine three different machine learning models, namely, Multinomial Logistic Regression, K-Nearest Neighbors, and Random Forest. Based on our initial observations, the random forest classifier outperformed the other models in recognizing different activities. Thus, we conduct the rest of our experiment using only the random forest classifier.
Random Forest is an ensemble model consisting of a set of decision trees each of which votes for specific class, which in this case is the activity-ID [
50]. Through the mean of predicted class probabilities across all decision trees, the Random Forest yields the final prediction of an instance. In this study, we set the total number of trees to 300, and to prevent the classifier from being overfitted, we assign a maximum depth of each of these trees to 25. One advantage about using random forest as a classifier is that this model provides extra information about feature importance, which is useful in recognizing the most essential features.
To evaluate the level of contribution for each of the 3D-ACC, ECG and PPG signals, we take advantage of the early fusion technique and introduce seven scenarios presented in
Table 4. Subsequently, we feed the classifier with feature matrices constructed based on each of these scenarios. We use the Python Scikit-learn library for our implementation [
51].
4.2. Performance Evaluation
In our study, we evaluate two types of models, the subject-specific model and the cross-subject model. In the following, we provide detailed explanation about these two models and evaluation techniques.
4.2.1. Subject-Specific Model
Subject-specific models are the most accurate types of models, as they train and test using the data belonging to same user. Hence, it is important that we evaluate if bio-signals can be useful to make such models even better.
To evaluate the performance of our subject-specific model, we employ a
k-fold cross-validation technique [
52].
K-fold cross-validation is a widely-used method for performance evaluation and consists in randomly segmenting the dataset into
k parts (folds). The machine learning model is trained on
partitions and is tested on the remaining partition; this procedure repeats
k times, always testing the model on a different fold. For each of the
k runs, the evaluation procedure is done based on the scoring parameter. Finally, the average value of obtained scores is reported as the overall performance of the classifier.
As stated in
Section 2.2, we have an imbalanced dataset, therefore, it is important to specify how to split the dataset into folds. We use the stratified
k-fold method to preserve the proportion of each class label in each fold to be similar to the proportion of each class label in the entire set. Regarding scoring parameters, we evaluate our models with two metrics, namely, F1-score and area under the receiver operating characteristic (ROC) curve [
53,
54]. Since our study is a multi-class classification problem, we aggregate the mentioned scores using an average weighted by support.
In our case, to evaluate the subject-specific model, we consider one feature set related to only one subject and split it into a train set (
) and a test set (
). Subsequently, we apply the 10-fold CV technique on the training set and store the resulting F1-score and AUC measurements per fold. Finally, we apply the trained model on the test set, then, we record its classification performance in terms of F1-Score and AUC. Our objective of evaluating the model performance on the train set, and then on the test set, was to confirm that the model is not overfitting the data. An overfitted model fits perfectly on the train set, but has poor performance on the test set [
55]. We repeat the described procedure 14 times, as many as the number of subjects. Eventually, we calculate the average F1-Score and AUC, over all subjects’ results and will report its performance in
Section 5.1.
Subject-specific model is a subject-dependent technique, since we train the model on features related to one subject and then test the model using the remaining features belonging to the same subject; also known as “personal model” in the study of Weiss et al. [
56].
4.2.2. Cross-Subject Model
Cross-subject models are not as accurate as the subject-specific models [
21], however, since such models are cheaper, in practice, these are more commonly used. Cross-subject models are cheaper because they do not require the user’s personal data, instead, are trained using data from other individuals. Therefore, knowing that bio-signals can contribute to this type of model is important to improve the generalization of the model.
To evaluate the performance of our cross-subject model, we use the Leave-One-Subject-Out (LOSO) evaluation method [
57,
58]. This method consists in training models on a group of subjects and testing the model on unseen individual data (test set). Similar to the
k-fold, in a group with
n subjects, the model is trained in
subjects and tested on the remaining subject’s data. This process repeats
n times, to cover all subjects in the test set, always using the other subjects’ data for training.
