AI-Assisted In-House Power Monitoring for the Detection of Cognitive Impairment in Older Adults
Abstract
:1. Introduction
2. Related Work
3. Materials and Methods
3.1. Study Design and Participants
3.2. Cognitive Assessment
3.3. Other Clinical Data
3.4. Experimental Setup
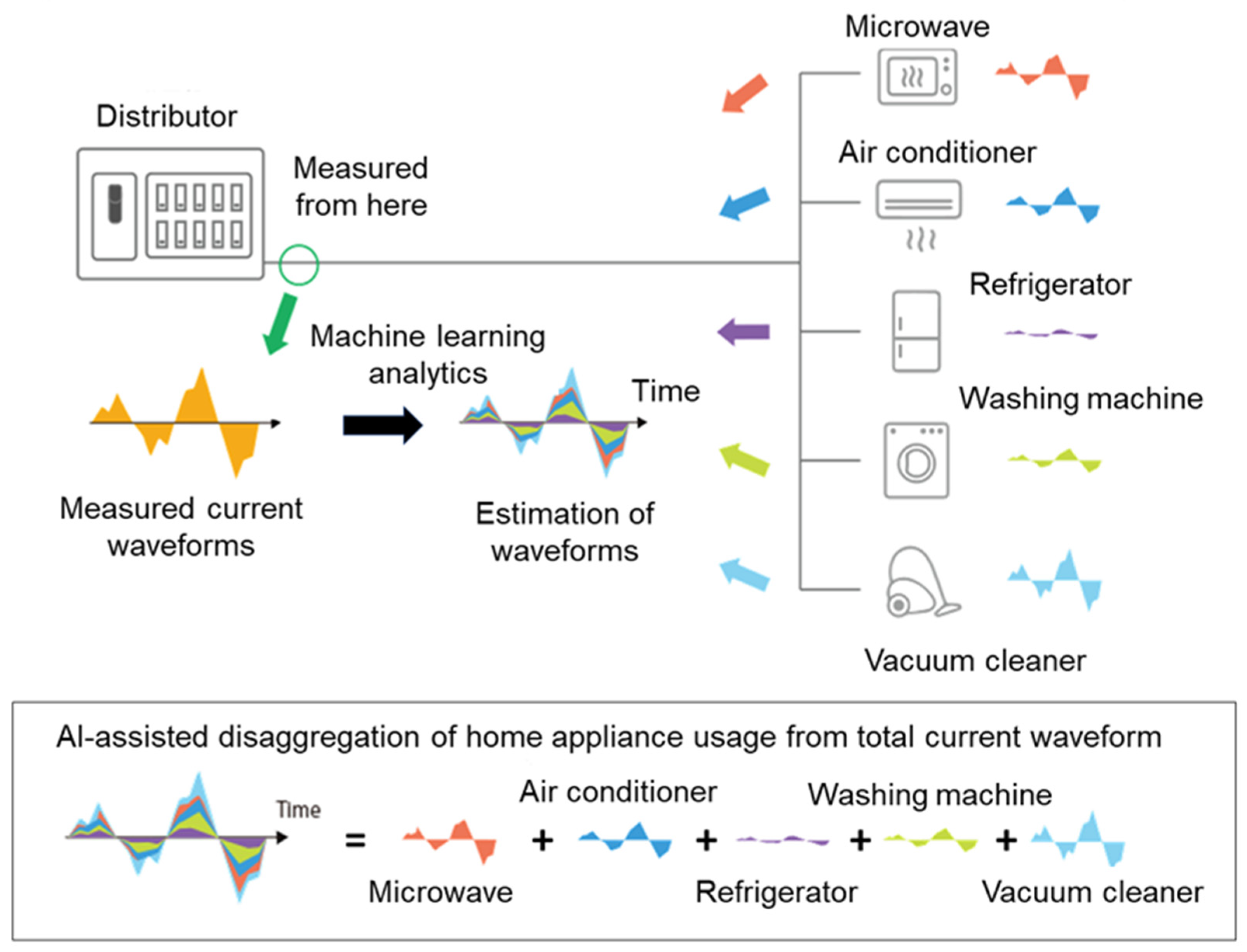
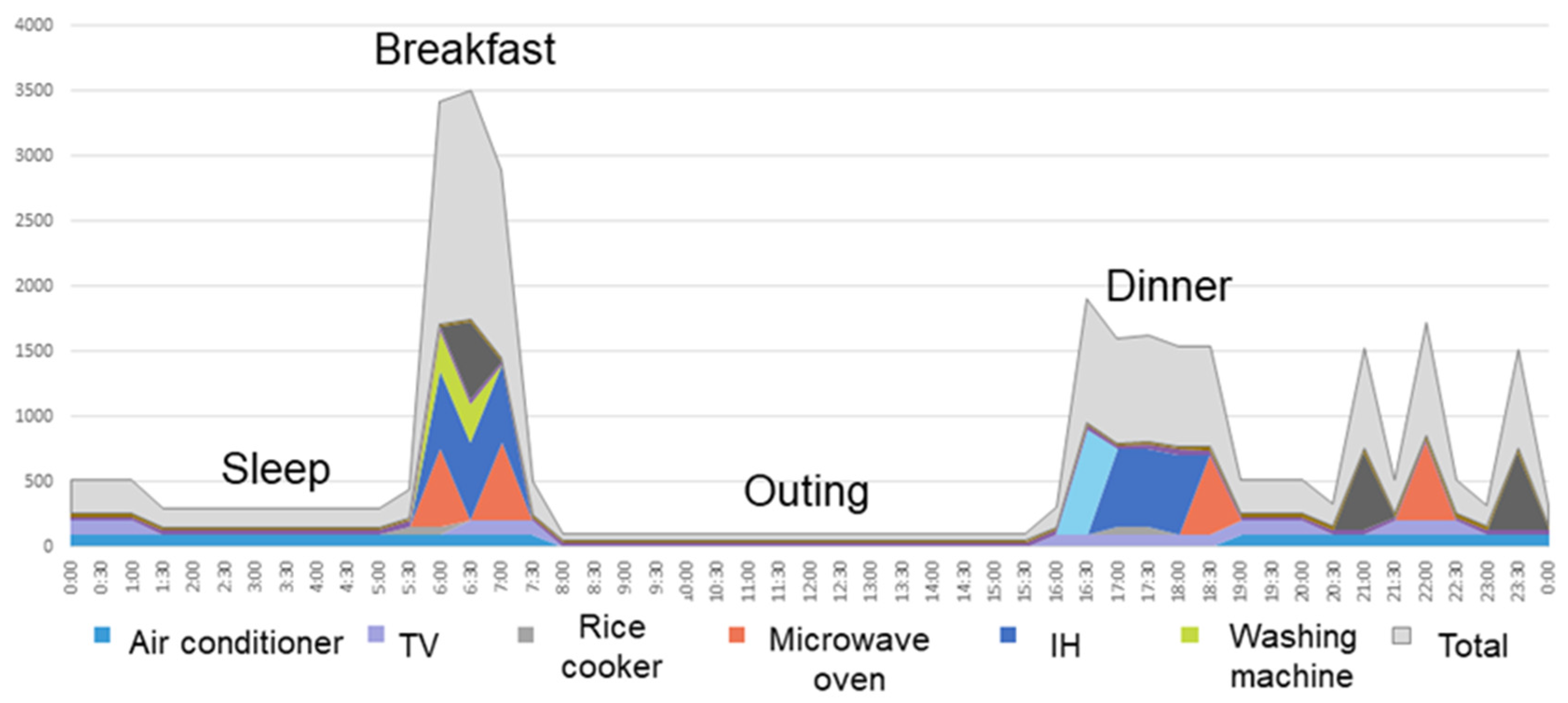
