Structure, Surface Morphology, Chemical Composition, and Sensing Properties of SnO2 Thin Films in an Oxidizing Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
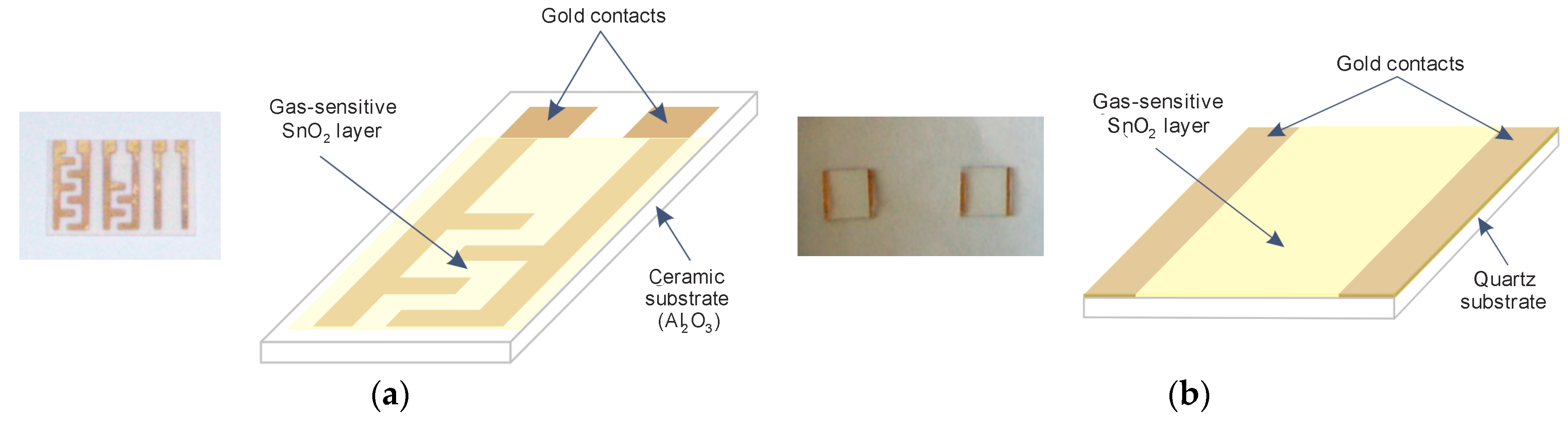
2.1. Production of SnO2 Thin Layers
2.2. Measurement of Gas Response
2.3. Characterization of SnO2 Thin Layers
3. Results and Discussion
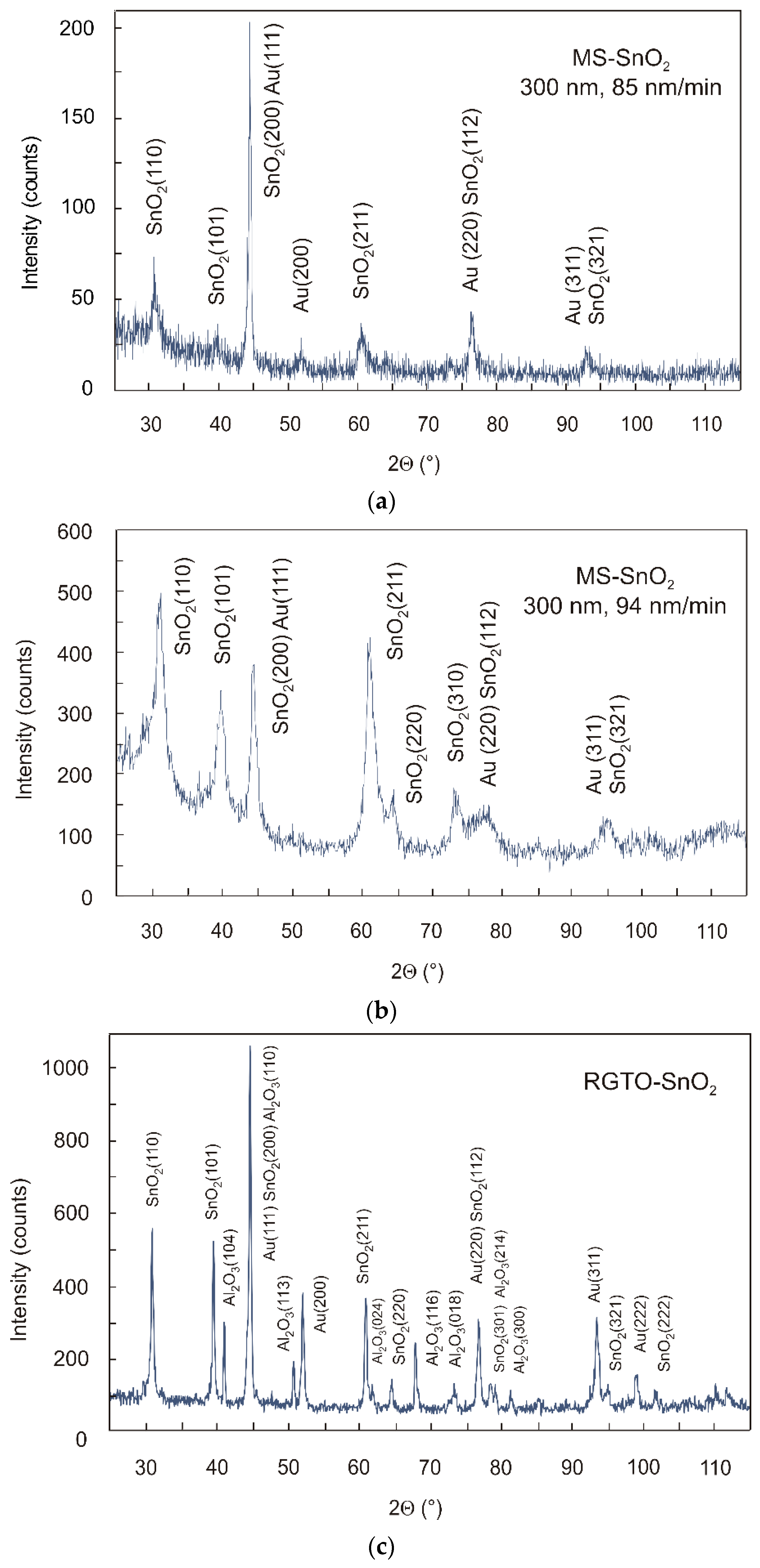
3.1. X-ray Diffractometry
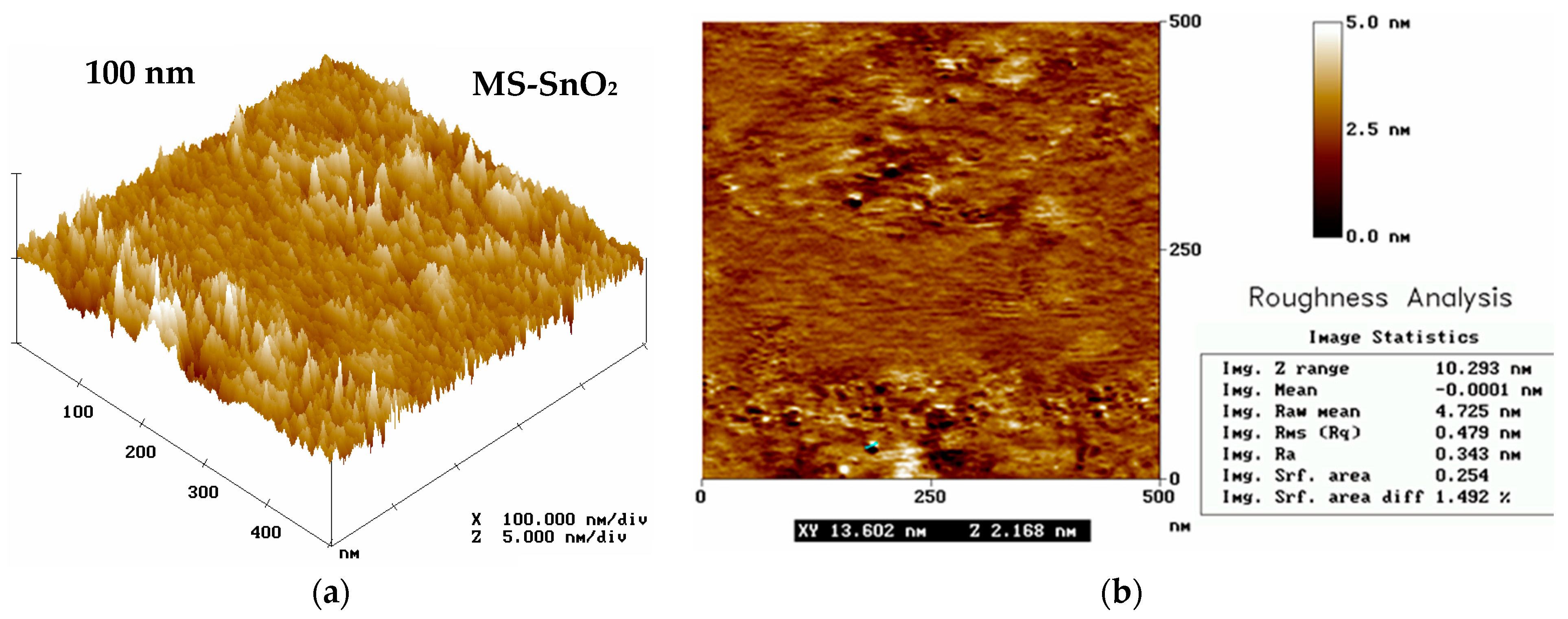
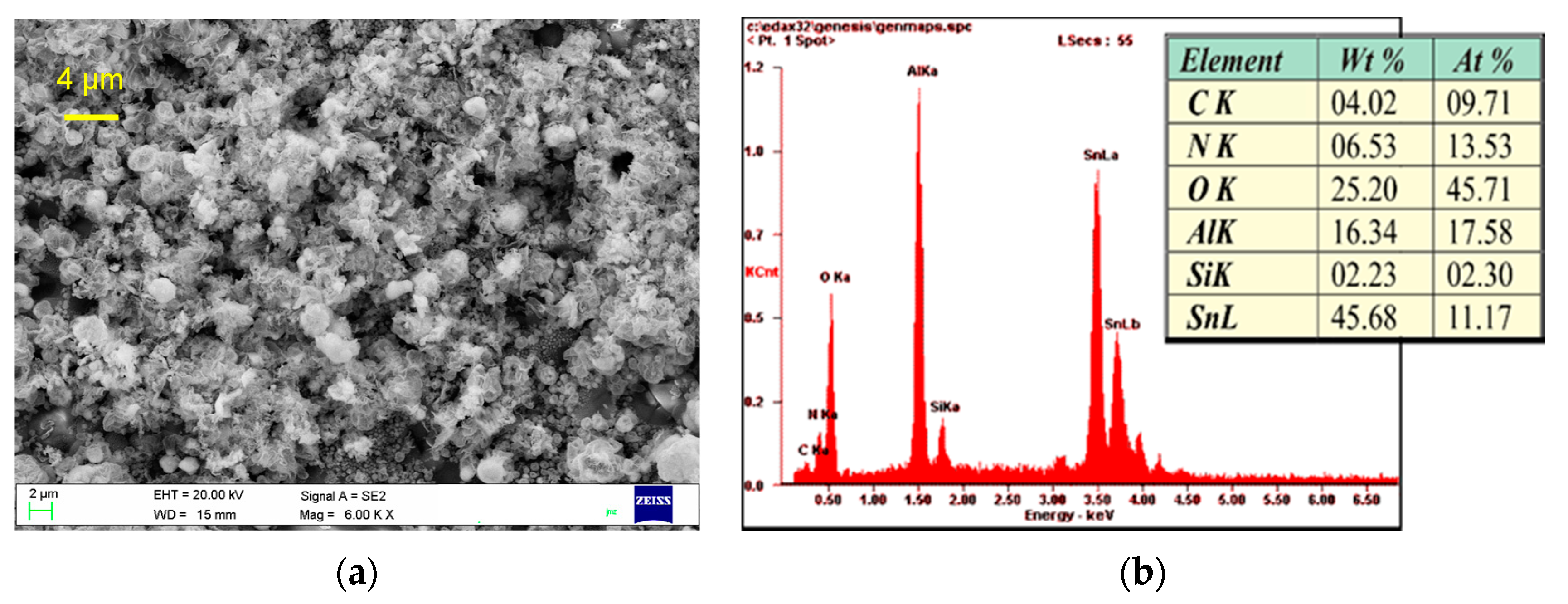
3.2. Atomic Force (AFM) and Scanning Electron (SEM) Microscopy
3.3. Auger Electron Spectroscopy (AES)
3.4. X-ray Photoelectron Spectroscopy (XPS)
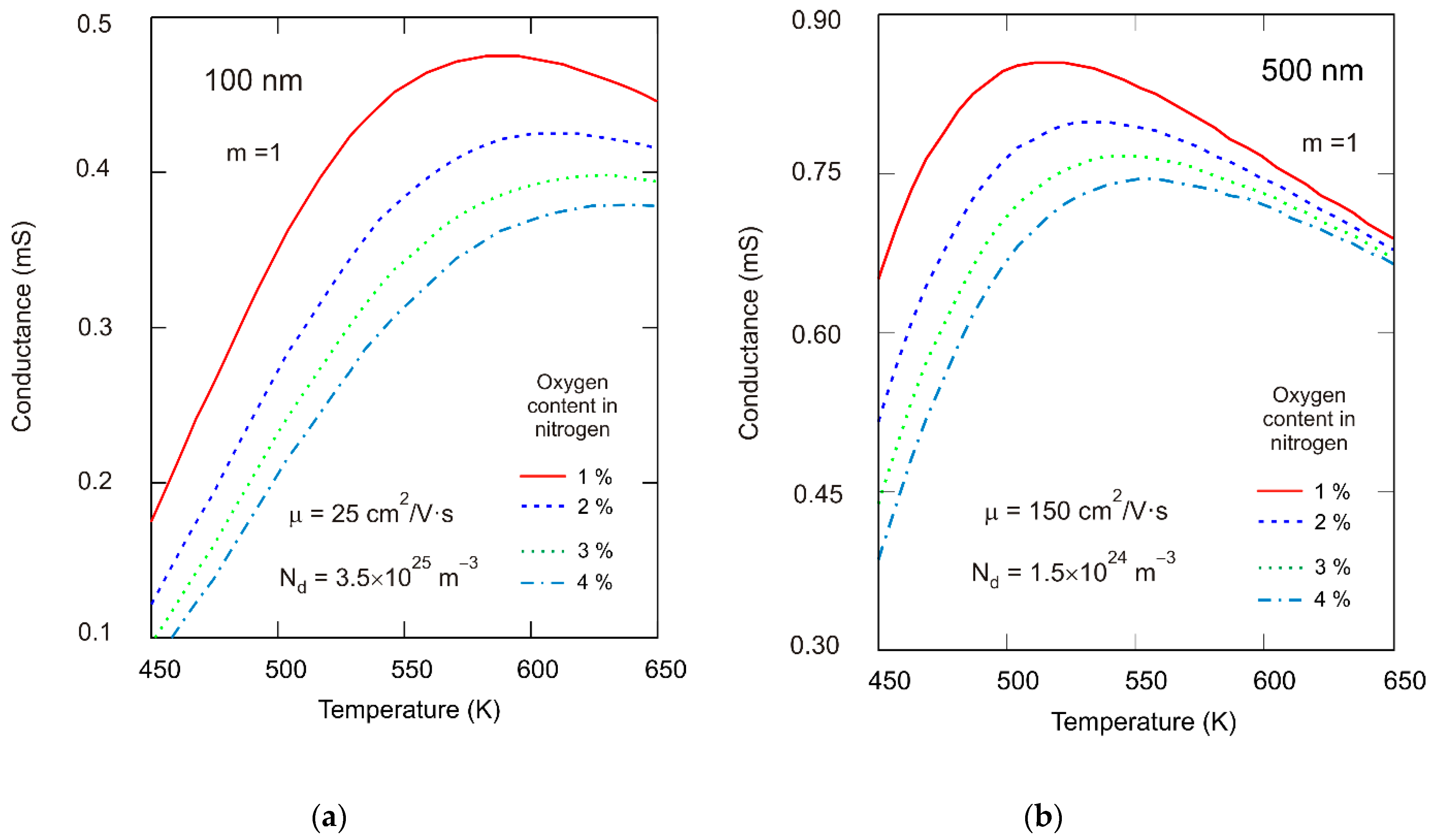
3.5. Gas Sensing Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sberveglieri, G. Recent developments in semiconducting thin-films gas sensors. Sens. Actuators B Chem. 1995, 23, 103–109. [Google Scholar] [CrossRef]
- Wilson, R.L.; Simion, C.E.; Blackman, C.S.; Carmalt, C.J.; Stanoiu, A.; Di Maggio, F.; Covington, J.A. The Effect of Film Thickness on the Gas Sensing Properties of Ultra-Thin TiO2 Films Deposited by Atomic Layer Deposition. Sensors 2018, 18, 735. [Google Scholar] [CrossRef] [Green Version]
- Geörgy, E.; Pérez del Pino, A. Tunable optical and nano-scale electrical properties of WO3 and Ag-WO3 nanocomposite thin films. J. Mater. Sci. 2011, 46, 3560–3567. [Google Scholar] [CrossRef]