In our case, to evaluate the cross-specific model, we create a larger feature table consisting of 13 feature sets related to all subjects but one. We use this table as an input matrix to train the random forest model. Then, we test our model by the feature table of the remaining subject. Again, we aggregate F1-score and AUC measurement using average weighted by support. We rerun this process 14 times. Then, we calculate the average F1-Score and AUC over all subjects’ results and will report its performance in
Section 5.2.
The aforementioned evaluation method, minimizes the risk of overfitting, moreover, it is subject-independent; and is also called “impersonal model” in the study of Wiess et al. [
56]. Hence, if a classification model performs well given the LOSO evaluation method, then this model generalizes well to other subjects.
5. Results
In this section, we report the results obtained from aforementioned evaluation strategies. We answer our initial questions about how informative are each of the sources of signals and whether sensor fusion improves the performance of a HAR classifier. We organize the result section based on the scenarios presented in
Table 4, that is, using only one source of signal (scenario 1, 2 and 3), considering a combination of two signals (scenario 4, 5, and 6), and scenario 7 which is related to 3D-ACC, ECG and PPG signals fusion. We conclude each subsection by reporting our observations regarding the activity performance of the HAR models.
5.1. RQ1: What Is the Contribution Level of Signals under Study in Subject-Specific HAR Systems?
As stated in
Section 4.2.1, we train and test subject-specific models and we are interested in the contribution level of each source of signals in these models. We report the results of the subject-specific model evaluation in
Figure 5.
One type of signal. Considering only one source of signal, the 3D-ACC signal (Scenario 1) outperforms the other two bio-signals in recognizing human activities (Scenarios 1–3). Interestingly, a model using exclusively the ECG signal (Scenario 2) performs relatively satisfactory, with a comparable AUC performance to a 3D-ACC trained model, yielding a much better performance than the model trained solely with a PPG signal (Scenario 3).
Two signals fusion. When combining the 3D-ACC with the ECG signal (Scenario 4), the performance of the model surpasses the model using only 3D-ACC (Scenario 1). As shown in
Figure 5, adding ECG to 3D-ACC improves the human activity recognition by
in terms of F1-Score. Our results suggest that including the ECG signal in HAR systems that are based solely on the 3D-ACC can slightly improve the model performance. However, adding a PPG signal to the 3D-ACC signal (Scenario 5) does not provide any significant enhancement for our HAR models. Combining solely bio-signals (PPG and ECG in Scenario 6) does not yield a model with superior performance compared to just a 3D-ACC model, even if they outperform models trained with one bio-signal (Scenarios 2 and 3).
Three signals fusion. Regarding Scenario 7, when we consider all three sources of signals, we realize that human activity recognition performance remains almost the same compared to Scenario 4 when we only took 3D-ACC and ECG signals into account. Therefore, we conclude that PPG signal fusion did not add any strength to the classifiers in our evaluation. In addition, it is obvious from
Figure 5 that the PPG signal is not very informative, not exclusively nor in combination with other sources of signals for subject-dependent HAR systems.
