BCI-Based Control for Ankle Exoskeleton T-FLEX: Comparison of Visual and Haptic Stimuli with Stroke Survivors
Abstract
1. Introduction
2. Materials and Methods
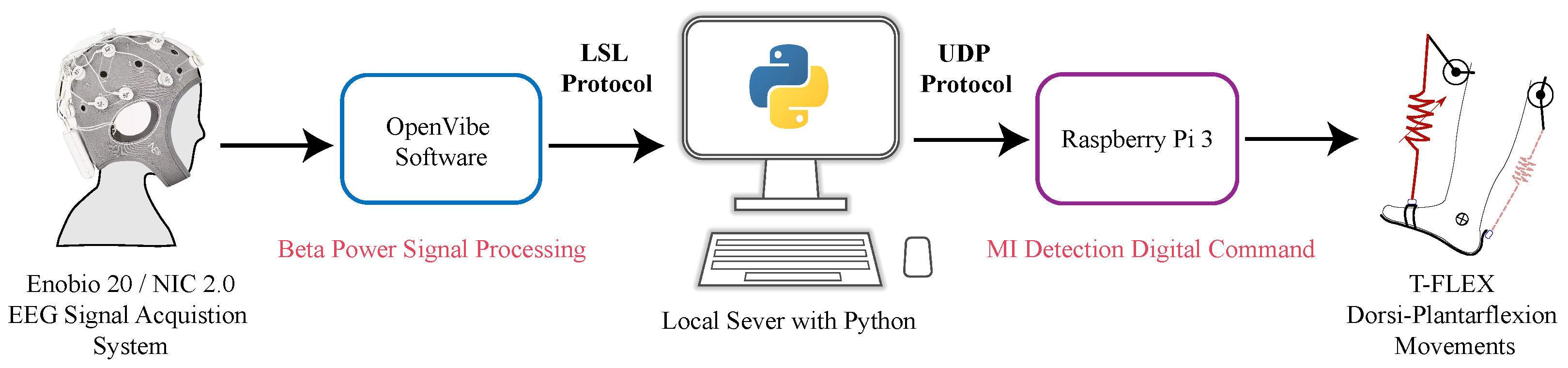
2.1. BCI-Exoskeleton System
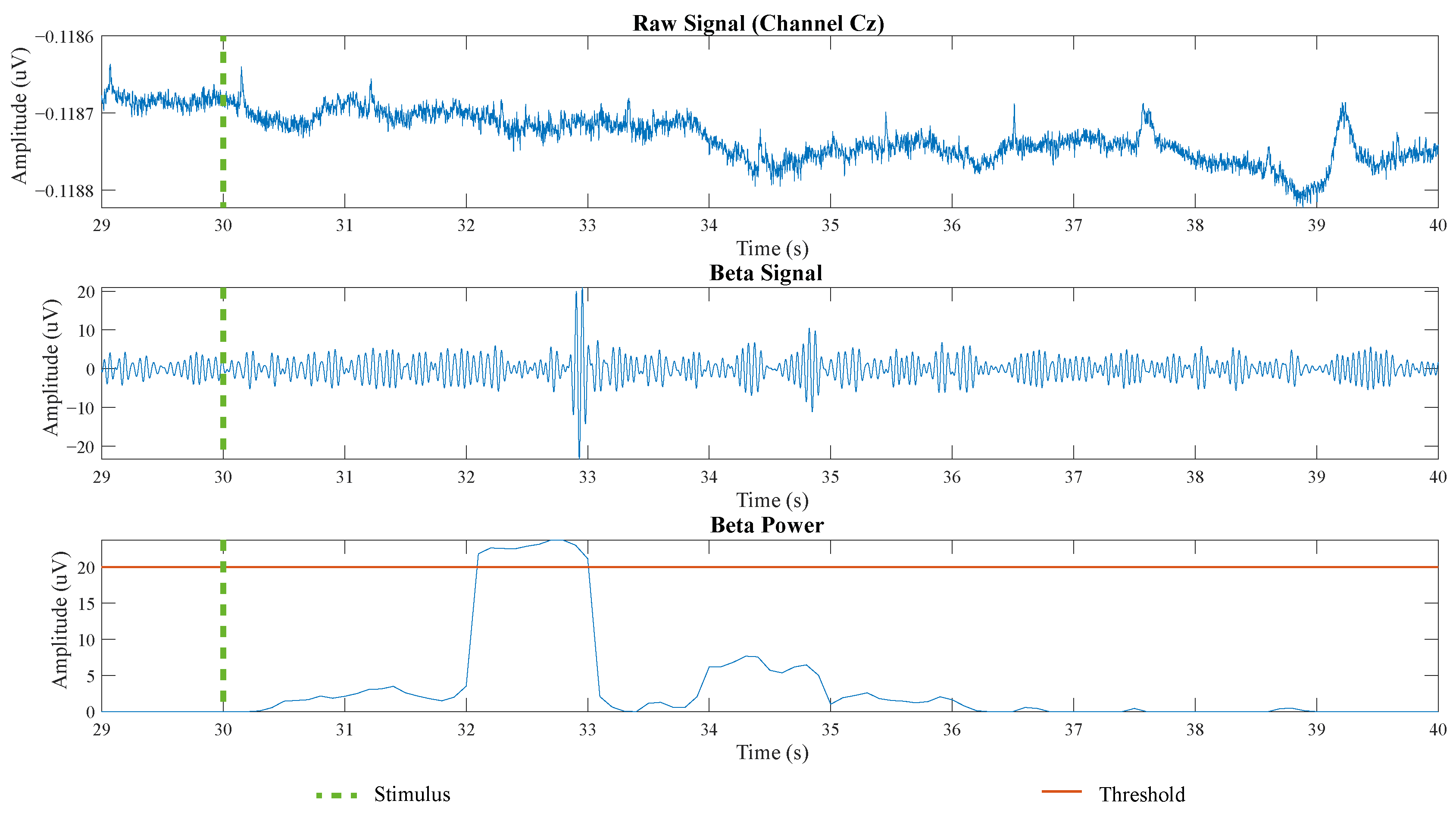
2.1.1. BCI Interface
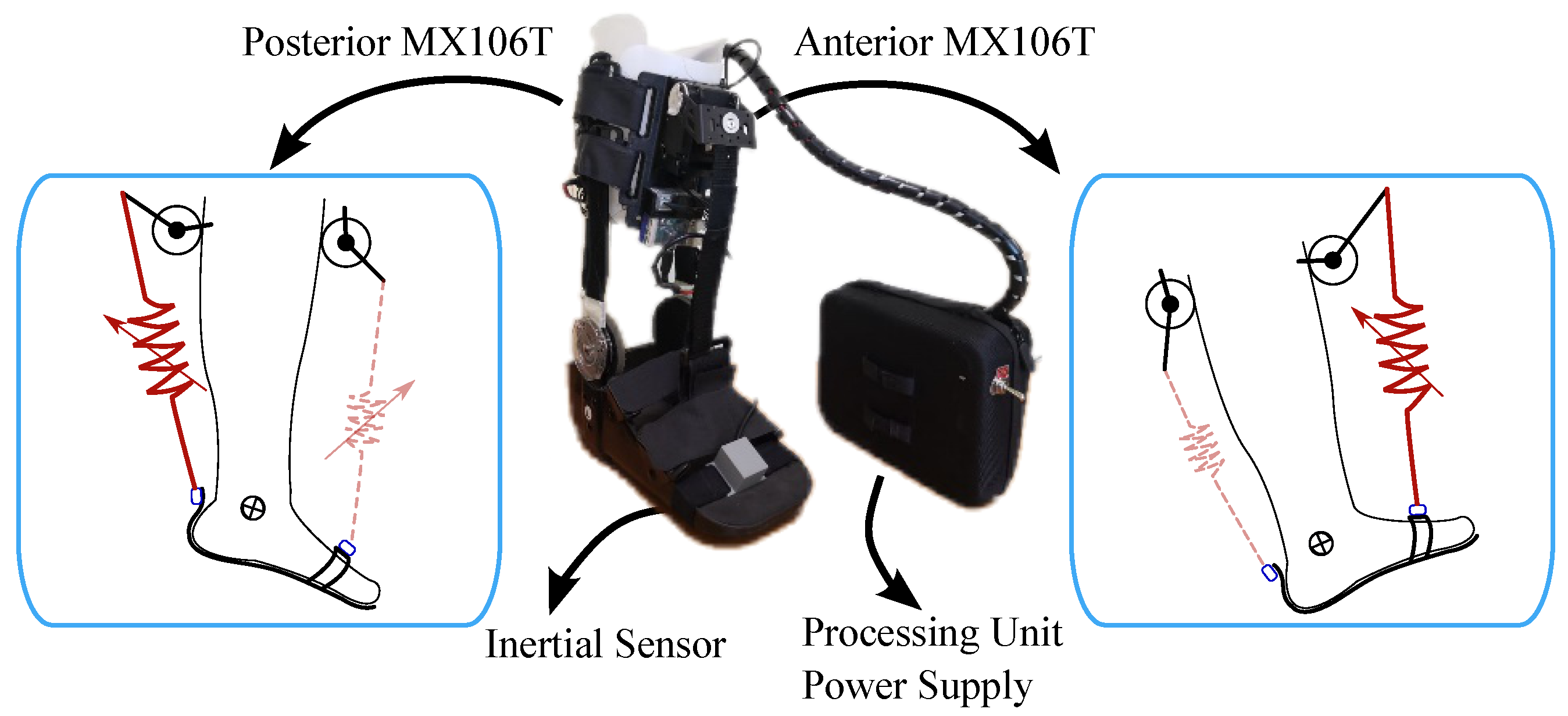
2.1.2. T-FLEX Ankle Exoskeleton
2.1.3. BCI—T-FLEX System Integration
2.1.4. Stimuli Strategies
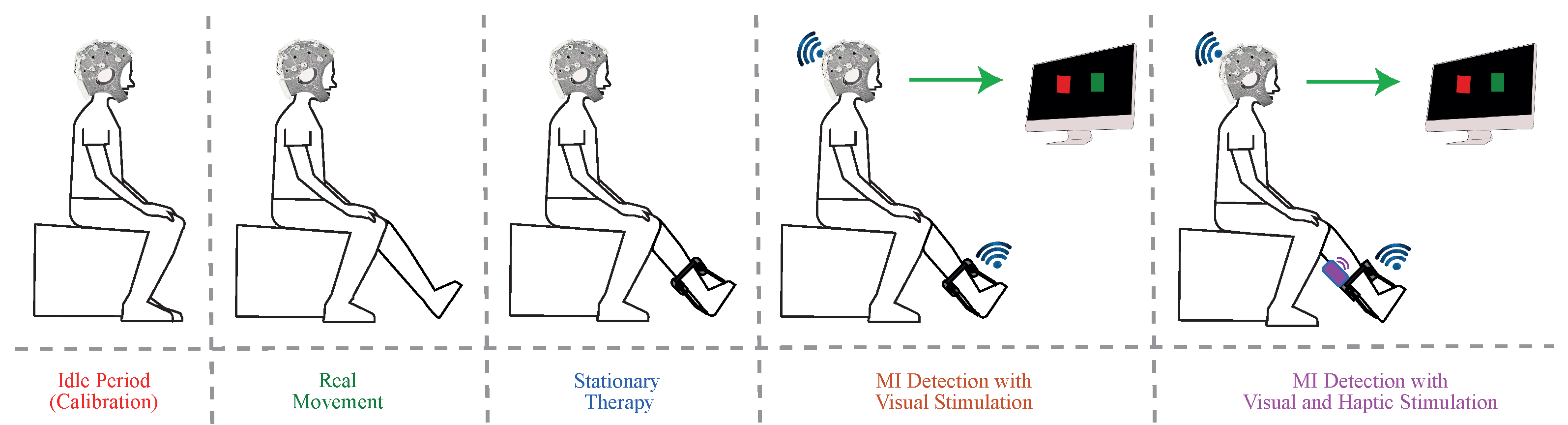
- Visual Stimulus System: The local server configured three types of instruction texts showed in a full-screen: (1) “Wait”, (2) “Idle”, and (3) “Move your feet”. On one hand, the main objective of the “Wait” text was to provide an initial 30 s waiting period to prepare the system. On the other hand, “Idle” and “Move your feet” texts, gave an explicit indication to the user to stay in a state of relaxation or a state of MI generation, with 10 s duration respectively (see Figure 3). In this way, only in the “Move your feet” stage, the local server received MI commands to activate T-FLEX.
- Haptic Stimulus System: The visual system worked with haptic one in sync with the “Move your Feet” periods to assist the patient in the MI generation (Figure 3). This haptic system, manually controlled by the supervisor, implemented the SunniMix rumble vibration motor (SM SunniMix, USA) with a vibration frequency in a range between 36 and 40 Hz (2200 to 2500 r/min). This motor attached the system through a structure made of Ethylene Vinyl Acetate (EVA), a box made of Acrylonitrile Butadiene Styrene (ABS) coated it, and finally, velcro material allowed the adhesion to the anterior tibialis muscle area.
2.2. Experimental Validation
2.2.1. Participants
- Inclusion Criteria: Patients between the ages range of 18 to 70 years with a pathology associated with the foot-ankle complex due to a neurological injury and with partial independence to mobilize.
- Exclusion Criteria: Candidates with hypertension, uncontrolled epilepsy, pain in the lower limbs, and severe spasticity (level 4 of the Ashworth Scale) were excluded from the study, as well as patients with the presence of wound or pressure ulcers that could have made nonfeasible the use of the device.
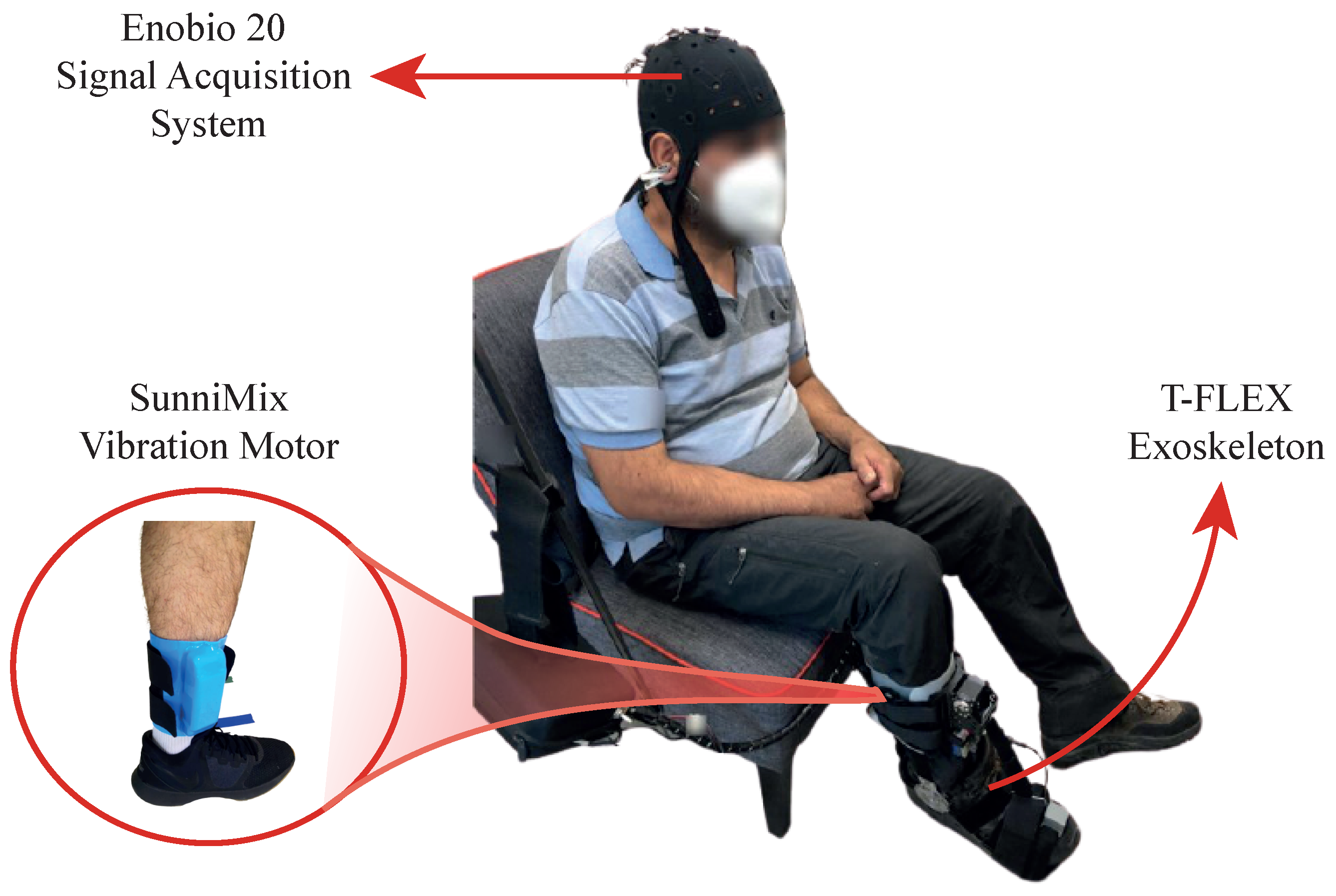
2.2.2. Experimental Setup
2.2.3. Experimental Procedure
2.2.4. Experimental Analysis
3. Results
3.1. Participants
3.2. Accuracy Results
3.3. Power Spectral Density Results
3.4. User Perception Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCI | Brain-Computer Interface |
| EEG | Electroencephalography |
| MI | Motor Imagination |
| MIV | Motor Imagination with Visual Stimulus |
