Using Wearable Sensor Technology to Measure Motion Complexity in Infants at High Familial Risk for Autism Spectrum Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedures
2.1.1. Quantitative and Standardized Motor Assessment
2.1.2. Developmental Evaluation
2.1.3. Sensor Data
2.1.4. Sensor Data Pre-Processing and Development of the Motion Complexity Index
2.1.5. Evaluation of Motion Complexity Score and Developmental Outcomes
3. Results
3.1. Motion Complexity Score
3.2. Developmental Outcomes
3.3. Relationship of the Motion Complexity Score and Developmental Outcomes
4. Discussion
4.1. Limitations
4.2. Next Steps
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thelen, E.; Bradshaw, G.; Ward, J.A. Spontaneous kicking in month-old infants: Manifestation of a human central locomotor program. Behav. Neural Biol. 1981, 32, 45–53. [Google Scholar] [CrossRef]
- Jeng, S.-F.; Chen, L.-C.; Yau, K.-I.T. Kinematic analysis of kicking movements in preterm infants with very low birth weight and full-term infants. Phys. Ther. 2002, 82, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Vaal, J.; van Soest, A.J.; Hopkins, B.; Sie, L.T.; van der Knaap, M.S. Development of spontaneous leg movements in infants with and without periventricular leukomalacia. Exp. Brain Res. 2000, 135, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Piaget, J. The Origins of Intelligence in Children; International Universities Press: New York, NY, USA, 1952. [Google Scholar]
- Karasik, L.B.; Tamis-lemonda, C.S.; Adolph, K.E. Transition from Crawling to Walking and Infants’ Actions with Objects and People. Child Dev. 2012, 82, 1199–1209. [Google Scholar] [CrossRef]
- Thelen, E. Motor development as foundation and future of developmental psychology. Int. J. Behav. Dev. 2000, 24, 385–397. [Google Scholar] [CrossRef]
