A Pilot Trial to Evaluate the Accuracy of a Novel Non-Invasive Glucose Meter
Abstract
:1. Introduction
2. Materials and Methods
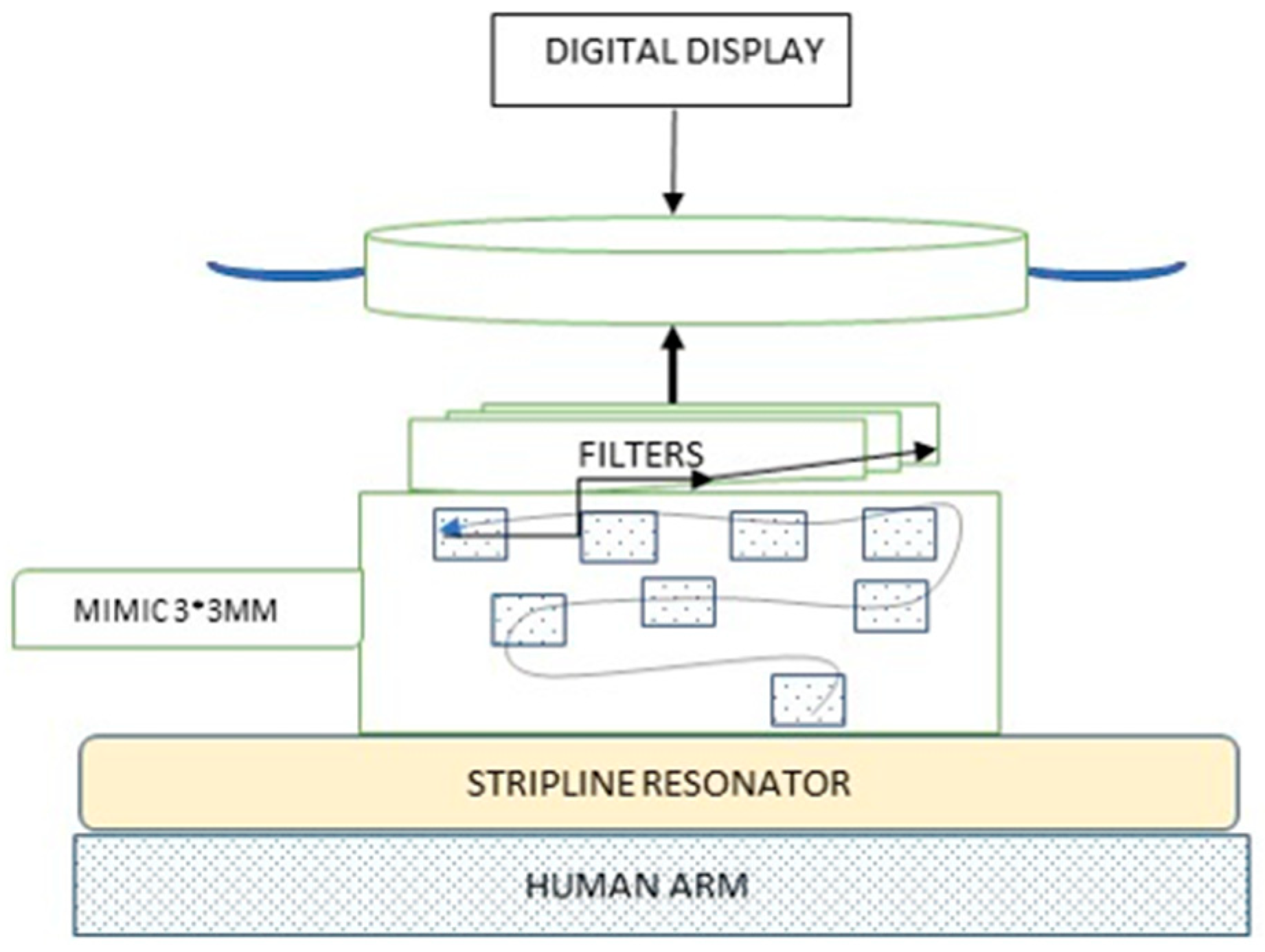
2.1. The Device
2.2. Study Participants
2.3. Study Location
2.4. Study Procedure Overview
2.5. Study Procedure Step by Step
- Patients reported at 8:00 a.m. after an 8 h fast (no caloric intake, water permitted). A peripheral intravenous catheter was placed in the antecubital fossa. Baseline blood glucose was collected and sent for analysis at the core laboratory.