| MIVH | Motor Imagination with Visual and Haptic Stimuli |
| ERP | Event-Related Potential |
| PSD | Power Spectral Density |
| ERS/ERD | Event-Related Desynchronization/Synchronization |
References
- Ang, K.K.; Guan, C. Brain-computer interface in stroke rehabilitation. Comput. Sci. Eng. 2013, 7, 139–146. [Google Scholar] [CrossRef]
- Verma, R.; Arya, K.; Sharma, P.; Garg, R.K. Understanding gait control in post-stroke: Implications for management. J. Bodyw. Mov. Ther. 2012, 16, 14–21. [Google Scholar] [CrossRef]
- Stevens, F.; Weerkamp, N.J.; Cals, J.W. Foot drop. BMJ 2015, 350, h1736. [Google Scholar] [CrossRef]
- Forghany, S.; Tyson, S.; Nester, C.; Preece, S.; Jones, R. Foot posture after stroke: Frequency, nature and clinical significance. Clin. Rehabil. 2011, 25, 1050–1055. [Google Scholar] [CrossRef]
- Whitehead, S.; Baalbergen, E. Post-stroke rehabilitation. S. Afr. Med. J. 2019, 109, 81–83. [Google Scholar] [CrossRef]
- Mikolajczyk, T.; Ciobanu, I.; Badea, D.I.; Iliescu, A.; Pizzamiglio, S.; Schauer, T.; Seel, T.; Seiciu, P.L.; Turner, D.L.; Berteanu, M. Advanced technology for gait rehabilitation: An overview. Adv. Mech. Eng. 2018, 10, 1687814018783627. [Google Scholar] [CrossRef]
- Chia Bejarano, N.; Maggioni, S.; De Rijcke, L.; Cifuentes, G.C.; Reinkensmeyer, D. Robot-Assisted Rehabilitation Therapy: Recovery Mechanisms and Their Implications for Machine Design. In Emerging Therapies in Neurorehabilitation II; Springer: Berlin/Heidelberg, Germany, 2016; Volume 10, pp. 197–223. [Google Scholar] [CrossRef]
- Tariq, M.; Trivailo, P.M.; Simic, M. EEG-based BCI control schemes for lower-limb assistive-robots. Front. Hum. Neurosci. 2018, 12, 312. [Google Scholar] [CrossRef]
- He, Y.; Eguren, D.; Azorin, J.; Grossman, R.; Luu, T.P.; Contreras-Vidal, J. Brain–machine interfaces for controlling lower-limb powered robotic systems. J. Neural Eng. 2018, 15, 021004. [Google Scholar] [CrossRef]
- Ortiz, M.; Ferrero, L.; Iáñez, E.; Azorín, J.M.; Contreras-Vidal, J.L. Sensory integration in human movement: A new brain-machine interface based on gamma band and attention level for controlling a lower-limb exoskeleton. Front. Bioeng. Biotechnol. 2020, 8, 735. [Google Scholar] [CrossRef]
- Silvoni, S.; Ramos-Murguialday, A.; Cavinato, M.; Volpato, C.; Cisotto, G.; Turolla, A.; Piccione, F.; Birbaumer, N. Brain-Computer Interface in Stroke: A Review of Progress. Clin. EEG Neurosci. Off. J. EEG Clin. Neurosci. Soc. ENCS 2011, 42, 245–252. [Google Scholar] [CrossRef]
- García, M.; Iáñez, E.; Contreras-Vidal, J.; Azorin, J. Analysis of the EEG Rhythms Based on the Empirical Mode Decomposition During Motor Imagery When Using a Lower-Limb Exoskeleton. A Case Study. Front. Neurorobot. 2020, 14, 48. [Google Scholar] [CrossRef]