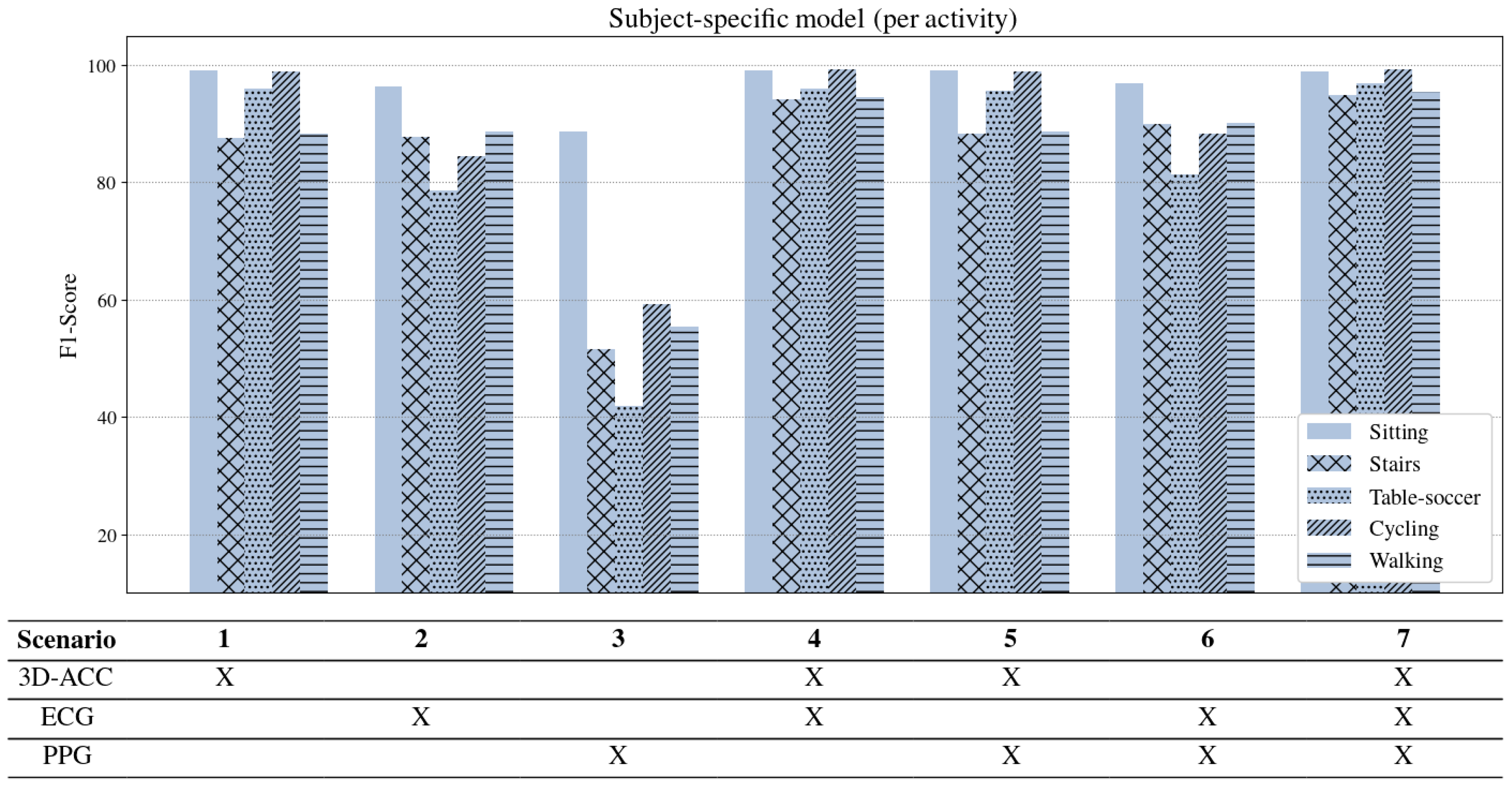
Per activity performance. Figure 6 represents results of the subject-specific model per activity. It is noticeable that ascending/descending stairs and walking are the two activities that our models have difficulty distinguishing when using only the 3D-ACC signal. However, feeding the model with features extracted from both 3D-ACC and ECG signals (Scenario 4), improves the “stairs” and “walking” distinction significantly by
and
F1-score, respectively. An important takeaway from
Figure 6 is that bio-signals have reliable power in distinguishing stationary activities such as sitting from non-stationary ones such as walking and cycling. When comparing the contribution of the PPG signal to the 3D-ACC per activity, we note that the combination did not yield any improvement in distinguishing the mentioned activities. In fact, the model performance when combining 3D-ACC and PPG is highly similar to its performance when we apply only a 3D-ACC signal (as in Scenario 1), which indicates an inadequacy of the PPG signal features in distinguishing any information not already captured by the 3D-ACC.