- Iverson, J.M. Developing language in a developing body: The relationship between motor development and language development. J. Child Lang. 2010, 37, 229–261. [Google Scholar] [CrossRef]
- Campos, J.J.; Anderson, D.I.; Barbu-roth, M.A.; Hubbard, E.M.; Matthew, J.; Witherington, D. Travel Broadens the Mind. Infancy 2000, 1, 149–219. [Google Scholar] [CrossRef]
- Adolph, K.E.; Franchak, J.M. The development of motor behavior. Wiley Interdiscip. Rev. Cogn. Sci. 2017, 8. [Google Scholar] [CrossRef]
- Leonard, H.C. The impact of poor motor skills on perceptual, social and cognitive development: The case of developmental coordination disorder. Front. Psychol. 2016, 7, 1–4. [Google Scholar] [CrossRef]
- Bhat, A.N.; Galloway, J.C.; Landa, R.J. Relationship between early motor delay and later communication delay in infants at risk for autism. Infant Behav. Dev. 2012, 35, 836–846. [Google Scholar] [CrossRef]
- Flanagan, J.E.; Landa, R.; Bhat, A.; Bauman, M. Head lag in infants at risk for autism: A preliminary study. Am. J. Occup. Ther. 2012, 66, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.N.; Landa, R.J.; Galloway, J.C.; Ganz, M.; Ghaziuddin, M.; Butler, E.; Green, D.; Baird, G.; Barnett, A.; Miyahara, M.; et al. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys. Ther. 2011, 91, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Baranek, G.T.; Danko, C.D.; Skinner, M.L.; Donald, B., Jr.; Hatton, D.D.; Roberts, J.E.; Mirrett, P.L. Video Analysis of Sensory-Motor Features in Infants with Fragile X Syndrome at 9–12 Months of Age. J. Autism Dev. Disord. 2005, 35, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.-L.; Schoemaker, M.M.; Albaret, J.-M.; Geuze, R.H. What is the evidence of impaired motor skills and motor control among children with attention deficit hyperactivity disorder (ADHD)? Systematic review of the literature. Res. Dev. Disabil. 2015, 36, 338–357. [Google Scholar] [CrossRef]