- The HGR glucose meter was calibrated at the beginning of the day, before initiating any study procedures using Abbot FreeStyle® glucose meters and standard solutions.
- Blood glucose was measured non-invasively using the HGR device by placing the reading plate of the device on the anterior part of the wrist. The capillary blood glucose of study participants 2–5 was recorded at the baseline and at all the time points, using two Abbot FreeStyle® glucose meters.
- Study participants consumed 75 g of glucose (standard solution for an oGTT made by adding 75 g glucose [FLORIS, Industrial Park Misgav, Israel] to 180 mL water) within 15 min.
- Additional sets of measurements (venous blood, non-invasive reading and capillary blood) were taken at 15, 30, 45, and 60 min following glucose ingestion.
- Between 60 and 180 min samples were collected every 30 min, with a total of 9 sets of measurements per subject from 0 to 180 min.
2.6. Study Outcomes
- The median related absolute difference (RAD) between HGR glucose measurements and reference glucose levels. RAD was calculated using the following formula:RAD = (Obgi − Rbgi)/RbgiObgi is the ith HGR estimated glucoseRbgi is the ith reference blood glucose
- The percentage of the readings within ±5%, ±10%, ±15% and ±20% of the reference readings were calculated.
- The Pearson correlation between HGR glucose meter readings and reference glucose values was calculated using GraphPad Prism V.6.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- DHHS. National Diabetes Statistics Report, 2020. Available online: https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html (accessed on 5 October 2021).
- American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S85–S99. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.J.; Stalvey, M.S.; Silverstein, J.H. Predictors of control of diabetes: Monitoring may be the key. J. Pediatr. 2004, 144, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Tsuzaki, K.; Yoshioka, F.; Okada, H.; Kishi, J.; Yamada, K.; Sakane, N. The relationship between the frequency of self-monitoring of blood glucose and glycemic control in patients with type 1 diabetes mellitus on continuous subcutaneous insulin infusion or on multiple daily injections. J. Diabetes Investig. 2015, 6, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Machry, R.V.; Rados, D.V.; de Gregório, G.R.; Rodrigues, T.C. Self-monitoring blood glucose improves glycemic control in type 2 diabetes without intensive treatment: A systematic review and meta-analysis. Diabetes Res. Clin. Pr. 2018, 142, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.; Polonsky, W.H.; Parkin, C.G.; Jelsovsky, Z.; Petersen, B.; Wagner, R.S. The impact of structured blood glucose testing on attitudes toward self-management among poorly controlled, insulin-naïve patients with type 2 diabetes. Diabetes Res. Clin. Pr. 2012, 96, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, W.V.; Mobashsher, A.T.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, N.; Yabe, D.; Murotani, K.; Ueno, S.; Kuwata, H.; Hamamoto, Y.; Kurose, T.; Takahashi, N.; Akashi, T.; Matsuoka, T.; et al. Mental distress and health-related quality of life among type 1 and type 2 diabetes patients using self-monitoring of blood glucose: A cross-sectional questionnaire study in Japan. J. Diabetes Investig. 2018, 9, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.-S.; Ong, W.M.; Ng, C.J. Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: A qualitative study. Patient Preference Adherence 2014, 8, 237–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatman, E.L.; Jenkins, A.; Ahmedani, M.Y.; Ogle, G.D. Blood glucose meters and test strips: Global market and challenges to access in low-resource settings. Lancet Diabetes Endocrinol. 2019, 7, 150–160. [Google Scholar] [CrossRef]
- Hellmund, R.; Weitgasser, R.; Blissett, D. Cost calculation for a flash glucose monitoring system for UK adults with type 1 diabetes mellitus receiving intensive insulin treatment. Diabetes Res. Clin. Pr. 2018, 138, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolla, A.S.; Priefer, R. Blood glucose monitoring- an overview of current and future non-invasive devices. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; So, C.-F.; Choi, K.-S.; Wong, T.K. Recent advances in noninvasive glucose monitoring. Med. Devices Evid. Res. 2012, 5, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, T.; Foster, R.; Hao, Y. Radio-Frequency and Microwave Techniques for Non-Invasive Measurement of Blood Glucose Levels. Diagnostics 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S Food and Drug Administration. Self-Monitoring Blood Glucose Test Systems for Over-the-Counter Use Guidance for Industry and Food and drug administration staff; Food and Drug Administration: Rockville, MD, USA, 2020.