5.2. RQ2: What Is the Contribution Level of Signals under Study in Cross-Subject HAR Systems?
In
Section 4.2.2, we mentioned the cross-subject models and the fact that these models tend to perform worse than subject-specific models, given that cross-subject are more general. Therefore, for the current evaluation setup, we observe a more significant contribution from bio-signals.
Figure 7 shows the overall performance of the signals under study in terms of F1-score and AUC measurements (aggregated as stated above). As expected, the performance of cross-subject models has an overall lower performance than the subject-specific models. This decrease in performance is expected as cross-subject models are trained on other individual’s data, and individuals perform activities differently. Moreover, other factors, such as subject’s height, weights, gender and level of fitness may contribute in this variation.
One type of signal. According to
Figure 7, the 3D-ACC signal provides the most informative data to our cross-subject models, yielding a performance with a F1-score of 83.16 (Scenario 1). Contrasting with the results observed for subject-specific models, a model trained only with ECG (Scenario 2) did not yield comparable performance with a 3D-ACC model (Scenario 1). Still, cross-subject models trained using only the ECG signal (Scenario 2) outperform the models trained exclusively with the PPG signal (Scenario 3), by 13.49% in terms of F1-score.
Two signals fusion. The combination of 3D-ACC and ECG signals (Scenario 4) has shown to improve the performance of our cross-subject model by (F1-score), compared to using exclusively the 3D-ACC signal. Once again, a fusion of PPG and 3D-ACC signals has shown to not yield performance improvements (Scenario 5) to trained 3D-ACC models. Interestingly, combining both ECG and PPG (Scenario 6) yields a model with better performance than the models trained exclusively with ECG (Scenario 2) and PPG (Scenario 3), even if it still underperforms against the purely 3D-ACC trained model (Scenario 1). In the end, we conclude that the ECG signal can be complement well the 3D-ACC signal in HAR systems, while PPG did not provide informative data to our cross-subject models.
Three signals fusion. Once we fuse all three sources of signals, we observe a decrease in the model’s performance compared to just combining ACC-3D and ECG signals (Scenario 4). This further corroborates with the notion that adding the PPG signal to the combination of 3D-ACC and ECG signals is disadvantageous.
Per activity performance. Figure 8 shows the performance of the models broken down per activity. First, we note that “cycling”, “table-soccer” and “sitting” activities remain rather stable in all models trained with 3D-ACC, exclusively or in combination. Second, cross-subject models often miss-classify “stairs” and “walk” activities. Once we fuse both the 3D-ACC and ECG signal, the model is better able to distinguish between the two activities, which explains the gain in the overall performance of the model. Third, models trained exclusively with bio-signals have very distinct performance profiles per activity. Note that PPG is reasonably good at distinguishing the “sitting” activity, as this is the least physically demanding activity in our dataset (lower heart rate). ECG models, on the other hand, outperform PPG models in all other activities, further corroborating that the ECG signal is, on average, more informative for cross-subject HAR models than the PPG signal.
6. Discussion
To elaborate more on the impact of the ECG signal in the performance of HAR models, we dive into the confusion matrices related to all subjects in both subject-specific and cross-subject models.
6.1. Subject-Specific
As stated earlier, considering only 3D-ACC signals, models already reach a high recognition performance of
F1-score. Thus, most of the instances in confusion matrices are labeled accurately. However, the activities of using “Stair” and “Walking” were frequently confused with each other. Therefore, we contrast the confusion matrices of the model which include only 3D-ACC (Scenario 1) against the models including both 3D-ACC and ECG signals (Scenario 4). We observe significant improvement in distinguishing the mentioned activities after adding the ECG signal.
