Association between the Degree of Pre-Synaptic Dopaminergic Pathway Degeneration and Motor Unit Firing Behavior in Parkinson’s Disease Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Muscle Strength Testing and Physical Symptom Assessment
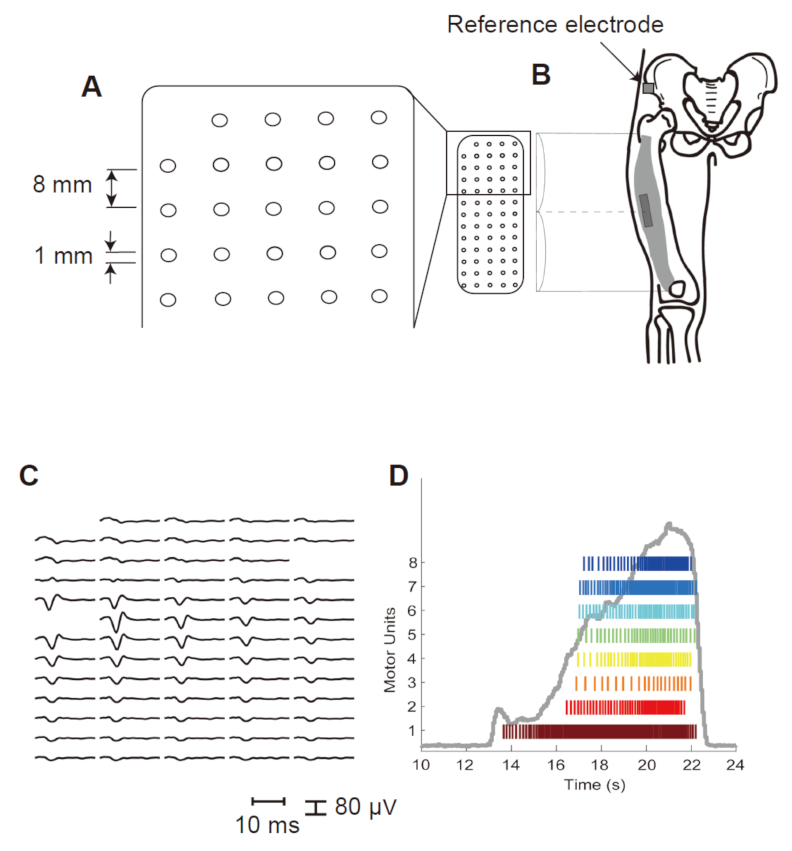
2.3. Electromyography (EMG) Recording
2.4. Data Processing
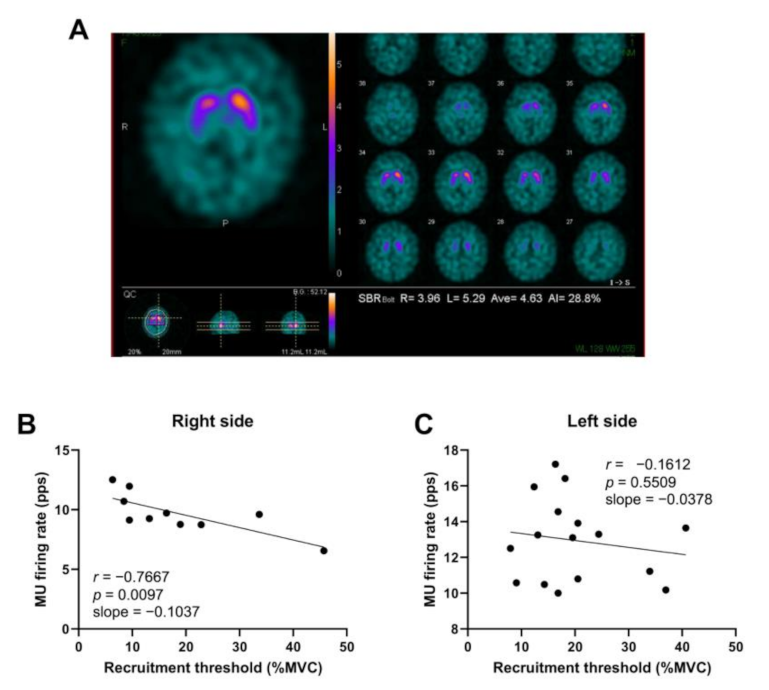
2.5. DAT-SPECT
2.6. Statistical Analysis
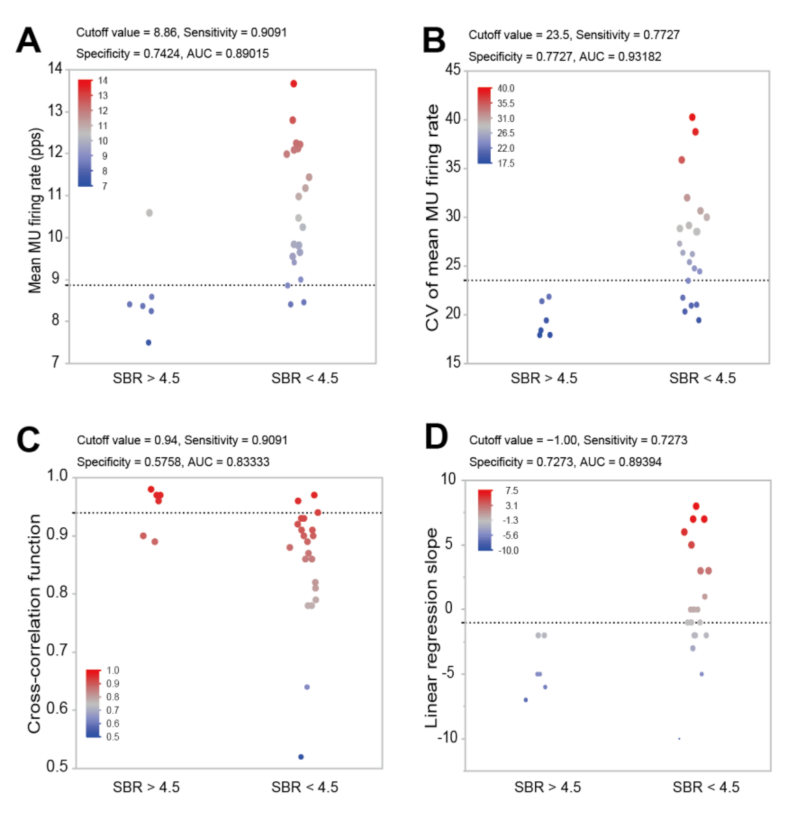
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Rijk, M.C.; Launer, L.J.; Berger, K.; Breteler, M.M.; Dartigues, J.F.; Baldereschi, M.; Fratiglioni, L.; Lobo, A.; Martinez-Lage, J.; Trenkwalder, C.; et al. Prevalence of Parkinson’s disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000, 54, S21–S23. [Google Scholar]
- Stevens-Lapsley, J.; Kluger, B.M.; Schenkman, M. Quadriceps muscle weakness, activation deficits, and fatigue with Parkinson disease. Neurorehabil. Neural Repair. 2012, 26, 533–541. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Kang, J.H.; Irwin, D.J.; Chen-Plotkin, A.S.; Siderowf, A.; Caspell, C.; Coffey, C.S.; Waligórska, T.; Taylor, P.; Pan, S.; Frasier, M.; et al. Association of cerebrospinal fluid β-amyloid 1-42, T-tau, P-tau181, and α-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol. 2013, 70, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Negro, F.; Muceli, S.; Castronovo, A.M.; Holobar, A.; Farina, D. Multi-channel intramuscular and surface EMG decomposition by convolutive blind source separation. J. Neural Eng. 2016, 13, 026027. [Google Scholar] [CrossRef]
- Holobar, A.; Farina, D. Blind source identification from the multichannel surface electromyogram. Physiol. Meas. 2014, 35, R143–R165. [Google Scholar] [CrossRef] [PubMed]
- Holobar, A.; Farina, D.; Gazzoni, M.; Merletti, R.; Zazula, D. Estimating motor unit discharge patterns from high-density surface electromyogram. Clin. Neurophysiol. 2009, 120, 551–562. [Google Scholar] [CrossRef]
- Holobar, A.; Zazula, D. Correlation-based decomposition of surface electromyograms at low contraction forces. Med. Biol. Eng. Comput. 2004, 42, 487–495. [Google Scholar] [CrossRef]
- Holobar, A.; Gallego, J.A.; Kranjec, J.; Rocon, E.; Romero, J.P.; Benito-León, J.; Pons, J.L.; Glaser, V. Motor Unit-Driven Identification of Pathological Tremor in Electroencephalograms. Front. Neurol. 2018, 9, 879. [Google Scholar] [CrossRef] [PubMed]