- Naberezhnyi, D.; Rumyantseva, M.; Filatova, D.; Batuk, M.; Hadermann, J.; Baranchikov, A.; Khmelevsky, N.; Aksenenko, A.; Konstantinova, E.; Gaskov, A. Effects of Ag Additive in Low Temperature CO Detection with In2O3 Based Gas Sensors. Nanomaterials 2018, 8, 801. [Google Scholar] [CrossRef] [Green Version]
- Comini, E. Metal oxide nanowire chemical sensors: Innovation and quality of life. Mater. Today 2016, 19, 559–567. [Google Scholar] [CrossRef]
- Li, Z.J.; Li, H.; Wu, Z.L.; Wang, M.K.; Luo, J.T.; Torun, H.; Hu, P.A.; Yang, C.; Grudmann, M.; Liu, X.T.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef] [Green Version]
- Henrich, V.E.; Cox, P.A. The Surface Science of Metal Oxides; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Ikohura, K.; Watson, J. The Stannic Oxide Gas Sensor, Principles and Applications; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of tin oxide surface with O2, H2O and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen ionosorption and spectroscopic evidence for adsorbed oxygen. Chemphyschem 2006, 7, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Tournier, G.; Pijolat, C. Influence of oxygen concentration in the carrier gas on the response of tin dioxide sensor under hydrogen and methane. Sens. Actuators B Chem. 1999, 61, 43–50. [Google Scholar] [CrossRef]