- Roberts, J.E.; Mccary, L.M.; Shinkareva, S.V.; Bailey, D.B. Infant Development in Fragile X Syndrome: Cross-Syndrome Comparisons. J. Autism Dev. Disord. 2016, 46, 2088–2099. [Google Scholar] [CrossRef]
- Ulrich, B.D.; Ulrich, D.A. Spontaneous Leg Movements of Infants with Down Syndrome and Nondisabled Infants. Child Dev. 1995, 66, 1844–1855. [Google Scholar] [CrossRef]
- Piven, J.; Elison, J.T.; Zylka, M.J. Toward a conceptual framework for early brain and behavior development in Autism. Mol. Psychiatry 2017, 22, 1–10. [Google Scholar] [CrossRef]
- Ozonoff, S.; Young, G.S.; Carter, A.; Messinger, D.; Yirmiya, N.; Zwaigenbaum, L.; Bryson, S.; Carver, L.J.; Constantino, J.N.; Hutman, T.; et al. Recurrence Risk for Autism Spectrum Disorders: A Baby Siblings Research Consortium Study. Pediatrics 2011, 128, 488–495. [Google Scholar] [CrossRef]
- Lebarton, E.; Iverson, J. Associations between Gross Motor and Communicative Development in At-Risk Infants. Infant Behav. Dev. 2016, 59–67. [Google Scholar] [CrossRef]
- Elison, J.T.; Wolff, J.J.; Reznick, J.S.; Botteron, K.N.; Estes, A.M.; Gu, H.; Hazlett, H.C.; Meadows, A.J.; Paterson, S.J.; Zwaigenbaum, L.; et al. Repetitive Behavior in 12-Month-Olds Later Classified With Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2015, 53, 1216–1224. [Google Scholar] [CrossRef]
- Wilson, R.B.; Mccracken, J.T.; Rinehart, N.J.; Jeste, S.S. What’s missing in autism spectrum disorder motor assessments? J. Neurodev. Disord. 2018. [Google Scholar] [CrossRef]
- Harbourne, R.T.; Stergiou, N. Nonlinear analysis of the development of sitting postural control. Dev. Psychobiol. 2003, 42, 368–377. [Google Scholar] [CrossRef]
