Fast-Response Colorimetric UVC Sensor Made of a Ga2O3 Photocatalyst with a Hole Scavenger
Abstract
1. Introduction
2. Experimental
2.1. Reduction of Methylene Blue (MB) with Ga2O3 Photocatalysts with 254 nm Radiation
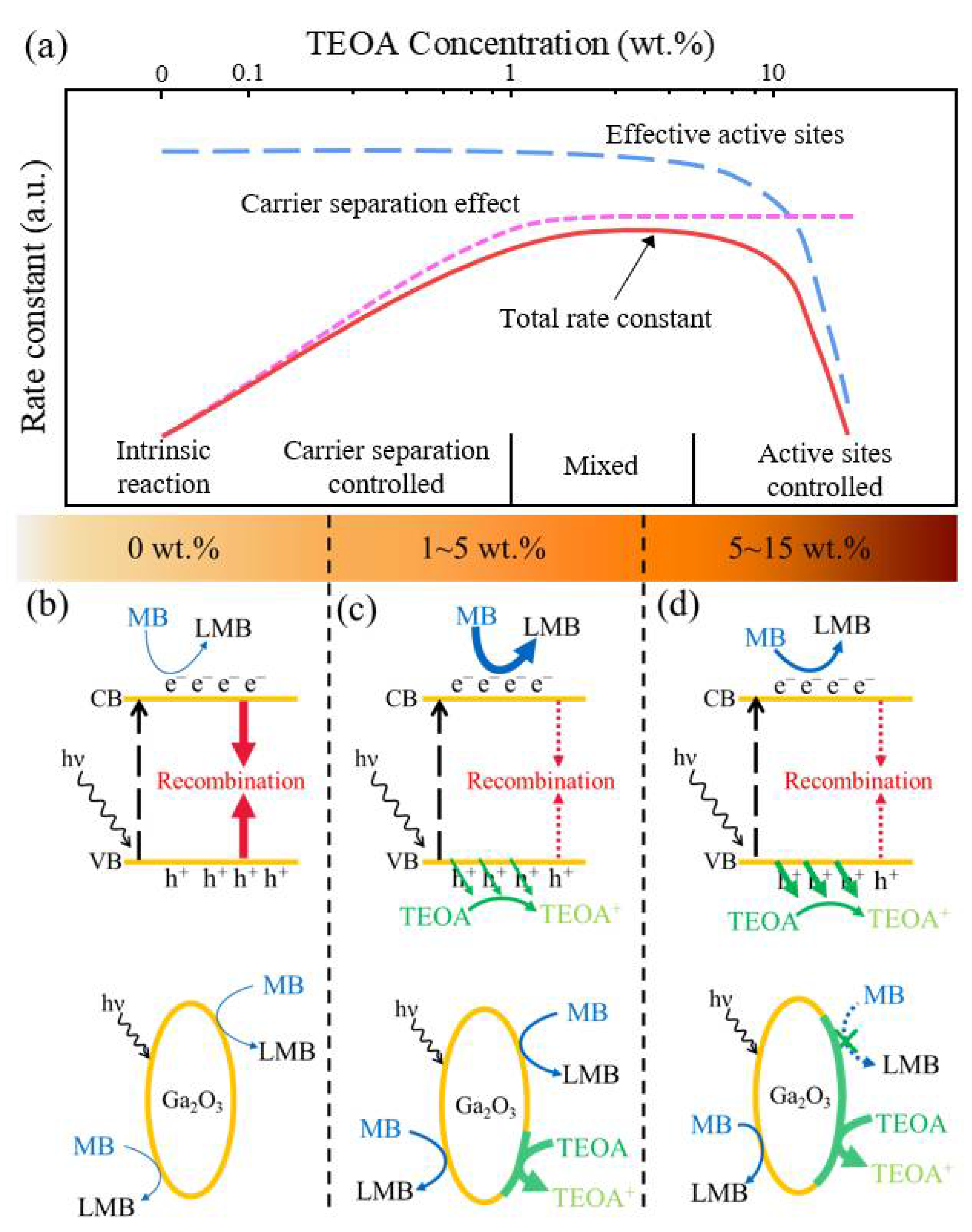
2.2. Triethanolamine (TEOA) as a Hole Scavenger
2.3. Comparison of the Colorimetric UVC Detection Using UV-Vis Spectrophotometers and the R Value from the RGB Photodetector
2.4. Kinetic Model Analysis for TEOA Effects on the MB Reduction with Ga2O3
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Begum, M.; Hocking, A.; Miskelly, D. Inactivation of food spoilage fungi by ultra violet (UVC) irradiation. Int. J. Food Microbiol. 2009, 129, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.G.; Castenholz, R.W. Scytonemin, a cyanobacterial sheath pigment, protects against UVC radiation: Implications for early photosynthetic life. J. Phycol. 1999, 35, 673–681. [Google Scholar] [CrossRef]
- Lin, S.; Lin, H.; Ma, C.; Cheng, Y.; Ye, S.; Lin, F.; Li, R.; Xu, J.; Wang, Y. High-security-level multi-dimensional optical storage medium: Nanostructured glass embedded with LiGa5O8: Mn2+ with photostimulated luminescence. Light. Sci. Appl. 2020, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Xu, X.; Yang, W.; Chen, J.; Fang, X. Materials and Designs for Wearable Photodetectors. Adv. Mater. 