- Povalej Bržan, P.; Gallego, J.A.; Romero, J.P.; Glaser, V.; Rocon, E.; Benito-León, J.; Bermejo-Pareja, F.; Posada, I.J.; Holobar, A. New Perspectives for Computer-Aided Discrimination of Parkinson’s Disease and Essential Tremor. Complexity 2017, 2017, 4327175. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Watanabe, K.; Holobar, A.; Maeda, N.; Maruyama, H.; Tanaka, S. Identification of the laterality of motor unit behavior in female patients with parkinson’s disease using high-density surface electromyography. Eur. J. Neurosci. 2021, 53, 1938–1949. [Google Scholar] [CrossRef]
- Kägi, G.; Bhatia, K.P.; Tolosa, E. The role of DAT-SPECT in movement disorders. J. Neurol. Neurosurg. Psychiatry 2010, 81, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Solla, P.; Cannas, A.; Ibba, F.C.; Loi, F.; Corona, M.; Orofino, G.; Marrosu, M.G.; Marrosu, F. Gender differences in motor and non-motor symptoms among Sardinian patients with Parkinson’s disease. J. Neurol. Sci. 2012, 323, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Haaxma, C.A.; Bloem, B.R.; Borm, G.F.; Oyen, W.J.; Leenders, K.L.; Eshuis, S.; Booij, J.; Dluzen, D.E.; Horstink, M.W. Gender differences in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 819–824. [Google Scholar] [CrossRef]
- Szewczyk-Krolikowski, K.; Tomlinson, P.; Nithi, K.; Wade-Martins, R.; Talbot, K.; Ben-Shlomo, Y.; Hu, M.T. The influence of age and gender on motor and non-motor features of early Parkinson’s disease: Initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat. Disord. 2014, 20, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Watanabe, K.; Kawade, S.; Takahashi, T.; Kimura, H.; Maruyama, H.; Hyngstrom, A. The effect of a portable electrical muscle stimulation device at home on muscle strength and activation patterns in locomotive syndrome patients: A randomized control trial. J. Electromyogr. Kinesiol. 2019, 45, 46–52. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Watanabe, K.; Takahashi, T.; Hosomi, N.; Orita, N.; Mikami, Y.; Maruyama, H.; Kimura, H.; Matsumoto, M. Sex differences in variances of multi-channel surface electromyography distribution of the vastus lateralis muscle during isometric knee extension in young adults. Eur. J. Appl. Physiol. 2017, 117, 583–589. [Google Scholar] [CrossRef]