- Iverson, J.M.; Shic, F.; Wall, C.A.; Chawarska, K.; Curtin, S.; Estes, A.; Gardner, J.M.; Hutman, T.; Landa, R.J.; Levin, A.R.; et al. Early motor abilities in infants at heightened versus low risk for ASD: A Baby Siblings Research Consortium (BSRC) study. J. Abnorm. Psychol. 2019, 128, 69–80. [Google Scholar] [CrossRef]
- Abrishami, M.S.; Nocera, L.; Mert, M.; Trujillo-Priego, I.A.; Purushotham, S.; Shahabi, C.; Smith, B.A. Identification of Developmental Delay in Infants Using Wearable Sensors: Full-Day Leg Movement Statistical Feature Analysis. Point Care Technol. 2019, 7. [Google Scholar] [CrossRef]
- Harbourne, R.T.; Stergiou, N. Movement variability and the use of nonlinear tools: Principles to guide physical therapist practice. Phys. Ther. 2009, 89, 267–282. [Google Scholar] [CrossRef]
- Hadders-algra, M. Early Human Development Reduced variability in motor behaviour: An indicator of impaired cerebral connectivity? Early Hum. Dev. 2008, 84, 787–789. [Google Scholar] [CrossRef]
- Smith, B.A.; Teulier, C.; Sansom, J.; Stergiou, N.; Ulrich, B.D. Approximate Entropy Values Demonstrate Impaired Neuromotor Control of Spontaneous Leg Activity in Infants with Myelomeningocele. Pediatr. Phys. Ther. 2011, 23, 241. [Google Scholar] [CrossRef]
- Smith, B.; Vanderbilt, D.; Applequist, B.; Kyvelidou, A. Sample Entropy Identifies Differences in Spontaneous Leg Movement Behavior between Infants with Typical Development and Infants at Risk of Developmental Delay. Technologies 2017, 5, 55. [Google Scholar] [CrossRef]