2019, 31, e1808138. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gao, N.; Ren, J.; Qu, X. Polyoxometalate-based Rewritable Paper. Chem. Mater. 2015, 27, 7573–7576. [Google Scholar] [CrossRef]
- Casini, B.; Tuvo, B.; Cristina, M.L.; Spagnolo, A.M.; Totaro, M.; Baggiani, A.; Privitera, G. Evaluation of an Ultraviolet C (UVC) Light-Emitting Device for Disinfection of High Touch Surfaces in Hospital Critical Areas. Int. J. Environ. Res. Public Health 2019, 16, 3572. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-I.; Yoon, J.; Schroeder, C.; Bradforth, S.E.; Cockburn, M.; Pfeifer, G.P. Wavelength dependence of ultraviolet radiation-induced DNA damage as determined by laser irradiation suggests that cyclobutane pyrimidine dimers are the principal DNA lesions produced by terrestrial sunlight. FASEB J. 2011, 25, 3079–3091. [Google Scholar] [CrossRef]
- Mckinlay, A.F.; Diffey, B.L. A reference action spectrum for ultraviolet induced erythema in human skin. CIE J. 1987, 6, 17–22. [Google Scholar]
- Zou, W.; Sastry, M.; Gooding, J.J.; Ramanathan, R.; Bansal, V. Recent Advances and a Roadmap to Wearable UV Sensor Technologies. Adv. Mater. Technol. 2020, 5, 1–1901036. [Google Scholar] [CrossRef]