- Sahm, T.; Gurlo, A.; Barsan, N.; Weimar, U. Basics of oxygen and SnO2 interaction, work function change and conductivity measurements. Sens. Actuators B Chem. 2006, 118, 78–83. [Google Scholar] [CrossRef]
- Foltynowicz, Z.; Rikhie, A. Oxygen Scavengers Applications in the Dairy Industry. HSOA J. Dairy Res. Technol. 2020, 3, 1–6. [Google Scholar] [CrossRef]
- Trivellin, N.; Barbisan, D.; Badocco, D.; Pastore, P.; Meneghesso, G.; Meneghini, M.; Zanoni, E.; Belgioioso, G.; Cenedese, A. Study and Development of a Fluorescence Based Sensor System for Monitoring Oxygen in Wine Production: The WOW Project. Sensors 2018, 18, 1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Li, Y.; Migliaccio, K.; Olczyk, T.; Alva, A. Oxygen Amendment on Growth and Nitrogen Use Efficiency of Flooded Italian Basil, International. J. Veg. Sci. 2013, 19, 217–227. [Google Scholar] [CrossRef]
- Ilsley, A.H.; Runciman, W.B. An Evaluation of Fourteen Oxygen Analysers for Use in Patient Breathing. Circuits Anaesth Intens Care 1986, 14, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ding, W. Design of Intelligent Monitoring System for Aquaculture Water Dissolved Oxygen. In Proceedings of the 2018 IEEE 3rd Advanced Information Technology, Electronic and Automation Control Conference (IAEAC), Chongqing, China, 12–14 October 2018; pp. 414–418. [Google Scholar]