3.5. Measurement System for Home Appliance Usage Time
3.6. Statistical Analyses
4. Results
4.1. Participants
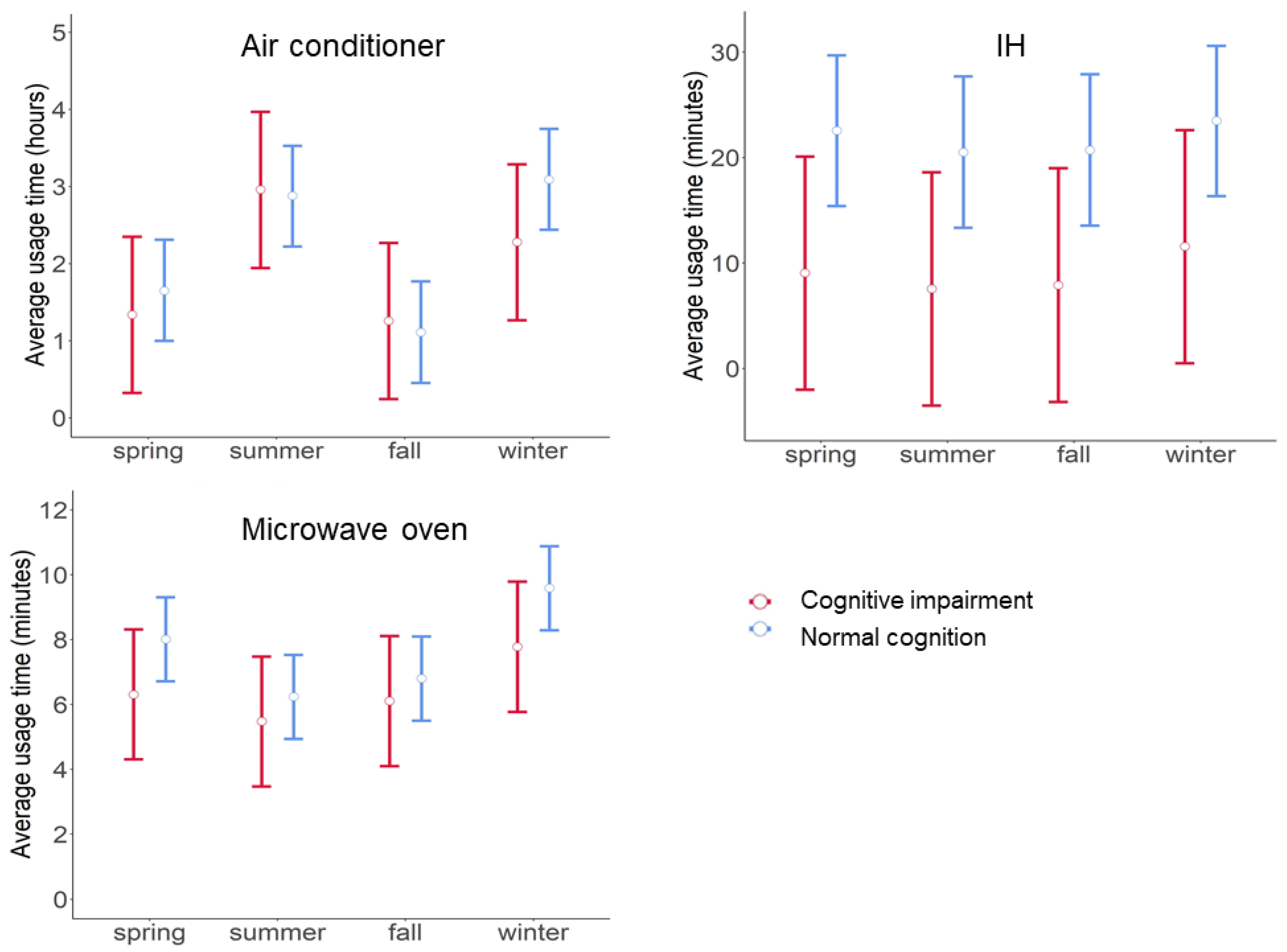
4.2. Relationships between Electric Appliance Usage Time and Cognitive Status
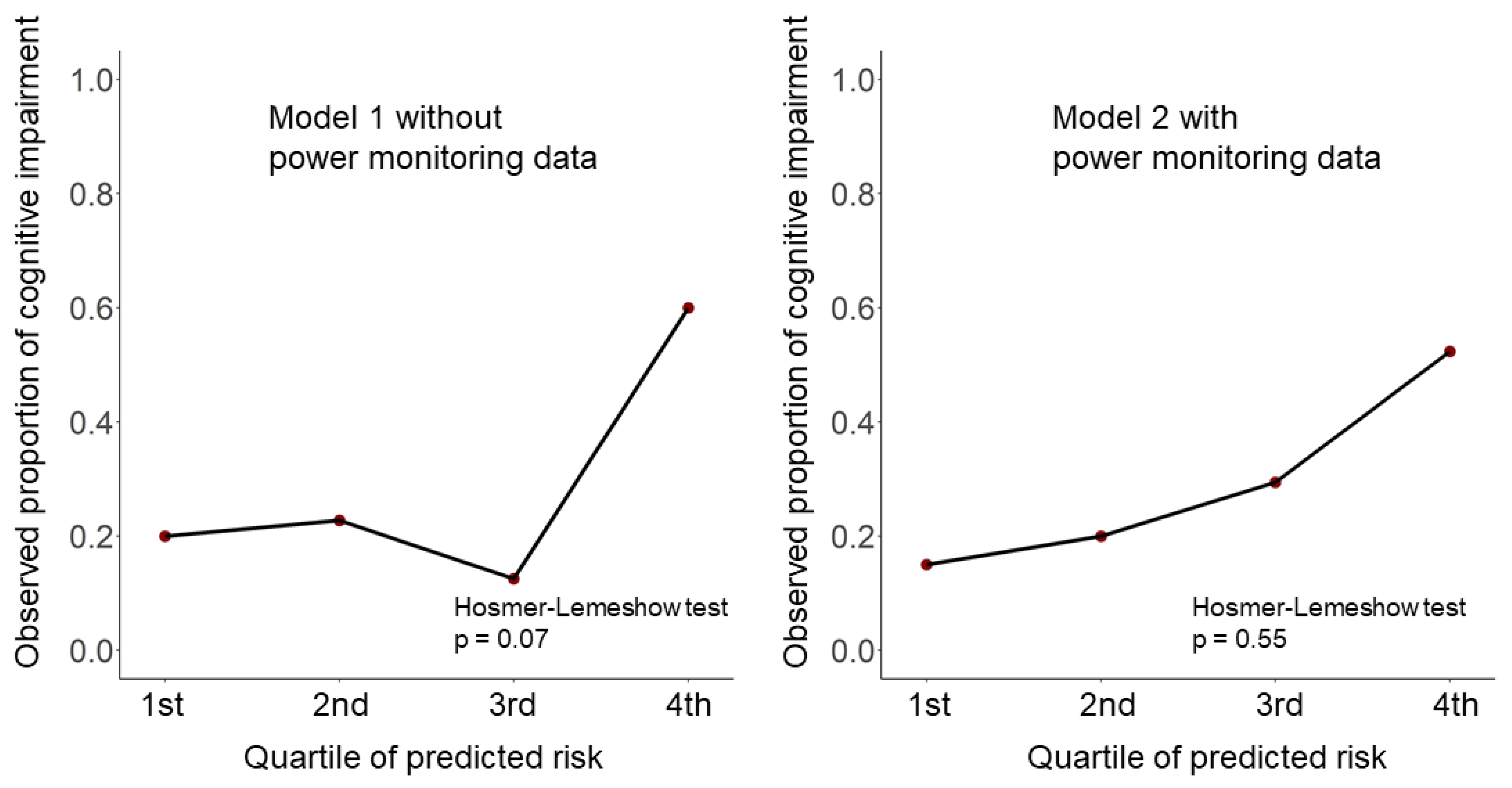
4.3. Prediction Models for Cognitive Impairment with and without In-House Power Monitoring Data
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| F Score | Precision | Recall | Specificity | Negative Predictive Value | Cohen Kappa | Accuracy | |
|---|---|---|---|---|---|---|---|
| Air conditioner | 0.88 | 0.91 | 0.90 | 0.94 | 0.91 | 0.80 | 0.95 |
| Microwave oven | 0.96 | 0.97 | 0.95 | 1.00 | 1.00 | 0.96 | 1.00 |
| Washing machine | 0.88 | 0.87 | 0.90 | 1.00 | 1.00 | 0.88 | 0.99 |
| Rice cooker | 0.89 | 0.94 | 0.85 | 1.00 | 1.00 | 0.89 | 1.00 |
| IH | 0.90 | 0.92 | 0.91 | 0.99 | 1.00 | 0.89 | 0.99 |
| TV | 0.69 | 0.81 | 0.68 | 0.91 | 0.83 | 0.59 | 0.83 |
References
- World Alzheimer Report 2019: Attitudes to Dementia; Alzheimer’s Disease International: London, UK, 2019; Available online: https://www.alzint.org/u/WorldAlzheimerReport2019.pdf (accessed on 28 July 2021).