- Watanabe, K.; Miyamoto, T.; Tanaka, Y.; Fukuda, K.; Moritani, T. Type 2 diabetes mellitus patients manifest characteristic spatial EMG potential distribution pattern during sustained isometric contraction. Diabetes Res. Clin. Pract. 2012, 97, 468–473. [Google Scholar] [CrossRef][Green Version]
- Nishikawa, Y.; Watanabe, K.; Takahashi, T.; Orita, N.; Kimura, H.; Matsumoto, M.; Maruyama, H. Spatial electromyography distribution pattern of the vastus lateralis muscle during ramp up contractions in Parkinson’s disease patients. J. Electromyogr. Kinesiol. 2017, 37, 125–131. [Google Scholar] [CrossRef]
- Merletti, R.; Holobar, A.; Farina, D. Analysis of motor units with high-density surface electromyography. J. Electromyogr. Kinesiol. 2008, 18, 879–890. [Google Scholar] [CrossRef]
- Holobar, A.; Minetto, M.A.; Farina, D. Accurate identification of motor unit discharge patterns from high-density surface EMG and validation with a novel signal-based performance metric. J. Neural Eng. 2014, 11, 016008. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Gazzoni, M.; Holobar, A.; Miyamoto, T.; Fukuda, K.; Merletti, R.; Moritani, T. Motor unit firing pattern of vastus lateralis muscle in type 2 diabetes mellitus patients. Muscle Nerve 2013, 48, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Holobar, A.; Kouzaki, M.; Ogawa, M.; Akima, H.; Moritani, T. Age-related changes in motor unit firing pattern of vastus lateralis muscle during low-moderate contraction. Age 2016, 38, 48. [Google Scholar] [CrossRef] [PubMed]
- Fuglevand, A.J.; Winter, D.A.; Patla, A.E. Models of recruitment and rate coding organization in motor-unit pools. J. Neurophysiol. 1993, 70, 2470–2488. [Google Scholar] [CrossRef] [PubMed]
- Tossici-Bolt, L.; Hoffmann, S.M.; Kemp, P.M.; Mehta, R.L.; Fleming, J.S. Quantification of [123I]FP-CIT SPECT brain images: An accurate technique for measurement of the specific binding ratio. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hou, Y.; Hallett, M.; Zhang, J.; Chan, P. Lateralization of brain activity pattern during unilateral movement in Parkinson’s disease. Hum. Brain Mapp. 2015, 36, 1878–1891. [Google Scholar] [CrossRef]
- De Luca, C.J.; Hostage, E.C. Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J. Neurophysiol. 2010, 104, 1034–1046. [Google Scholar] [CrossRef]
- De Luca, C.J.; Erim, Z. Common drive of motor units in regulation of muscle force. Trends Neurosci. 1994, 17, 299–305. [Google Scholar] [CrossRef]