- Xu, R.; Jiang, N.; Mrachacz-Kersting, N.; Lin, C.; Prieto, G.; Moreno, J.; Pons, J.; Dremstrup, K.; Farina, D. A Closed-Loop Brain-Computer Interface Triggering an Active Ankle-Foot Orthosis for Inducing Cortical Neural Plasticity. IEEE Trans. Biomed. Eng. 2014, 61, 2092–2101. [Google Scholar] [CrossRef]
- Guillot, A.; Collet, C. Construction of the Motor Imagery Integrative Model in Sport: A review and theoretical investigation of motor imagery use. Int. Rev. Sport Exerc. Psychol. 2008, 1, 31–44. [Google Scholar] [CrossRef]
- Bai, O.; Huang, D.; Fei, D.Y.; Kunz, R. Effect of real-time cortical feedback in motor imagery-based mental practice training. NeuroRehabilitation 2014, 34, 355–363. [Google Scholar] [CrossRef]
- Lotte, F.; Cellard, A. Illustration of Electrophysiological Phenomena with OpenViBE. In Brain-Computer Interfaces 2: Technology and Applications; John Wiley: Hoboken, NJ, USA, 2016; Chapter 11; pp. 204–206. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Neuper, C.; Brunner, C.; Lopes da Silva, F. Beta rebound after different types of motor imagery in man. Neurosci. Lett. 2005, 378, 156–159. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ushiba, J. EEG-based classification of imaginary left and right foot movements using beta rebound. Clin. Neurophysiol. 2013, 124, 2153–2160. [Google Scholar] [CrossRef]
- Bizovičar, N.; Dreo, J.; Koritnik, B.; Zidar, J. Decreased movement-related beta desynchronization and impaired post-movement beta rebound in amyotrophic lateral sclerosis. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2014, 125, 1689–1699. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Rimbert, S.; Lindig-León, C.; Fedotenkova, M.; Bougrain, L. Modulation of beta power in EEG during discrete and continuous motor imageries. In Proceedings of the 2017 8th International IEEE/EMBS Conference on Neural Engineering (NER), Shanghai, China, 25–28 May 2017; pp. 333–336. [Google Scholar] [CrossRef]
- Lisi, G.; Noda, T.; Morimoto, J. Decoding the ERD/ERS: Influence of afferent input induced by a leg assistive robot. Front. Syst. Neurosci. 2014, 8, 85. [Google Scholar] [CrossRef]
- Tang, Z.; Sun, S.; Zhang, S.; Chen, Y.; Li, C.; Chen, S. A Brain-Machine Interface Based on ERD/ERS for an Upper-Limb Exoskeleton Control. Sensors 2016, 16, 2050. [Google Scholar] [CrossRef]
- Del Felice, A.; Masiero, S.; Bosco, A.; Izzi, F.; Piccione, F.; Formaggio, E. 32. Quantitative EEG evaluation during robot-assisted foot movement. Clin. Neurophysiol. 2016, 127, e330–e331. [Google Scholar] [CrossRef]