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, M.S.; DeKosky, S.; Dickson, D.W.; Dubois, B.; Feldman, H.; Fox, N.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Ward, A.; Arrighi, H.M.; Michels, S.; Cedarbaum, J.M. Mild cognitive impairment: Disparity of incidence and prevalence estimates. Alzheimers Dement. 2012, 8, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lussier, M.; Adam, S.; Chikhaoui, B.; Consel, C.; Gagnon, M.; Gilbert, B.; Giroux, S.; Guay, M.; Hudon, C.; Imbeault, H.; et al. Smart Home Technology: A New Approach for Performance Measurements of Activities of Daily Living and Prediction of Mild Cognitive Impairment in Older Adults. J. Alzheimers Dis. 2019, 68, 85–96. [Google Scholar] [CrossRef]
- Lyons, B.E.; Eaustin, D.; Eseelye, A.; Epetersen, J.; Yeargers, J.; Eriley, T.; Esharma, N.; Mattek, N.C.; Ewild, K.; Edodge, H.; et al. Pervasive Computing Technologies to Continuously Assess Alzheimer’s Disease Progression and Intervention Efficacy. Front. Aging Neurosci. 2015, 7, 102. [Google Scholar] [CrossRef]
- Piau, A.; Wild, K.; Mattek, N.; Kaye, J. Current State of Digital Biomarker Technologies for Real-Life, Home-Based Monitoring of Cognitive Function for Mild Cognitive Impairment to Mild Alzheimer Disease and Implications for Clinical Care: Systematic Review. J. Med. Internet Res. 2019, 21, e12785. [Google Scholar] [CrossRef]
- Lussier, M.; Lavoie, M.; Giroux, S.; Consel, C.; Guay, M.; Macoir, J.; Hudon, C.; Lorrain, D.; Talbot, L.; Langlois, F.; et al. Early Detection of Mild Cognitive Impairment with in-home Monitoring Sensor Technologies Using Functional Measures: A Systematic Review. IEEE J. Biomed. Health Inform. 2018, 23, 838–847. [Google Scholar] [CrossRef]
- Blackman, S.; Matlo, C.; Bobrovitskiy, C.; Waldoch, A.; Fang, M.L.; Jackson, P.; Mihailidis, A.; Nygård, L.; Astell, A.; Sixsmith, A. Ambient Assisted Living Technologies for Aging Well: A Scoping Review. J. Intell. Syst. 2016, 25, 55–69. [Google Scholar] [CrossRef]
- Nilsson, M.Y.; Andersson, S.; Magnusson, L.; Hanson, E. Ambient assisted living technology-mediated interventions for older people and their informal carers in the context of healthy ageing: A scoping review. Health Sci. Rep. 2021, 4, 225. [Google Scholar] [CrossRef]
- Grguric, A.; Khan, O.; Ortega-Gil, A.; Markakis, E.; Pozdniakov, K.; Kloukinas, C.; Medrano-Gil, A.; Gaeta, E.; Fico, G.; Koloutsou, K. Reference Architectures, Platforms, and Pilots for European Smart and Healthy Living—Analysis and Comparison. Electronics 2021, 10, 1616. [Google Scholar] [CrossRef]
- Nikoloudakis, Y.; Panagiotakis, S.; Markakis, E.; Pallis, E.; Mastorakis, G.; Mavromoustakis, C.X.; Dobre, C. A Fog-Based Emergency System for Smart Enhanced Living Environments. IEEE Cloud Comput. 2016, 3, 54–62. [Google Scholar] [CrossRef]
- Stavropoulos, T.G.; Meditskos, G.; Kompatsiaris, I. DemaWare2: Integrating sensors, multimedia and semantic analysis for the ambient care of dementia. Pervasive Mob. Comput. 2017, 34, 126–145. [Google Scholar] [CrossRef]