- Piper, M.; Darrah, J. Motor Assessment of the Developing Infant; Saunders Publishing: Philadelphia, PA, USA, 1994. [Google Scholar]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part 1): Modules 1–4; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Mullen, E.M. Mullen scales of early learning: AGS edition. Am. Guid. Serv. 1995, 18, 92. [Google Scholar]
- Sparrow, S.; Balla, D.; Cicchetti, D. The Vineland Adaptive Behavior Scales: Interview edition, survey. Major Psychol. Assess. Instrum. 1984, 2, 199–231. [Google Scholar]
- Trujillo-Priego, I.A.; Smith, B.A. Kinematic characteristics of infant leg movements produced across a full day. J. Rehabil. Assist. Technol. Eng. 2017, 4, 205566831771746. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Lane, C.J.; Perkins, E.; Schiesel, D.; Smith, B.A. Determining if wearable sensors affect infant leg movement frequency: Brief Report. Dev. Neurorehabil. 2018, 21, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.; Trujillo-Priego, I.A.; Lane, C.J.; Finley, J.M.; Horak, F.B. Daily Quantity of Infant Leg Movement: Wearable Sensor Algorithm and Relationship to Walking Onset. Sensors 2015, 15, 19006–19020. [Google Scholar] [CrossRef] [PubMed]
- Welch, P.D. The use of Fast Fourier Transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Hadders-Algra, M. General Movements: A window for early identification of children at high risk for developmental disorders. J. Pediatr. 2004, 145, S12–S18. [Google Scholar] [CrossRef] [PubMed]
- Jeng, S.F.; Fetters, L.; Chen, Y.P.; Jonsdottir, J.; Tronick, E.Z. Kicking coordination captures differences between full-term and premature infants with white matter disorder. Hum. Mov. Sci. 2004, 22, 729–748. [Google Scholar]
- Wolff, J.J.; Botteron, K.N.; Dager, S.R.; Elison, J.T.; Estes, A.M.; Gu, H.; Hazlett, H.C.; Pandey, J.; Paterson, S.J.; Schultz, R.T.; et al. Longitudinal patterns of repetitive behavior in toddlers with autism. J. Child Psychol. Psychiatry Allied Discip. 2014, 55, 945–953. [Google Scholar] [CrossRef]