- Chaves de Melo, G.; Sternlicht, V.; Forner-Cordero, A. EEG Analysis in Coincident Timing Task Towards Motor Rehabilitation. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Montreal, QC, Canada, 20–24 July 2020; Volume 2020, pp. 3027–3030. [Google Scholar] [CrossRef]
- Lotte, F.; Faller, J.; Guger, C.; Renard, Y.; Pfurtscheller, G.; Lécuyer, A.; Leeb, R. Combining BCI with Virtual Reality: Towards New Applications and Improved BCI. In Towards Practical Brain-Computer Interfaces: Bridging the Gap from Research to Real-World Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 197–220. [Google Scholar] [CrossRef]
- Andreev, A.; Barachant, A.; Lotte, F.; Congedo, M. Recreational Applications of OpenViBE: Brain Invaders and Use-the-Force; John Wiley: Hoboken, NJ, USA, 2016; Chapter 14; pp. 241–258. [Google Scholar] [CrossRef]
- Lotte, F.; Renard, Y.; Lécuyer, A. Self-Paced Brain-Computer Interaction with Virtual Worlds: A Quantitative and Qualitative Study “Out of the Lab”. In Proceedings of the 4th International Brain Computer Interface Workshop and Training Course, Graz, Austria, 18–21 September 2008. [Google Scholar]
- Allison, B.; Neuper, C. Could anyone use a BCI? In Applying Our Minds to Human-Computer Interaction; Springer: Berlin/Heidelberg, Germany, 2010; pp. 35–54. [Google Scholar]
- Lotte, F.; Florian, L.; Mühl, C. Flaws in current human training protocols for spontaneous Brain-Computer Interfaces: Lessons learned from instructional design. Front. Hum. Neurosci. 2013, 7, 568. [Google Scholar] [CrossRef] [PubMed]
- Emami, Z.; Chau, T. The effects of visual distractors on cognitive load in a motor imagery brain-computer interface. Behav. Brain Res. 2020, 378, 112240. [Google Scholar] [CrossRef]
- Alimardani, M.; Nishio, S.; Ishiguro, H. Brain-Computer Interface and Motor Imagery Training: The Role of Visual Feedback and Embodiment. In Evolving BCI Therapy; IntechOpen: London, UK, 2018. [Google Scholar]
- Gomez-Pilar, J.; Corralejo, R.; Luis, L.; Alvarez, D.; Hornero, R. Neurofeedback training with a motor imagery-based BCI: Neurocognitive improvements and EEG changes in the elderly. Med. Biol. Eng. Comput. 2016, 54, 1655–1666. [Google Scholar] [CrossRef]
- Vernon, D.; Egner, T.; Cooper, N.; Compton, T.; Neilands, C.; Sheri, A.; Gruzelier, J. The effect of training distinct neurofeedback protocols on aspects of cognitive performance. Int. J. Psychophysiol. 2003, 47, 75–85. [Google Scholar] [CrossRef]
- Butler, A.; Page, S. Mental Practice With Motor Imagery: Evidence for Motor Recovery and Cortical Reorganization After Stroke. Arch. Phys. Med. Rehabil. 2007, 87, S2–S11. [Google Scholar] [CrossRef]