- Naeem, U.; Anthony, R.; Tawil, A.-R.; Azam, M.A.; Preston, D. The Role of Ambient Intelligent Environments for Tracking Functional Decline. In Assistive Technologies for Physical and Cognitive Disabilities; Theng, L.B., Ed.; IGI Global: Hershey, PA, USA, 2015; Chapter 8; pp. 152–172. [Google Scholar] [CrossRef]
- Chalmers, C.; Fergus, P.; Montañez, C.A.C.; Sikdar, S.; Ball, F.; Kendall, B. Detecting Activities of Daily Living and Routine Behaviours in Dementia Patients Living Alone Using Smart Meter Load Disaggregation. IEEE Trans. Emerg. Top. Comput. 2020, 1. [Google Scholar] [CrossRef]
- Ishizu, K.; Mizumoto, T.; Yamaguchi, H.; Higashino, T. Home activity pattern estimation using aggregated electricity con-sumption data. Sens. Mater. 2021, 33, 69–88. [Google Scholar]
- Hallé, S.; Gaboury, S.; Bouchard, B. Towards User Activity Recognition Through Energy Usage Analysis and Complex Event Processing. In Proceedings of the 9th ACM International Conference on PErvasive Technologies Related to Assistive Environments, Corfu Island, Greece, 29 June–1 July 2016; Volume 3, p. 3. [Google Scholar]
- Ruzzelli, A.G.; Nicolas, C.; Schoofs, A.; O’Hare, G.M.P. Real-Time Recognition and Profiling of Appliances through a Single Electricity Sensor. In Proceedings of the 2010 7th Annual IEEE Communications Society Conference on Sensor, Mesh and Ad Hoc Communications and Networks (SECON), Boston, MA, USA, 21–25 June 2010; pp. 1–9. [Google Scholar]
- Sugishita, M.; Koshizuka, Y.; Sudou, S.; Sugishita, K.; Hemmi, I.; Karasawa, H. The Validity and Reliability of the Japanese Version of the Mini-Mental State Examination (MMSE-J) with the original procedure of the Attention and Calculation task (2001). Jpn. J. Cogn. Neurosci. 2018, 20, 91–110. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Kaufer, D.I.; Williams, C.S.; Braaten, A.J.; Gill, K.; Zimmerman, S.; Sloane, P.D. Cognitive Screening for Dementia and Mild Cognitive Impairment in Assisted Living: Comparison of 3 Tests. J. Am. Med. Dir. Assoc. 2008, 9, 586–593. [Google Scholar] [CrossRef]
- Damian, A.M.; Jacobson, S.A.; Hentz, J.G.; Belden, C.M.; Shill, H.A.; Sabbagh, M.N.; Caviness, J.N.; Adler, C.H. The Montreal Cognitive Assessment and the Mini-Mental State Examination as Screening Instruments for Cognitive Impairment: Item Analyses and Threshold Scores. Dement. Geriatr. Cogn. Disord. 2011, 31, 126–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yesavage, J.A. Geriatric depression scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar]
- Almeida, O.P.; Almeida, S.A. Short versions of the geriatric depression scale: A study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int. J. Geriatr. Psychiatry 1999, 14, 858–865. [Google Scholar] [CrossRef]
- Kivipelto, M.; Ngandu, T.; Laatikainen, T.; Winblad, B.; Soininen, H.; Tuomilehto, J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurol. 2006, 5, 735–741. [Google Scholar] [CrossRef]
- Ngandu, T.; von Strauss, E.; Helkala, E.-L.; Winblad, B.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Education and dementia: What lies behind the association? Neurology 2007, 69, 1442–1450. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: http://www.r-project.org/index.html (accessed on 28 July 2021).