- Wei, Y.; Jiao, Y.; An, D.; Li, D.; Li, W.; Wei, Q. Review of Dissolved Oxygen Detection Technology: From Laboratory Analysis to Online Intelligent Detection. Sensors 2019, 19, 3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, H.; Liang, Y.; Wang, Y.; Wang, B.; Tong, H.; Yuan, Y.; Wei, S. Effect of Dissolved Oxygen on Pitting Corrosion Behavior of Low-Alloy Steel under Hydrostatic Pressure. Int. J. Electrochem. Sci. 2019, 14, 4812–4827. [Google Scholar] [CrossRef]
- Suematsu, K.; Ma, N.; Watanabe, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of Humid Aging on the Oxygen Adsorption in SnO2 Gas Sensors. Sensors 2018, 18, 254. [Google Scholar] [CrossRef] [Green Version]
- Suchorska-Woźniak, P.; Rac, O.; Fiedot, M.; Teterycz, H. Analysis of SnO2|WO3 Heterocontact Properties during the Detection of Hydrogen Sulphide. Sensors 2014, 14, 20480–20499. [Google Scholar] [CrossRef] [Green Version]
- Shaposhnik, A.V.; Shaposhnik, D.A.; Turishchev, S.Y.; Chuvenkova, O.A.; Ryabtsev, S.V.; Vasiliev, A.A.; Vilanova, X.; Hernandez-Ramirez, F.; Morante, J.R. Gas sensing properties of individual SnO2 nanowires and SnO2 sol–gel nanocomposites. Beilstein J. Nanotechnol. 2019, 10, 1380–1390. [Google Scholar] [CrossRef] [Green Version]
- Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Quasi-one dimensional metal oxide semiconductors: Preparation, characterisation and application as chemical sensors. Prog. Mater. Sci. 2009, 54, 1–67. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Q.; Lu, Z.; Wei, Z.; Xu, L.; Gui, Y. Recent Advances of SnO2-Based Sensors for Detecting Fault Characteristic Gases Extracted From Power Transformer Oil. Front. Chem. 2018, 6, 364. [Google Scholar] [CrossRef] [Green Version]
- Yuliarto, B.; Gumilar, G.; Septiani, N.L. SnO2 Nanostructure as Pollutant Gas Sensors: Synthesis, Sensing Performances, and Mechanism. Adv. Mater. Sci. Eng. 2015, 2015, 694823. [Google Scholar] [CrossRef] [Green Version]
- Korotcenkov, G. Current Trends in Nanomaterials for Metal Oxide-Based Conductometric Gas Sensors: Advantages and Limitations. Part 1: 1D and 2D Nanostructures. Nanomaterials 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Gulevich, D.; Rumyantseva, M.; Gerasimov, E.; Khmelevsky, N.; Tsvetkova, E.; Gaskov, A. Synergy Effect of Au and SiO2 Modification on SnO2 Sensor Properties in VOCs Detection in Humid Air. Nanomaterials 2020, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Shaikh, M.O. A Room Temperature H2 Sensor Fabricated Using High Performance Pt-Loaded SnO2 Nanoparticles. Sensors 2015, 15, 14286–14297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liewhiran, C.; Tamaekong, N.