- Frolov, A.A.; Mokienko, O.; Lyukmanov, R.; Biryukova, E.; Kotov, S.; Turbina, L.; Nadareyshvily, G.; Bushkova, Y. Post-stroke Rehabilitation Training with a Motor-Imagery-Based Brain-Computer Interface (BCI)-Controlled Hand Exoskeleton: A Randomized Controlled Multicenter Trial. Front. Neurosci. 2017, 11, 400. [Google Scholar] [CrossRef]
- Mokienko, O.; Lyudmila, C.; Frolov, A.; Bobrov, P. Motor Imagery and Its Practical Application. Neurosci. Behav. Physiol. 2014, 44, 483–489. [Google Scholar] [CrossRef]
- Soekadar, S.; Birbaumer, N.; Slutzky, M.; Cohen, L. Brain-Machine Interfaces In Neurorehabilitation of Stroke. Neurobiol. Dis. 2014, 83, 172–179. [Google Scholar] [CrossRef]
- Rodríguez-Ugarte, M.; Iáñez, E.; Ortiz, M.; Azorín, J.M. Improving Real-Time Lower Limb Motor Imagery Detection Using tDCS and an Exoskeleton. Front. Neurosci. 2018, 12, 757. [Google Scholar] [CrossRef]
- Neuper, C.; Schlögl, A.; Pfurtscheller, G. Enhancement of left-right sensorimotor EEG differences during feedback-regulated motor imagery. J. Clin. Neurophysiol. 1999, 16, 373–382. [Google Scholar] [CrossRef]
- Cincotti, F.; Kauhanen, L.; Aloise, F.; Palomäki, T.; Caporusso, N.; Jylänki, P.; Mattia, D.; Babiloni, F.; Vanacker, G.; Nuttin, M.; et al. Vibrotactile Feedback for Brain-Computer Interface Operation. Comput. Intell. Neurosci. 2007, 2007, 48937. [Google Scholar] [CrossRef][Green Version]
- Grigorev, N.; Savosenkov, A.; Lukoyanov, M.; Udoratina, A.; Shusharina, N.; Kaplan, A.; Hramov, A.; Kazantsev, V.; Gordleeva, S. A BCI-based vibrotactile neurofeedback training improves motor cortical excitability during motor imagery. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kauhanen, L.; Palomäki, T.; Jylänki, P.; Aloise, F.; Nuttin, M.; Millán, J.d.R. Haptic feedback compared with visual feedback for BCI. In Proceedings of the 3rd International Brain-Computer Interface Workshop & Training Course 2006, Graz, Austria, 21–24 September 2006. [Google Scholar]
- Enobio 20|Solutions|Neuroelectrics. Available online: https://www.neuroelectrics.com/solutions/enobio/20 (accessed on 16 May 2021).
- McFarland, D.J.; McCane, L.M.; David, S.V.; Wolpaw, J.R. Spatial filter selection for EEG-based communication. Electroencephalogr. Clin. Neurophysiol. 1997, 103, 386–394. [Google Scholar] [CrossRef]
- Bradshaw, L.; Wikswo, J. Spatial filter approach for evaluation of the surface Laplacian of the electroencephalogram and magnetoencephalogram. Ann. Biomed. Eng. 2001, 29, 202–213. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Neuper, C. Motor imagery activates primary sensorimotor area in humans. Neurosci. Lett. 1997, 239, 65–68. [Google Scholar] [CrossRef]