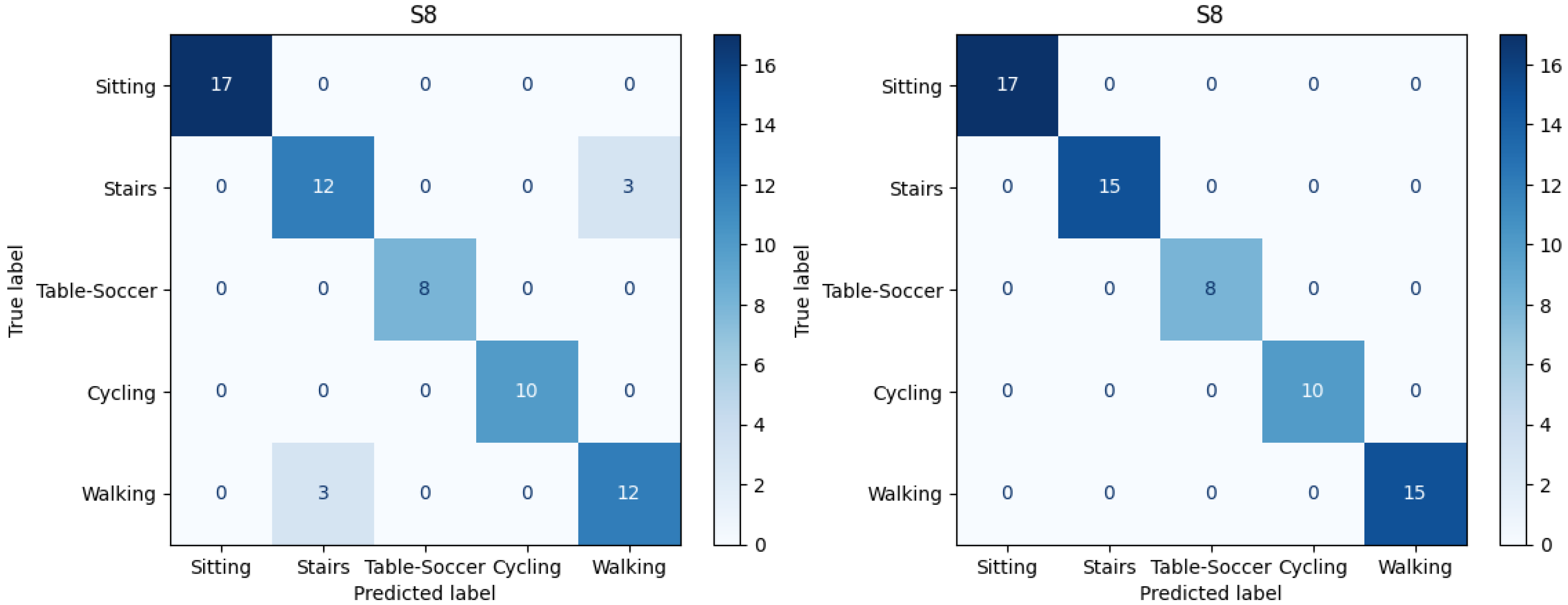
Figure 9 presents confusion matrices related to subject number 8 in the subject-specific model. On the left side of
Figure 9, we can observe the model performance when considering only 3D-ACC. Note the instances which are miss-classified and confused between “Stairs” and “Walking” activities. On the right side of
Figure 9, however, it is clear that after adding the ECG signal, the mentioned confusions are solved.
It is important to mention that for 10 out of 14 subjects we observe “Stairs-Walking” improvement after adding the ECG signal to 3D-ACC, however, in 3 out of 14 cases adding the ECG signal does not improve the “Stairs-Walking” classification. Moreover, in 1 case, the model perfectly distinguishes between “Stairs-Walking” by just using the 3D-ACC, leaving no space for improvement for the 3D-ACC and ECG fusion model.
6.2. Cross-Subject
Cross-subject models provide a more insightful analysis, since these models miss-classify activities more often, compared to subject-specific models. As depicted in
Figure 7, using only the 3D-ACC signal, we obtained an F1-score of
which is relatively lower than the model performance in the subject-specific setup. After a detailed investigation in confusion matrices of the 3D-ACC trained model, we once again identify that the activities “stairs” and “walking” are miss-labeled. In addition to the mentioned pair of activities, another pair is miss classified in cross-subject models, namely, “sitting” and “playing table soccer”.