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Rentería, M.A.; Vonk, J.M.; Felix, G.; Avila, J.F.; Zahodne, L.B.; Dalchand, E.; Frazer, K.M.; Martinez, M.N.; Shouel, H.L.; Manly, J.J. Illiteracy, dementia risk, and cognitive trajectories among older adults with low education. Neurology 2019, 93, e2247–e2256. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Meng, Z.; Liu, X.; Jin, Q.; Su, R. Decision Variants for the Automatic Determination of Optimal Feature Subset in RF-RFE. Genes 2018, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and Elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosmer, D.W.; Hosmer, T.; Cessie, S.L.; Lemeshow, S. A comparison of goodness-of-fit tests for the logistic regression model. Stat. Med. 1997, 16, 965–980. [Google Scholar] [CrossRef]
- Kramer, A.A.; Zimmerman, J.E. Assessing the calibration of mortality benchmarks in critical care: The Hosmer-Lemeshow test revisited. Crit. Care Med. 2007, 35, 2052–2056. [Google Scholar] [CrossRef] [PubMed]
- Borsje, P.; Wetzels, R.B.; Lucassen, P.L.; Pot, A.M.; Koopmans, R.T. The course of neuropsychiatric symptoms in community-dwelling patients with dementia: A systematic review. Int. Psychogeriatr. 2015, 27, 385–405. [Google Scholar] [CrossRef]
- Jekel, K.; Damian, M.; Wattmo, C.; Hausner, L.; Bullock, R.; Connelly, P.J.; Dubois, B.; Eriksdotter, M.; Ewers, M.; Graessel, E.; et al. Mild cognitive impairment and deficits in instrumental activities of daily living: A systematic review. Alzheimers Res. Ther. 2015, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, P.D.; Downey, L.E.; Golden, H.L.; Clark, C.; Slattery, C.F.; Paterson, R.W.; Rohrer, J.; Schott, J.; Rossor, M.; Warren, J.D. Pain and temperature processing in dementia: A clinical and neuroanatomical analysis. Brain 2015, 138, 3360–3372. [Google Scholar] [CrossRef]
- Alberdi, A.; Weakley, A.; Schmitter-Edgecombe, M.; Cook, D.J.; Aztiria, A.; Basarab, A.; Barrenechea, M. Smart Home-Based Prediction of Multidomain Symptoms Related to Alzheimer’s Disease. IEEE J. Biomed. Health Inform. 2018, 22, 1720–1731. [Google Scholar] [CrossRef] [Green Version]
- Akl, A.; Chikhaoui, B.; Mattek, N.; Kaye, J.; Austin, D.; Mihailidis, A. Clustering home activity distributions for automatic detection of mild cognitive impairment in older adults1. J. Ambient. Intell. Smart Environ. 2016, 8, 437–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connors, M.H.; Seeher, K.; Teixeira-Pinto, A.; Woodward, M.; Ames, D.; Brodaty, H. Dementia and caregiver burden: A three-year longitudinal study. Int. J. Geriatr. Psychiatry 2019, 35, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-T. Dementia Caregiver Burden: A Research Update and Critical Analysis. Curr. Psychiatry Rep. 2017, 19, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cermakova, P.; Nelson, M.; Secnik, J.; Garcia-Ptacek, S.; Johnell, K.; Fastbom, J.; Kilander, L.; Winblad, B.; Eriksdotter, M.; Religa, D. Living Alone with Alzheimer’s Disease: Data from SveDem, the Swedish Dementia Registry. J. Alzheimers Dis. 2017, 58, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