- Nam, C.; Jeon, Y.; Kim, Y.J.; Lee, I.; Park, K. Movement imagery-related lateralization of event-related (de)synchronization (ERD/ERS): Motor-imagery duration effects. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2011, 122, 567–577. [Google Scholar] [CrossRef]
- Manchola, M.; Serrano, D.; Gómez, D.; Ballen, F.; Casas, D.; Munera, M.; Cifuentes, C.A. T-FLEX: Variable stiffness ankle-foot orthosis for gait assistance. In Proceedings of the International Symposium on Wearable Robotics, Pisa, Italy, 16–20 October 2018; Springer: Berlin/Heidelberg, Germany, 2018; pp. 160–164. [Google Scholar]
- Casas, J.; Leal-Junior, A.; Díaz, C.R.; Frizera, A.; Múnera, M.; Cifuentes, C.A. Large-range polymer optical-fiber strain-gauge sensor for elastic tendons in wearable assistive robots. Materials 2019, 12, 1443. [Google Scholar] [CrossRef]
- Gomez-Vargas, D.; Pinto-Betnal, M.J.; Ballén-Moreno, F.; Múnera, M.; Cifuentes, C.A. Therapy with t-flex ankle-exoskeleton for motor recovery: A case study with a stroke survivor. In Proceedings of the 2020 8th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), New York, NY, USA, 29 November–1 December 2020; pp. 491–496. [Google Scholar]
- Gomez-Vargas, D.; Ballen-Moreno, F.; Barria, P.; Aguilar, R.; Azorín, J.M.; Munera, M.; Cifuentes, C.A. The Actuation System of the Ankle Exoskeleton T-FLEX: First Use Experimental Validation in People with Stroke. Brain Sci. 2021, 11, 412. [Google Scholar] [CrossRef]
- Pino, A.; Gomez-Vargas, D.; Múnera, M.; Cifuentes, C.A. Visual Feedback Strategy based on Serious Games for Therapy with T-FLEX Ankle Exoskeleton. In Proceedings of the International Symposium on Wearable Robotics (WeRob2020) and WearRAcon Europe, online, 13–16 October 2020. [Google Scholar]
- Klass, D.W. The Continuing Challenge of Artifacts in the EEG. Am. J. EEG Technol. 1995, 35, 239–269. [Google Scholar] [CrossRef]
- Jacobson, E. Progressive Relaxation. Am. J. Psychol. 1987, 100, 522–537. [Google Scholar] [CrossRef]
- Prakaksita, N.; Kuo, C.Y.; Kuo, C.H. Development of a motor imagery based brain-computer interface for humanoid robot control applications. In Proceedings of the 2016 IEEE International Conference on Industrial Technology (ICIT), Taipei, Taiwan, 14–17 March 2016; pp. 1607–1613. [Google Scholar] [CrossRef]
- Cho, H.; Ahn, M.; Kwon, M.; Jun, S. A Step-by-Step Tutorial for a Motor Imagery–Based BCI. In Brain—Computer Interfaces Handbook; CRC Press: Boca Raton, FL, USA, 2018; pp. 445–460. [Google Scholar]
- Dean, J.C. Proprioceptive Feedback and Preferred Patterns of Human Movement. Exerc. Sport Sci. Rev. 2013, 41, 36. [Google Scholar] [CrossRef]
- Fleury, M.; Lioi, G.; Barillot, C.; Lécuyer, A. A Survey on the Use of Haptic Feedback for Brain-Computer Interfaces and Neurofeedback. Front. Neurosci. 2019. [Google Scholar] [CrossRef]