- Kurz, W.; Yetisen, A.K.; Kaito, M.V.; Fuchter, M.J.; Jakobi, M.; Elsner, M.; Koch, A.W. UV-Sensitive Wearable Devices for Colorimetric Monitoring of UV Exposure. Adv. Opt. Mater. 2020, 8, 1901969. [Google Scholar] [CrossRef]
- Min, K.-P.; Kim, G.-W. Photo-Rheological Fluid-Based Colorimetric Ultraviolet Light Intensity Sensor. Sensors 2019, 19, 1128. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; González, A.; Jampaiah, D.; Ramanathan, R.; Taha, M.; Walia, S.; Sriram, S.; Bhaskaran, M.; Dominguez-Vera, J.M.; Bansal, V. Skin color-specific and spectrally-selective naked-eye dosimetry of UVA, B and C radiations. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Kim, S.; Han, K.I.; Lee, I.G.; Yoon, Y.; Park, W.K.; Hong, S.W.; Yang, W.S.; Hwang, W.S. A Zero-Power, Low-Cost Ultraviolet-C Colorimetric Sensor Using a Gallium Oxide and Reduced Graphene Oxide Hybrid via Photoelectrochemical Reactions. Catalysts 2017, 7, 248. [Google Scholar] [CrossRef]
- Wang, W.; Xie, N.; He, L.; Yin, Y. Photocatalytic colour switching of redox dyes for ink-free light-printable rewritable paper. Nat. Commun. 2014, 5, 5459. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Mubarak, N.S.A.; Ishak, M.A.M.; Ismail, K.; Nawawi, W.I. Kinetics of photocatalytic decolourization of cationic dye using porous TiO2 film. J. Taibah Univ. Sci. 2016, 10, 352–362. [Google Scholar] [CrossRef]