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Ultra-sensitive H2 sensors based on flame-spray-made Pd-loaded SnO2 sensing films. Sens. Actuators B Chem. 2013, 176, 893–905. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Boris, I. (Cu, Fe, Co, or Ni)-doped tin dioxide films deposited by spray pyrolysis: Doping influence on film morphology. J. Mater. Sci. 2008, 43, 2761–2770. [Google Scholar] [CrossRef]
- Izydorczyk, W.; Niemiec, N.; Waczyński, K.; Uljanow, J. Hydrogen sensing properties of SnO2 nanocrystalline thin films. In Proceedings of the 21st European Microelectronics Packaging Conference (EMPC 2017), Warsaw, Poland, 10–13 September 2017; pp. 1–5. [Google Scholar]
- Adamowicz, B.; Izydorczyk, W.; Izydorczyk, J.; Klimasek, A.; Jakubik, W.; Żywicki, J. Response of an optimised SnO2-based gas sensor to oxygen. Vacuum 2008, 82, 966–970. [Google Scholar] [CrossRef]
- Rantala, T.S.; Rantala, T.T.; Lantto, V. Computational studies for the interpretation of gas response of SnO2 (110) surface. Sens. Actuators B Chem. 2000, 65, 375–378. [Google Scholar] [CrossRef]