- Wolff, J.J.; Swanson, M.R.; Elison, J.T.; Gerig, G.; Pruett, J.R.; Styner, M.A.; Vachet, C.; Botteron, K.N.; Dager, S.R.; Estes, A.M.; et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol. Autism 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Brooks-kayal, A. Epilepsy and autism spectrum disorders: Are there common developmental mechanisms? Brain Dev. 2010, 32, 731–738. [Google Scholar] [CrossRef]
- Jeste, S.; Geschwind, D.H. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat. Rev. Neurol. 2014, 10, 74–81. [Google Scholar] [CrossRef]
- de la Torre-Ubieta, L.; Won, H.; Stein, J.L.; Geschwind, D.H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 2016, 22, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Pierce, K.; Schumann, C.M.; Redcay, E.; Buckwalter, J.A.; Kennedy, D.P.; Morgan, J. Mapping early brain development in autism. Neuron 2007, 56, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.E.; Hernandez, L.M.; Bookheimer, S.Y.; Dapretto, M. Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res. 2019, 12, 53–65. [Google Scholar] [CrossRef] [PubMed]
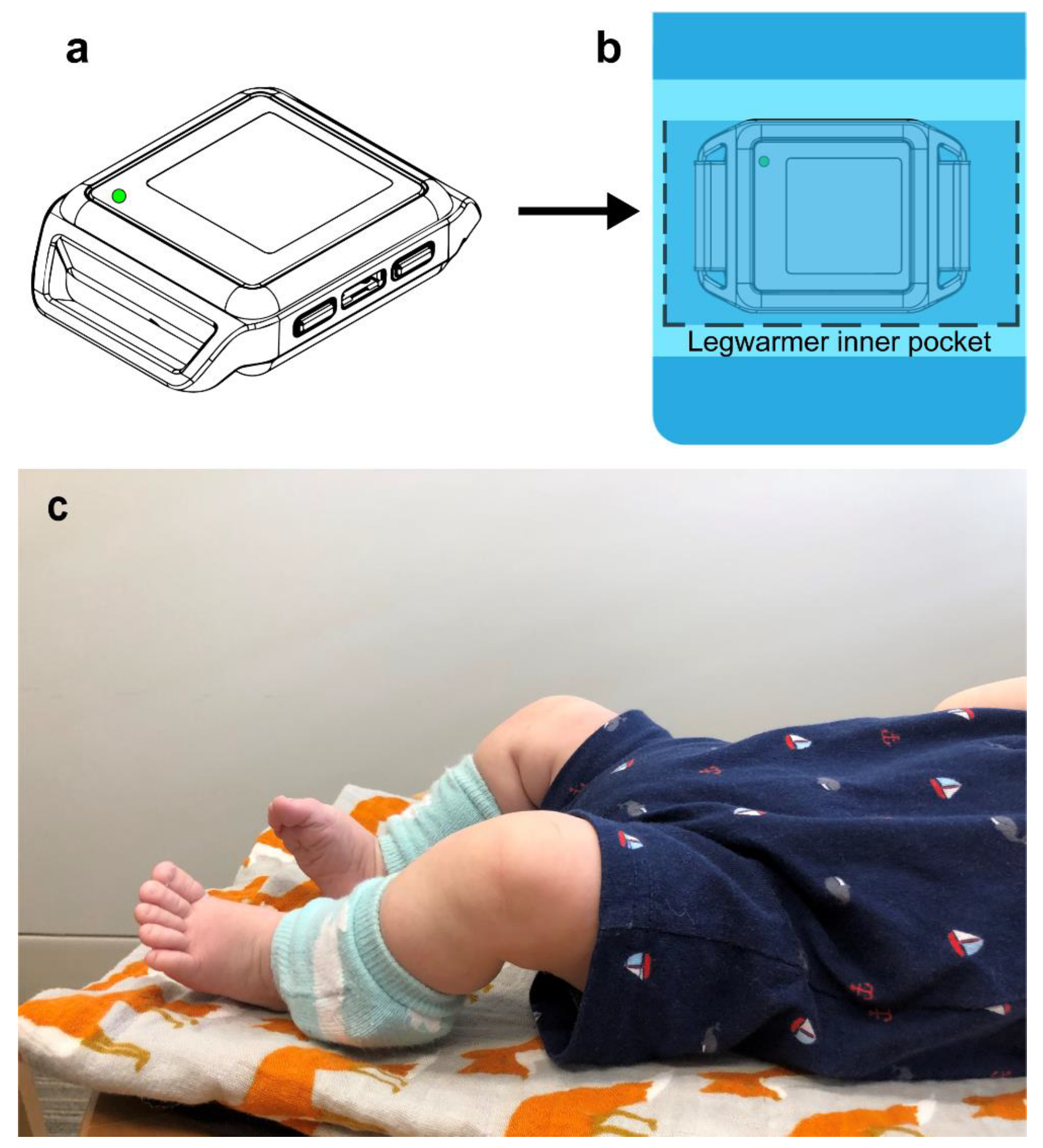
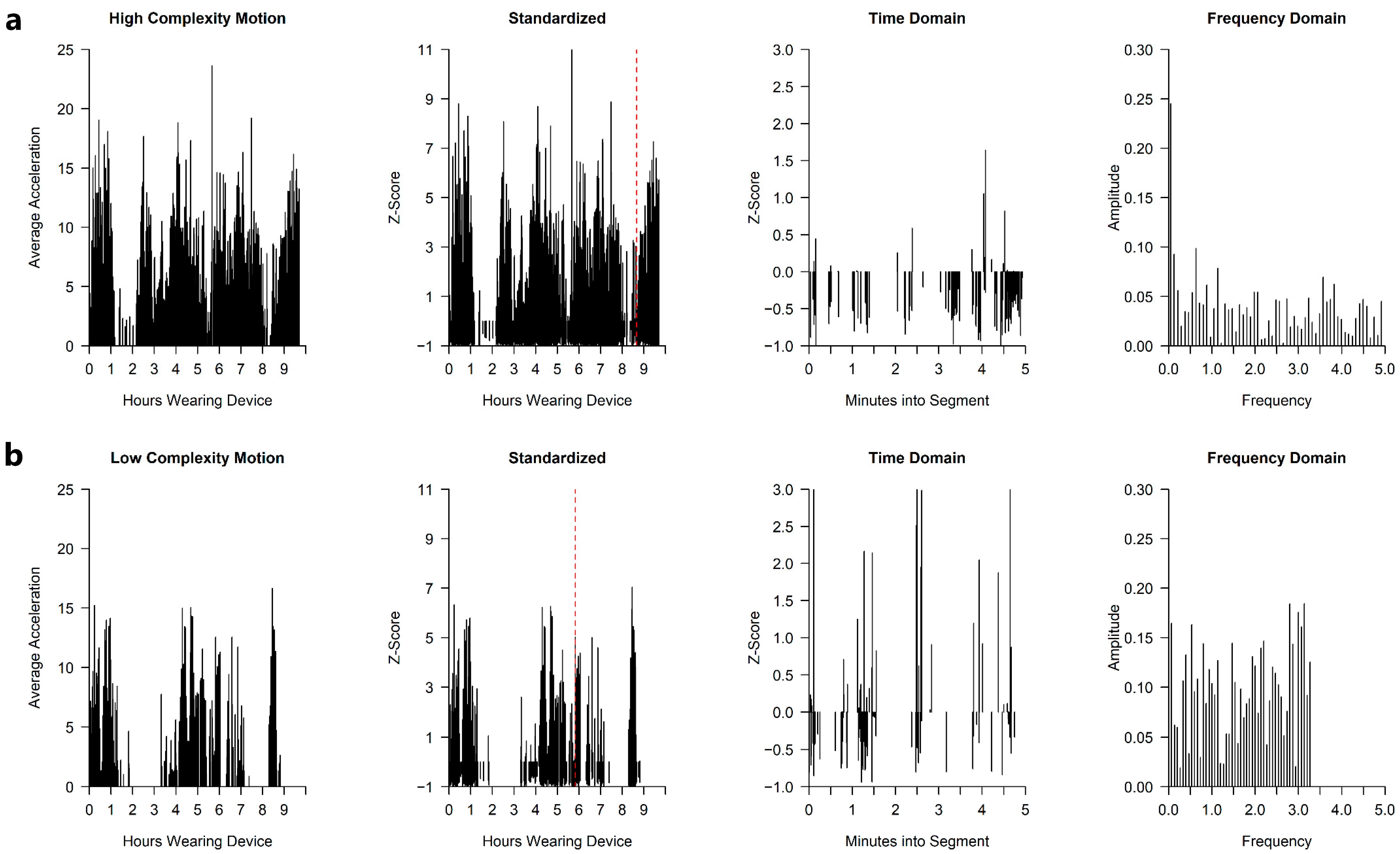
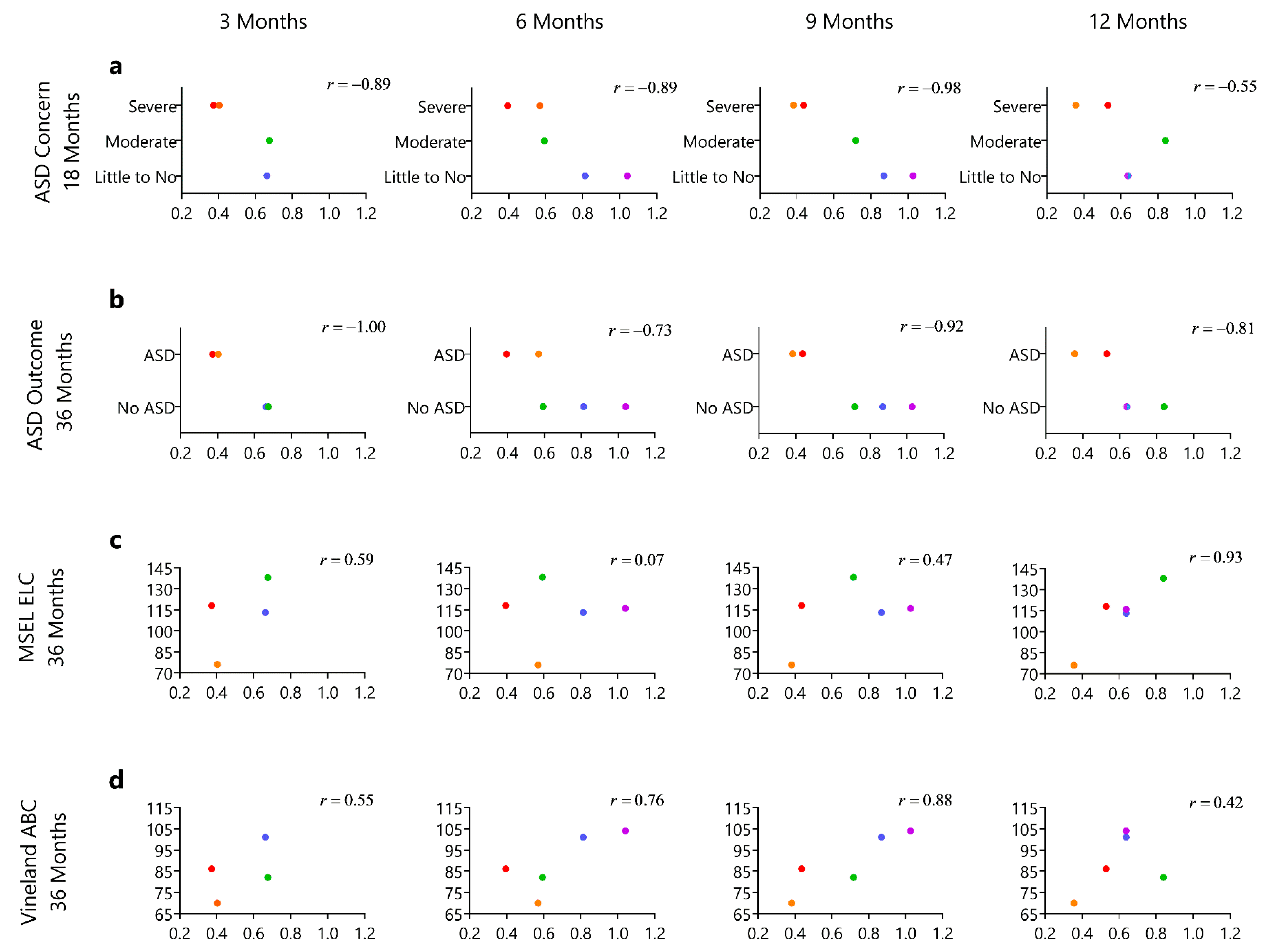
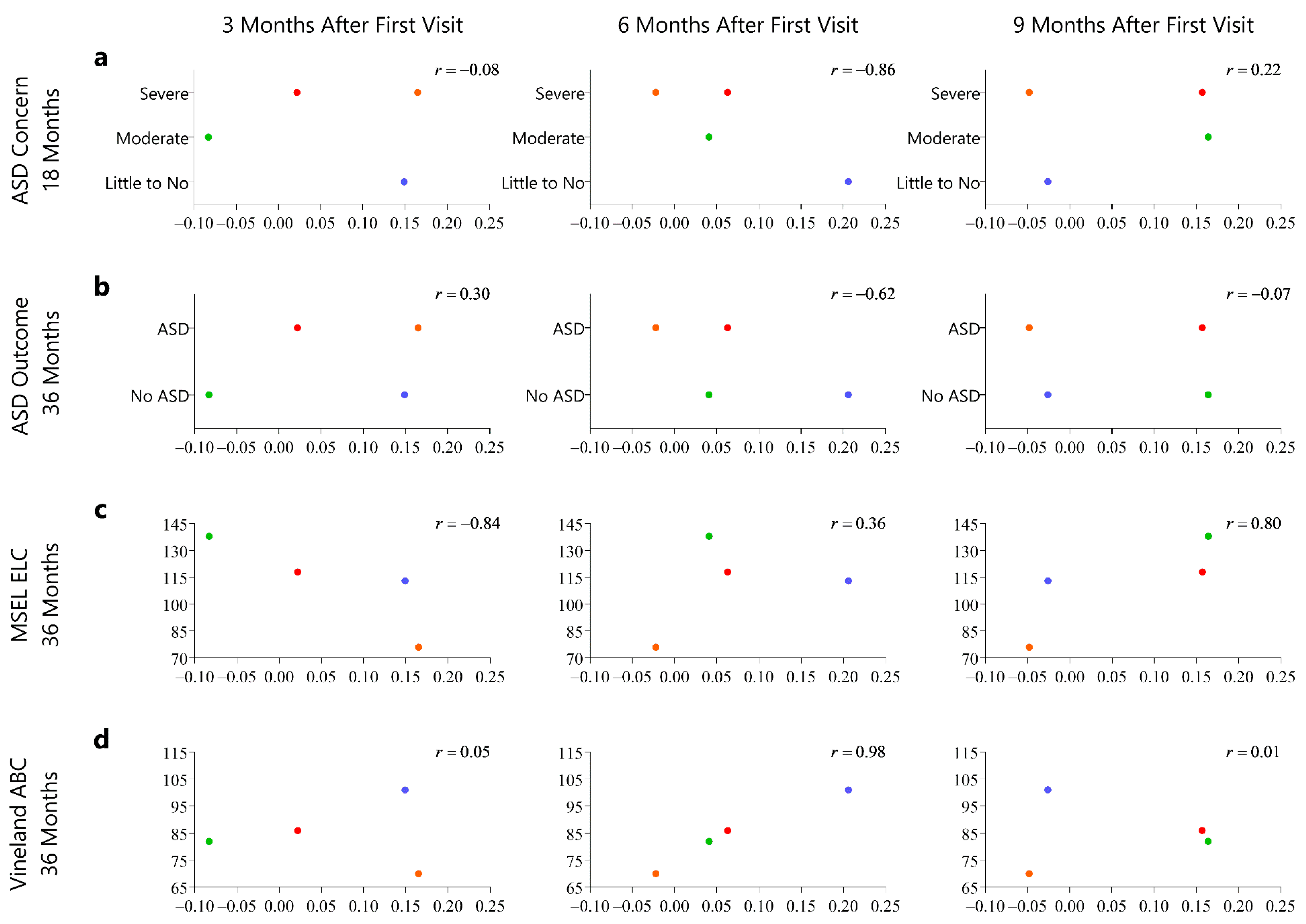
| Visit in Months | N | Age (days) | Weight (kg) | Body Length (cm) | Head (cm) | AIMS Percentile |
|---|---|---|---|---|---|---|
| 3 | 4 | 111 (32), (90–158) | 7.375 (1.4), (5.7–8.9) | 63.9 (5.0), (60.1–71.0) | 41.8 (1.5), (39.8–43.2) | 38 (20), (14–67) |
| 6 | 5 | 198 (29), (180–250) | 8.5 (1.9), (5.9–10.7) | 67.8 (5.3), (59.2–74.0) | 43.9 (1.5), (41.9–45.5) | 41 (27), (5–81) |
| 9 | 5 | 290 (9), (269–345) | 9.2 (2.0), (6.6–11.5) | 69.3 (3.9), (64.5–73.5) | 45.6 (1.7), (43.6–47.5) | 58 (25), (6–81) |