- Kim, Y.; Park, E.; Lee, A.; Im, C.H.; Kim, Y.H. Changes in network connectivity during motor imagery and execution. PLoS ONE 2018, 13, e0190715. [Google Scholar] [CrossRef] [PubMed]
- Portnova, G.; Girzhova, I.; Filatova, D.; Podlepich, V.; Tetereva, A.; Martynova, O. Brain Oscillatory Activity during Tactile Stimulation Correlates with Cortical Thickness of Intact Areas and Predicts Outcome in Post-Traumatic Comatose Patients. Brain Sci. 2020, 10, 720. [Google Scholar] [CrossRef]
- Zickler, C.; Riccio, A.; Leotta, F.; Hillian-Tress, S.; Halder, S.; Holz, E.; Staiger-Sälzer, P.; Hoogerwerf, E.J.; Desideri, L.; Mattia, D.; et al. A brain-computer interface as input channel for a standard assistive technology software. Clin. EEG Neurosci. 2011, 42, 236–244. [Google Scholar] [CrossRef] [PubMed]



| Subject | Age (Years) | Weight (Kg) | Height (cm) | BMI | Paretic Side |
|---|---|---|---|---|---|
| 1 | 55 | 84 | 173 | 28.1 | Right |
| 2 | 62 | 96 | 168 | 34 | Left |
| 3 | 63 | 79 | 161 | 30.5 | Right |
| 4 | 56 | 94 | 164 | 34.9 | Right |
| 5 | 61 | 69 | 166 | 25 | Left |
| Subject | Threshold | MIV Detection Time | MIVH Detection Time |
|---|---|---|---|
| 1 | 8 uV | 1798 ms | 2116 ms |
| 2 | 8 uV | 1272 ms | 1173 ms |
| 3 | 4 uV | 3326 ms | 1646 ms |
| 4 | 20 uV | 1226 ms | 1010 ms |
| 5 | 12 uV | 2418 ms | 986 ms |
| PSD (dB/Hz) Mean | ||||||
|---|---|---|---|---|---|---|
| Test | Subject | Fcz | C1 | Cz | C2 | Cpz |
| ST | 1 | 9.14 | 26.00 | 9.41 | 18.70 | 4.74 |
| 2 | 17.38 | 9.99 | 17.85 | 26.03 | 15.98 | |
| 3 | 0.86 | 1.12 | 0.97 | 4.20 | 0.86 | |
| 4 | 1.51 | 2.06 | 2.76 | 93.78 | NA * | |
| 5 | 0.52 | 0.57 | 0.56 | 0.52 | 0.36 | |
| MIV | 1 | 18.30 | 53.39 | 20.89 | 36.79 | 10.03 |
| 2 | 8.26 | 4.10 | 7.67 | 15.25 | 6.36 | |
| 3 | 0.17 | 0.24 | 0.15 | 0.16 | 0.13 | |
| 4 | 2.19 | 20.03 | 4.25 | 172.11 | NA * | |
| 5 | 6.07 | 6.55 | 7.78 | 6.47 | 5.42 | |
| MIVH | 1 | 8.98 | 13.30 | 10.17 | 24.89 | 6.19 |
| 2 | 24.45 | 12.35 | 24.89 | 55.95 | 21.41 | |
| 3 | 3.43 | 0.96 | 3.52 | 28.29 | 1.69 | |
| 4 | 7.95 | 2.99 | 16.55 | 85.16 | NA * | |
| 5 | 4.52 | 4.04 | 4.04 | 5.00 | 4.03 | |
| ST | 1 | 9.67 | 27.83 | 10.57 | 19.63 | 5.07 |
| 2 | 19.65 | 11.95 | 20.28 | 31.00 | 18.51 | |
| 3 | 1.05 | 1.15 | 1.11 | 4.29 | 1.05 | |
| 4 | 1.73 | 2.49 | 3.11 | 121.08 | NA * | |
| 5 | 0.79 | 0.80 | 0.90 | 0.81 | 0.51 | |
| MIV | 1 | 19.56 | 55.74 | 23.22 | 38.74 | 11.23 |
| 2 | 9.85 | 4.86 | 9.04 | 19.86 | 7.34 | |
| 3 | 0.18 | 0.29 | 0.17 | 0.19 | 0.15 | |
| 4 | 2.56 | 25.21 | 5.24 | 185.08 | NA * | |
| 5 | 8.86 | 10.06 | 11.75 | 9.84 | 8.02 | |
| MIVH | 1 | 9.91 | 13.26 | 10.96 | 26.81 | 7.31 |