- Saha, S.; Cano-Garcia, H.; Sotiriou, I.; Lipscombe, O.; Gouzouasis, I.; Koutsoupidou, M.; Palikaras, G.; Mackenzie, R.; Reeve, T.; Kosmas, P.; et al. A Glucose Sensing System Based on Transmission Measurements at Millimetre Waves using Micro strip Patch Antennas. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Kokabi, H.; Alquié, G.; Deshours, F.; Shubair, R.M. Low-cost portable microwave sensor for non-invasive monitoring of blood glucose level: Novel design utilizing a four-cell CSRR hexagonal configuration. Sci. Rep. 2020, 10, 1–20. [Google Scholar] [CrossRef]
- Cano-Garcia, H.; Saha, S.; Sotiriou, I.; Kosmas, P.; Gouzouasis, I.; Kallos, E. Millimeter-Wave Sensing of Diabetes-Relevant Glucose Concentration Changes in Pigs. J. Infrared Millimeter, Terahertz Waves 2018, 39, 761–772. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Parkes, J.L.; Kovatchev, B.P.; Kerr, D.; Bevier, W.C.; Brazg, R.L.; Christiansen, M.; Bailey, T.S.; Nichols, J.H.; Kohn, M.A. Investigation of the Accuracy of 18 Marketed Blood Glucose Monitors. Diabetes Care 2018, 41, 1681–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
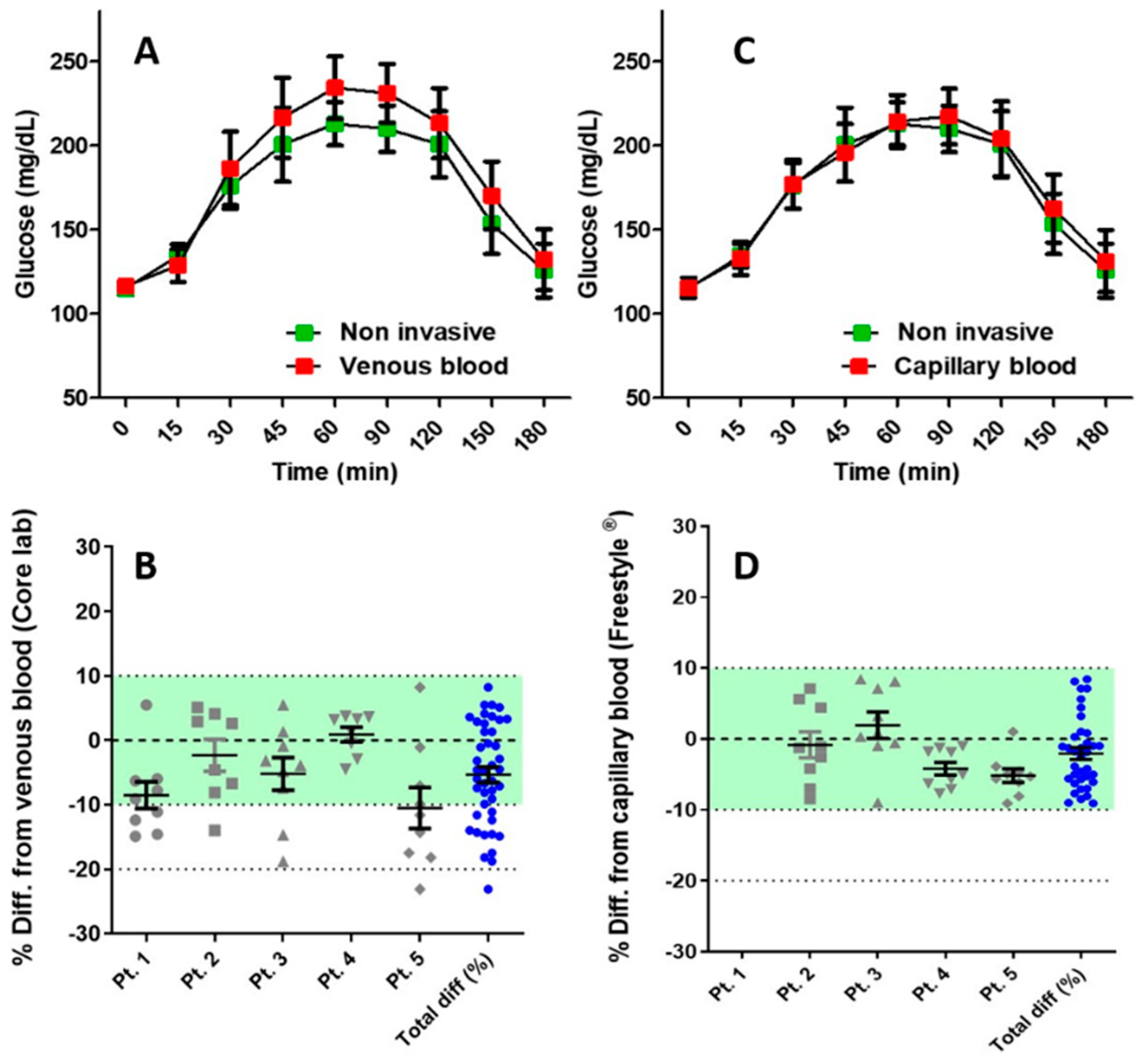

| Minimum | −23.08 |
| 25% Percentile | −11.59 |
| Median Relative Absolute Difference | −4.787 |