We once again compare the confusion matrices related models trained with 3D-ACC (Scenario 1) signal versus the model trained with both 3D-ACC and ECG signals (Scenario 4). We observe that the ECG signal significantly helps the model recognize “Stairs-Walking”, however, it does not add any value when it comes to distinguishing the “Sitting-Table-Soccer” pair.
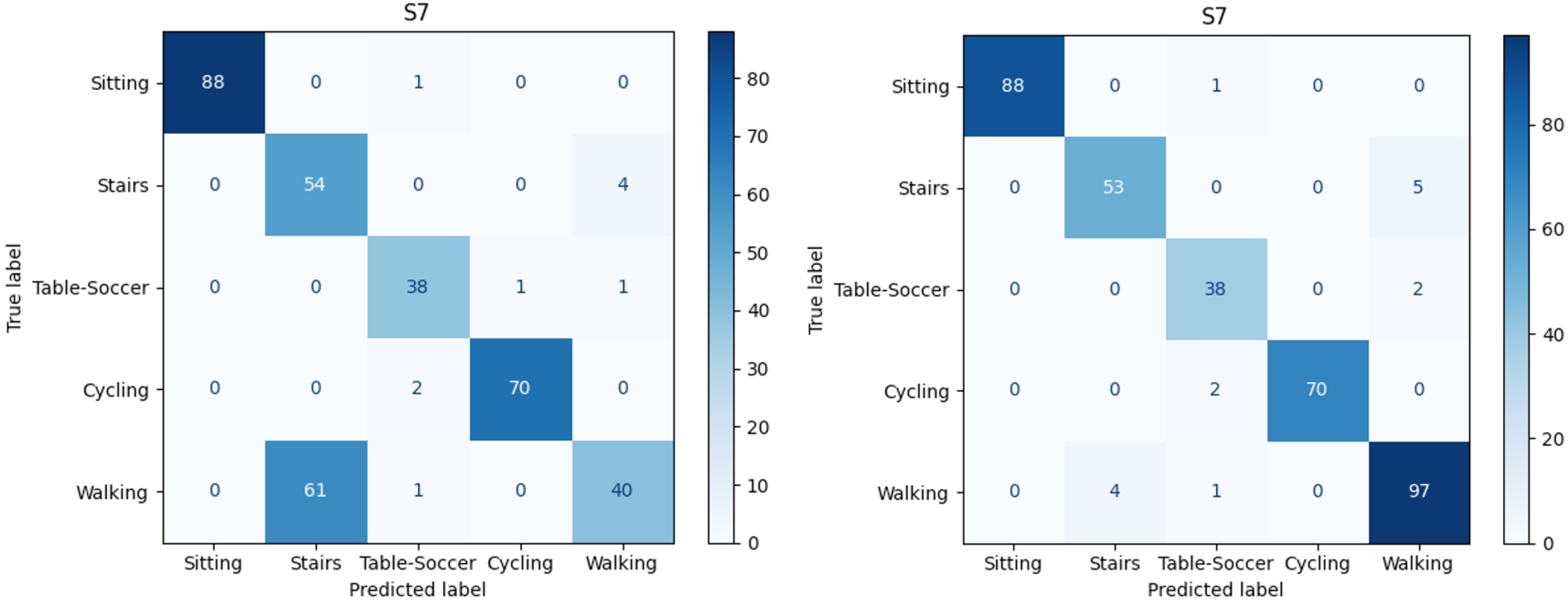
Figure 10 depicts both confusion matrices related to subject number 7 in the cross-subject model. The left side of
Figure 10 is related to the model performance when considering only 3D-ACC; note the huge portion of “Walking” instances which are miss-classified as “Stairs”. However, on the right side of
Figure 10, it is obvious that after adding the ECG signal, the “Stairs-Walking” detection enhances noticeably.
It is worth noting that for 9 out of 14 subjects, we observe “Stairs-Walking” improvement after adding the ECG signal to a pure 3D-ACC model. In 3 out of 14 cases, adding the ECG signal yielded no significant impact; and, in 2 out of 14 cases, the ECG signal addition resulted in a decline in the “Stairs-Walking” classification.