| 2 | 21.93 | 13.43 | 21.76 | 65.34 | 24.11 | |
| 3 | 4.13 | 1.24 | 4.77 | 33.19 | 2.24 | |
| 4 | 7.62 | 2.92 | 18.40 | 110.69 | NA * | |
| 5 | 4.30 | 4.38 | 3.97 | 4.78 | 3.63 | |
| Test Comparison | Fcz | C1 | Cz | C2 | Cpz |
|---|---|---|---|---|---|
| ST vs. MIV vs. MIVH | 0.704 | 0.498 | 0.562 | 0.549 | 0.368 |
| ST vs. MIV | 0.737 | 0.218 | 0.645 | 0.437 | 0.999 |
| ST vs. MIVH | 0.039 | 0.699 | 0.074 | 0.184 | 0.100 |
| MI vs. MIVH | 0.532 | 0.300 | 0.509 | 0.999 | 0.530 |
| QUEST Survey Responses | ||||||
|---|---|---|---|---|---|---|
| Criteria | S1 | S2 | S3 | S4 | S5 | Average |
| Dimensions | 4.00 | 5.00 | 3.00 | 4.00 | 5.00 | 4.20 |
| Weight | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Adjustment | 5.00 | 5.00 | 4.00 | 5.00 | 5.00 | 4.75 |
| Safety | 5.00 | 5.00 | 4.00 | 5.00 | 5.00 | 4.75 |
| Ease of use | 5.00 | 5.00 | 5.00 | 4.00 | 5.00 | 4.75 |
| Effectiveness | 5.00 | 5.00 | 5.00 | 4.00 | 5.00 | 4.75 |
| Information/Instuctions | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| QUEST Total Score | 4.85 | 5.00 | 4.42 | 4.57 | 5.00 | 4.76 |
| Extended QUEST Survey Responses | ||||||
| Reliability | 5.00 | 5.00 | 4.00 | 5.00 | 5.00 | 4.75 |
| Speed | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Learning | 4.00 | 5.00 | 5.00 | 5.00 | 5.00 | 4.75 |
| Aesthetic design | 4.00 | 5.00 | 4.00 | 5.00 | 5.00 | 4.25 |
| Added Items Total Score | 4.25 | 4.75 | 4.25 | 4.75 | 4.75 | 4.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barria, P.; Pino, A.; Tovar, N.; Gomez-Vargas, D.; Baleta, K.; Díaz, C.A.R.; Múnera, M.; Cifuentes, C.A. BCI-Based Control for Ankle Exoskeleton T-FLEX: Comparison of Visual and Haptic Stimuli with Stroke Survivors. Sensors 2021, 21, 6431. https://doi.org/10.3390/s21196431
Barria P, Pino A, Tovar N, Gomez-Vargas D, Baleta K, Díaz CAR, Múnera M, Cifuentes CA. BCI-Based Control for Ankle Exoskeleton T-FLEX: Comparison of Visual and Haptic Stimuli with Stroke Survivors. Sensors. 2021; 21(19):6431. https://doi.org/10.3390/s21196431
Chicago/Turabian StyleBarria, Patricio, Angie Pino, Nicolás Tovar, Daniel Gomez-Vargas, Karim Baleta, Camilo A. R. Díaz, Marcela Múnera, and Carlos A. Cifuentes. 2021. "BCI-Based Control for Ankle Exoskeleton T-FLEX: Comparison of Visual and Haptic Stimuli with Stroke Survivors" Sensors 21, no. 19: 6431. https://doi.org/10.3390/s21196431
APA StyleBarria, P., Pino, A., Tovar, N., Gomez-Vargas, D., Baleta, K., Díaz, C. A. R., Múnera, M., & Cifuentes, C. A. (2021). BCI-Based Control for Ankle Exoskeleton T-FLEX: Comparison of Visual and Haptic Stimuli with Stroke Survivors. Sensors, 21(19), 6431. https://doi.org/10.3390/s21196431