| 75% Percentile | 2.564 |
| Maximum | 8.209 |
| Mean Relative Absolute Difference | −5.339 |
| Std. Deviation | 7.908 |
| Std. Error | 1.206 |
| Within +/− 5% | Within +/− 10% | Within +/− 15% | Within +/− 20% | |
|---|---|---|---|---|
| Percent of glucose readings from reference glucose values | 60.45% | 79.04% | 92.99% | 97.64% |
| Percent of glucose readings from capillary glucose values | 94.3% | 100% | ---- | ---- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, Y.; Konvalina, N.; Tirosh, A. A Pilot Trial to Evaluate the Accuracy of a Novel Non-Invasive Glucose Meter. Sensors 2021, 21, 6704. https://doi.org/10.3390/s21206704
Schwarz Y, Konvalina N, Tirosh A. A Pilot Trial to Evaluate the Accuracy of a Novel Non-Invasive Glucose Meter. Sensors. 2021; 21(20):6704. https://doi.org/10.3390/s21206704
Chicago/Turabian StyleSchwarz, Yair, Noa Konvalina, and Amir Tirosh. 2021. "A Pilot Trial to Evaluate the Accuracy of a Novel Non-Invasive Glucose Meter" Sensors 21, no. 20: 6704. https://doi.org/10.3390/s21206704
APA StyleSchwarz, Y., Konvalina, N., & Tirosh, A. (2021). A Pilot Trial to Evaluate the Accuracy of a Novel Non-Invasive Glucose Meter. Sensors, 21(20), 6704. https://doi.org/10.3390/s21206704