6.3. Feature Importance
We have shown that fusing 3D-ACC and ECG signals yielded the best performance in classifying human activities in our study. However, which features from both signals were the most relevant to our model? In this section, we present the feature importance ranking of the model that combines 3D-ACC and ECG (Scenario 4) using the cross-subject model, as we want to investigate the best features across multiple subjects. We calculate the feature importance using the Mean Decrease in Impurity (MDI) of our random forest model [
59]. To aggregate the importance score for each model evaluated on a single subject, we calculate the average score for each feature over all the subjects and rank their importance score. As
Table 5 shows, out of top 20 features, 16 features are related to the 3D-ACC signal and 4 of them to the ECG signal. Naturally, as 3D-ACC provides the best signal of the individual signal models (scenario 1), we expect to see a dominance of 3D-ACC features in the top-20 ranking. Interestingly, frequency-domain features rank higher than the time-domain ones, confirming that frequency-based features provide informative data to our classifiers. Another pattern that emerges is that, for 3D-ACC, the Y and Z axes are relatively more important than the X-axis of the accelerometer sensor. As the original authors of the PPG-DaLiA dataset collected the accelerometer using a wristband, when the subject’s hand is positioned alongside the body, the X-axis captures movement in the forwards-backwards direction, the Y-axis captures up-down movements, and finally, the Z-axis captures left-right movements [
34].
6.4. Limitation and Future Work
As discussed in
Section 2.2, a variety of recorded signals are provided in the PPG-DaLiA dataset, however, out of IMU sensors (3D-ACC, gyroscope, and magnetometer), the available signals are limited to the 3D-ACC. This limitation prevents us from further investigating the contribution level of bio-signals when combined with gyroscope, and magnetometer signals. Furthermore, our results show that there are varying levels of contribution of the ECG signal in scenario 4. This variation may be due to fitness level, thus, the heart activity, of an individual. Although the PPG-DaLiA dataset was provided along with the fitness level score for each subject, the majority of the subjects had a score of above 4 (the scores are in the range of 1 to 6 depending on how often an individual exercises), which makes it difficult to check the impact of fitness level on heart and, consequently, on fused HAR systems. In our future work, we plan to check the impact of other contributing factors, such as fitness level, height, weight, and age on the performance of a fused HAR system in cross-subject setup. Moreover, as stated earlier, there are two main fusion methods, namely, early and late fusion, we want to further evaluate the contribution level of the signals under study in the late fusion approach.
7. Conclusions
In this paper, we perform a comparative study to evaluate the contribution level of different sources of signals in HAR systems. We use a dataset consisting of 3D-ACC, PPG, and ECG signals recorded while 14 subjects were performing daily activities.
After examining different window sizes, we identify that window size of seven seconds is the best segment duration to obtain sufficient information out of the mentioned signals. Afterwards, we extract time and frequency domain features from each segment, then standardize features so that all the features have zero mean and unit variance. Next, we omit features with more than correlation and select 45 features out of total 77 extracted features. We introduce seven scenarios of evaluation, including models trained solely by one source of signal and models trained by a combination of signal features. Finally, we evaluate two types of HAR models using Random Forest classifiers, a subject-specific model and a cross-subject model.
We conclude that in both subject-specific and cross-subject models, the 3D-ACC signal is the most informative signal if the HAR system design purpose is to record and use only one source of signal. However, our results suggest that the 3D-ACC and ECG signal combination improves recognizing activities such as walking and ascending/descending stairs. Moreover, we experimentally assess that features extracted from the PPG signal are not informative for HAR system, not exclusively, nor when applying signal fusion. Although, both bio-signals yield a satisfactory performance in distinguishing stationary activities (e.g., sitting) from non-stationary activities (e.g., walking, cycling). Overall, our results indicate that it might be beneficial to combine features from the ECG signal in scenarios in which pure 3D-ACC models struggle to distinguish between activities that have similar motion (walking vs. walking up/down the stairs) but differ significantly in their heart rate signature.