- Yoo, T.H.; Ryou, H.; Lee, I.G.; Cho, J.; Cho, B.J.; Hwang, W.S. Comparison of Ga2O3 and TiO2 Nanostructures for Photocatalytic Degradation of Volatile Organic Compounds. Catalysts 2020, 10, 545. [Google Scholar] [CrossRef]
- Pal, U.; Ghosh, S.; Chatterjee, D. Effect of sacrificial electron donors on hydrogen generation over visible light–irradiated nonmetal-doped TiO2 photocatalysts. Transit. Met. Chem. 2011, 37, 93–96. [Google Scholar] [CrossRef]
- Color Sensor—Programmable Color Light-To-Frequency Converter—TCS3200 ams|ams. Available online: https://ams.com/ko/tcs3200 (accessed on 9 December 2020).
- Delekar, S.D.; Yadav, H.; Achary, S.; Meena, S.; Pawar, S. Structural refinement and photocatalytic activity of Fe-doped anatase TiO2 nanoparticles. Appl. Surf. Sci. 2012, 263, 536–545. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Monaco, M.M.; Iervolino, G.; Vaiano, V. Photocatalytic Degradation of Azo Dye Reactive Violet 5 on Fe-Doped Titania Catalysts under Visible Light Irradiation. Catalysts 2019, 9, 645. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Ku, Y. Effect of solution pH on the adsorption and photocatalytic reaction behaviors of dyes using TiO2 and Nafion-coated TiO2. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 261–268. [Google Scholar] [CrossRef]