| MMSE > 27 Normal Cognition | MMSE ≤ 27 Cognitive Impairment | Total | |
|---|---|---|---|
| (N = 55) | (N = 23) | (N = 78) | |
| Continuous variables, median (IQRs) | |||
| Age | 75.0 (70.0, 78.5) | 78.0 (75.0, 83.5) | 76.0 (70.0, 80.0) |
| Men | 37 (67.3%) | 17 (73.9%) | 54 (69.2%) |
| MMSE | 29.0 (29.0, 30.0) | 26.0 (24.0, 27.0) | 29.0 (27.0, 30.0) |
| Years of education | 12.0 (11.5, 12.5) | 12.0 (10.5, 12.5) | 12.0 (11.0, 12.8) |
| Usage hours of air conditioner | 1.59 (0.44, 3.26) | 1.67 (0.31, 3.32) | 1.63 (0.35, 3.33) |
| Usage hours of microwave oven | 0.12 (0.06, 0.17) | 0.10 (0.04, 0.14) | 0.115 (0.06, 0.17) |
| Usage hours of IH | 0.15 (0.001, 0.71) | 0.00 (0, 0.12) | 0.02 (0, 0.56) |
| Categorical variables, n (%) | |||
| Everyday drinker | 21 (38.2%) | 10 (43.5%) | 31 (39.7%) |
| Current smoker | 3 (5.5%) | 2 (8.7%) | 5 (6.4%) |
| Living alone | 15 (27.3%) | 3 (13.0%) | 18 (23.1%) |
| Medical history | |||
| Diabetes mellitus | 5 (9.1%) | 3 (13.0%) | 8 (10.3%) |
| Hyperlipidemia | 16 (29.1%) | 11 (47.8%) | 27 (34.6%) |
| Hypertension | 23 (41.8%) | 11 (47.8%) | 34 (43.6%) |
| Stroke | 2 (3.6%) | 3 (13.0%) | 5 (6.4%) |
| Cancer | 10 (18.2%) | 4 (17.4%) | 14 (17.9%) |
| Myocardial infarction | 3 (5.5%) | 4 (17.4%) | 7 (9.0%) |
| Depressive symptoms | 13 (23.6%) | 3 (13.0%) | 16 (20.5%) |
| Model | Model 1 without Power Monitoring Data | Model 2 with Power Monitoring Data |
|---|---|---|
| Accuracy | 0.76 (0.65–0.85) | 0.82 (0.72–0.90) |
| Sensitivity | 0.30 (0.13–0.53) | 0.48 (0.27–0.69) |
| Specificity | 0.95 (0.85–0.99) | 0.96 (0.87–1.00) |
| Positive predictive value | 0.70 (0.35–0.93) | 0.85 (0.55–0.98) |
| Negative predictive value | 0.76 (0.65–0.86) | 0.82 (0.70–0.90) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakaoku, Y.; Ogata, S.; Murata, S.; Nishimori, M.; Ihara, M.; Iihara, K.; Takegami, M.; Nishimura, K. AI-Assisted In-House Power Monitoring for the Detection of Cognitive Impairment in Older Adults. Sensors 2021, 21, 6249. https://doi.org/10.3390/s21186249
Nakaoku Y, Ogata S, Murata S, Nishimori M, Ihara M, Iihara K, Takegami M, Nishimura K. AI-Assisted In-House Power Monitoring for the Detection of Cognitive Impairment in Older Adults. Sensors. 2021; 21(18):6249. https://doi.org/10.3390/s21186249
Chicago/Turabian StyleNakaoku, Yuriko, Soshiro Ogata, Shunsuke Murata, Makoto Nishimori, Masafumi Ihara, Koji Iihara, Misa Takegami, and Kunihiro Nishimura. 2021. "AI-Assisted In-House Power Monitoring for the Detection of Cognitive Impairment in Older Adults" Sensors 21, no. 18: 6249. https://doi.org/10.3390/s21186249
APA StyleNakaoku, Y., Ogata, S., Murata, S., Nishimori, M., Ihara, M., Iihara, K., Takegami, M., & Nishimura, K. (2021). AI-Assisted In-House Power Monitoring for the Detection of Cognitive Impairment in Older Adults. Sensors, 21(18), 6249. https://doi.org/10.3390/s21186249