| 12 | 5 | 379 (28), (358–429) | 10.3 (2.0), (7.6–12.8) | 74.8 (4.0), (69.0–80.2) | 46.4 (1.23), (44.5–47.9) | 56 (35), (1–90) |
| Infant | ADOS Score (18 m) | ADOS Outcome (18 m) | ADOS Score (36 m) | ADOS Outcome (36 m) | MSEL ELC (36 m) | VABS ABC (36 m) |
|---|---|---|---|---|---|---|
| HR1 | 17 | Autism (moderate-severe concern) | 9 | Autism | 118 | 86 |
| HR2 | 18 | Autism (moderate-severe concern) | 12 | Autism | 76 | 70 |
| HR3 | 3 | Little to no concern | 6 | Non-spectrum | 113 | 101 |
| HR4 | 12 | Mild-moderate concern | 4 | Non-spectrum | 138 | 82 |
| HR5 | 1 | Little to no concern | 0 | Non-Spectrum | 116 | 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, R.B.; Vangala, S.; Elashoff, D.; Safari, T.; Smith, B.A. Using Wearable Sensor Technology to Measure Motion Complexity in Infants at High Familial Risk for Autism Spectrum Disorder. Sensors 2021, 21, 616. https://doi.org/10.3390/s21020616
Wilson RB, Vangala S, Elashoff D, Safari T, Smith BA. Using Wearable Sensor Technology to Measure Motion Complexity in Infants at High Familial Risk for Autism Spectrum Disorder. Sensors. 2021; 21(2):616. https://doi.org/10.3390/s21020616
Chicago/Turabian StyleWilson, Rujuta B., Sitaram Vangala, David Elashoff, Tabitha Safari, and Beth A. Smith. 2021. "Using Wearable Sensor Technology to Measure Motion Complexity in Infants at High Familial Risk for Autism Spectrum Disorder" Sensors 21, no. 2: 616. https://doi.org/10.3390/s21020616
APA StyleWilson, R. B., Vangala, S., Elashoff, D., Safari, T., & Smith, B. A. (2021). Using Wearable Sensor Technology to Measure Motion Complexity in Infants at High Familial Risk for Autism Spectrum Disorder. Sensors, 21(2), 616. https://doi.org/10.3390/s21020616