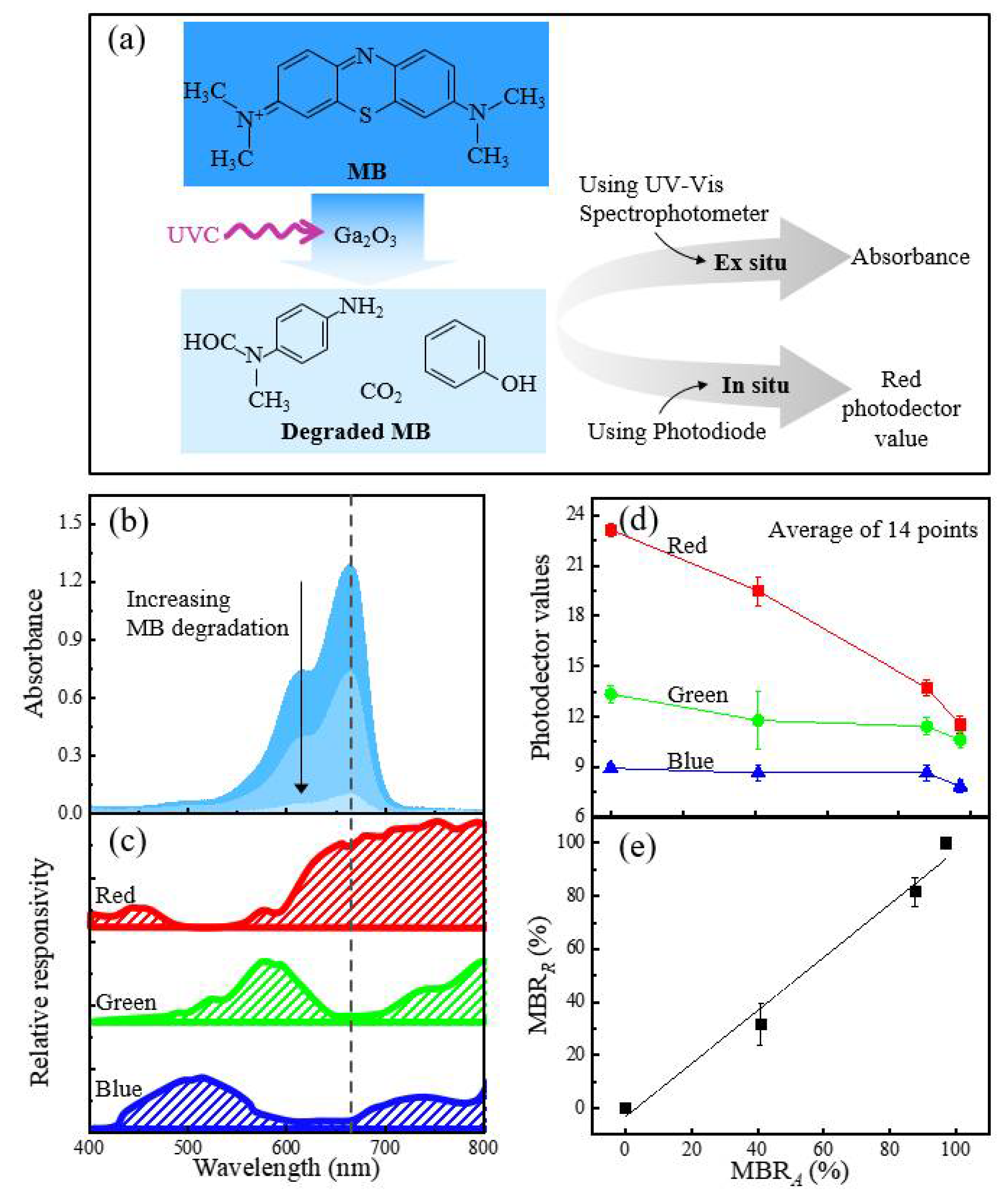
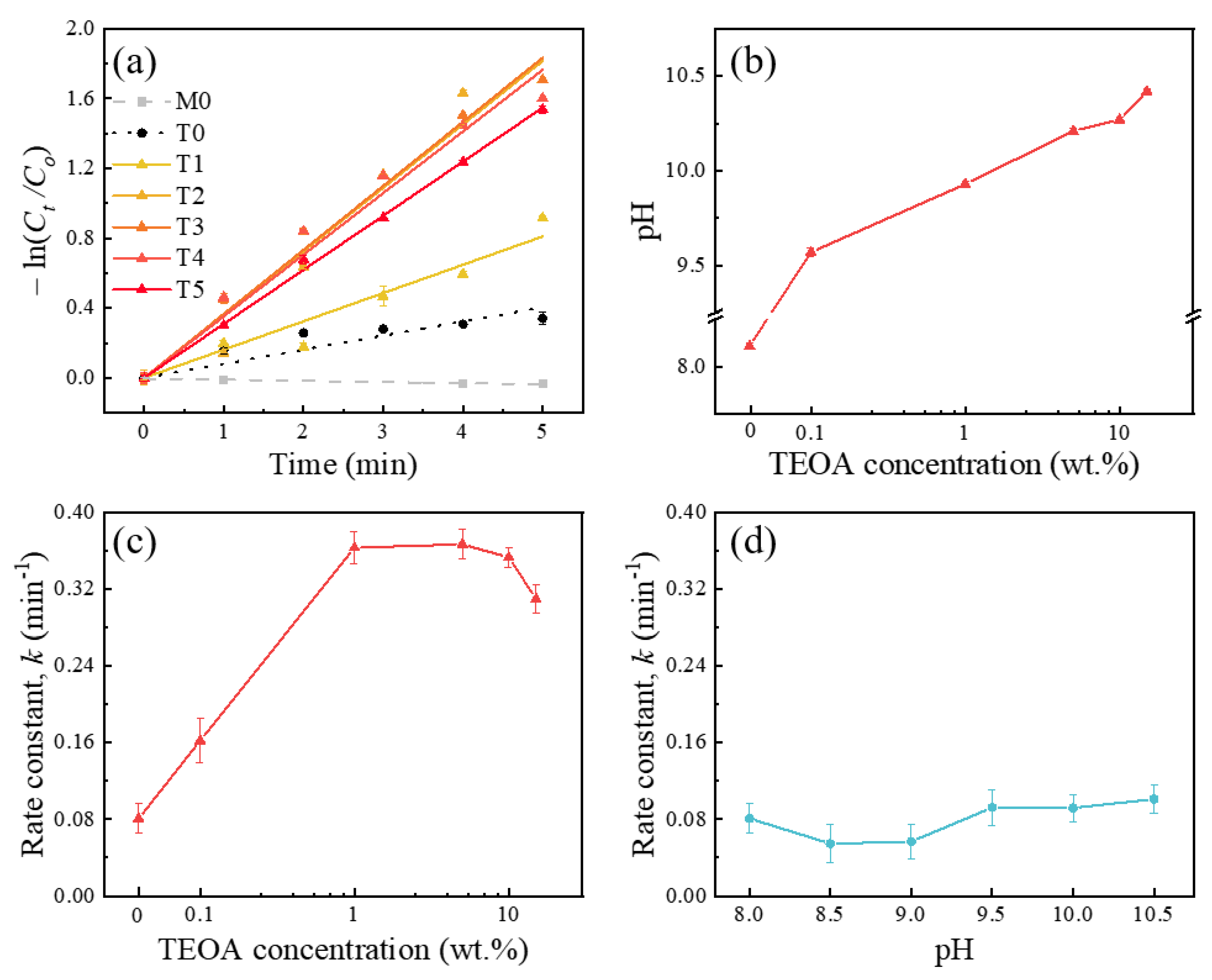

| Index | M0 | T0 | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|---|
| Ga2O3 (mg) | 0 | 9 | 9 | 9 | 9 | 9 | 9 |
| TEOA (wt.%) | 0 | 0 | 0.1 | 1 | 5 | 10 | 15 |
| R2 * | 0.97 | 0.95 | 0.98 | 0.99 | 0.99 | 0.99 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryou, H.; Kim, S.; Shin, M.; Cho, J.; Hwang, W.S. Fast-Response Colorimetric UVC Sensor Made of a Ga2O3 Photocatalyst with a Hole Scavenger. Sensors 2021, 21, 387. https://doi.org/10.3390/s21020387
Ryou H, Kim S, Shin M, Cho J, Hwang WS. Fast-Response Colorimetric UVC Sensor Made of a Ga2O3 Photocatalyst with a Hole Scavenger. Sensors. 2021; 21(2):387. https://doi.org/10.3390/s21020387
Chicago/Turabian StyleRyou, Heejoong, Sunjae Kim, Myunghun Shin, Junsang Cho, and Wan Sik Hwang. 2021. "Fast-Response Colorimetric UVC Sensor Made of a Ga2O3 Photocatalyst with a Hole Scavenger" Sensors 21, no. 2: 387. https://doi.org/10.3390/s21020387
APA StyleRyou, H., Kim, S., Shin, M., Cho, J., & Hwang, W. S. (2021). Fast-Response Colorimetric UVC Sensor Made of a Ga2O3 Photocatalyst with a Hole Scavenger. Sensors, 21(2), 387. https://doi.org/10.3390/s21020387