- Sun, T.Y.; Chen, J.J.; Lin, T.S. Analysis of motor unit firing patterns in patients with central or peripheral lesions using singular-value decomposition. Muscle Nerve 2000, 23, 1057–1068. [Google Scholar] [CrossRef]
- Paulus, W.; Jellinger, K. The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1991, 50, 743–755. [Google Scholar] [CrossRef]
- Lindvall, O.; Björklund, A.; Skagerberg, G. Dopamine-containing neurons in the spinal cord: Anatomy and some functional aspects. Ann. Neurol. 1983, 14, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114 Pt 5, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, D.S.; Enoka, R.M. Motor unit behavior in Parkinson’s disease. Phys. Ther. 1994, 74, 61–70. [Google Scholar] [CrossRef] [PubMed]


| Variables | Females with Parkinson’s Disease |
|---|---|
| Age, years | 72.6 ± 7.2 |
| Height, cm | 157.1 ± 10.2 |
| Weight, kg | 58.3 ± 6.8 |
| Disease duration, year | 3.5 ± 2.1 |
| MMSE | 26.6 ± 1.4 |
| UPDRS Part III | 9.9 ± 9.1 |
| L-dopa, mg/day | 300 (100–300) |
| Knee extensor muscle strength, Nm More-/Less-affected side | 54.3 ± 9.9/72.9 ± 20.5 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, Y.; Watanabe, K.; Holobar, A.; Takahashi, T.; Maeda, N.; Maruyama, H.; Tanaka, S.; Hyngstrom, A.S. Association between the Degree of Pre-Synaptic Dopaminergic Pathway Degeneration and Motor Unit Firing Behavior in Parkinson’s Disease Patients. Sensors 2021, 21, 6615. https://doi.org/10.3390/s21196615
Nishikawa Y, Watanabe K, Holobar A, Takahashi T, Maeda N, Maruyama H, Tanaka S, Hyngstrom AS. Association between the Degree of Pre-Synaptic Dopaminergic Pathway Degeneration and Motor Unit Firing Behavior in Parkinson’s Disease Patients. Sensors. 2021; 21(19):6615. https://doi.org/10.3390/s21196615
Chicago/Turabian StyleNishikawa, Yuichi, Kohei Watanabe, Aleš Holobar, Tetsuya Takahashi, Noriaki Maeda, Hirofumi Maruyama, Shinobu Tanaka, and Allison S Hyngstrom. 2021. "Association between the Degree of Pre-Synaptic Dopaminergic Pathway Degeneration and Motor Unit Firing Behavior in Parkinson’s Disease Patients" Sensors 21, no. 19: 6615. https://doi.org/10.3390/s21196615
APA StyleNishikawa, Y., Watanabe, K., Holobar, A., Takahashi, T., Maeda, N., Maruyama, H., Tanaka, S., & Hyngstrom, A. S. (2021). Association between the Degree of Pre-Synaptic Dopaminergic Pathway Degeneration and Motor Unit Firing Behavior in Parkinson’s Disease Patients. Sensors, 21(19), 6615. https://doi.org/10.3390/s21196615