- Suematsu, K.; Yuasa, M.; Kida, T.; Yamazoe, N.; Shimanoe, K. Determination of Oxygen Adsorption Species on SnO2: Exact Analysis of Gas Sensing Properties Using a Sample Gas Pretreatment System. J. Electrochem. Soc. 2014, 161, B123–B128. [Google Scholar] [CrossRef]
- Savu, R.; Ponce, M.A.; Joanni, E.; Bueno, P.R.; Castro, M.; Cilense, M.; Varela, J.A.; Longo, E. Grain size effect on the electrical response of SnO2 thin and thick film gas sensors. Mater. Res. 2009, 12, 83–87. [Google Scholar] [CrossRef]
- Khan, A.F.; Mehmood, M.; Rana, A.M.; Bhatti, M.T. Effect of annealing on electrical resistivity of rf-magnetron sputtered nanostructured SnO2 thin films. Appl. Surf. Sci. 2009, 255, 8562–8565. [Google Scholar] [CrossRef]
- Suematsu, K.; Yamada, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Evaluation of Oxygen Adsorption Based on the Electric Properties of SnO2 Semiconductor Gas Sensors. Sens. Mater. 2016, 28, 1211–1217. [Google Scholar]
- Sberveglieri, G.; Faglia, G.; Groppeli, S.; Nelli, P.; Camanzi, A. A New Technique for Growing Large Surface Area SnO2 Thin Film (RGTO-Technique). Semicond. Sci. Technol. 1990, 5, 1231–1233. [Google Scholar] [CrossRef]
- Szuber, J.; Uljanow, J.; Karczewska-Buczek, T.; Jakubik, W.; Waczynski, K.; Kwoka, M.; Kończak, S. On the correlation between morphology and gas sensing properties of RGTO SnO2 thin films. Thin Solid Films 2005, 490, 54–58. [Google Scholar] [CrossRef]
- Diéguez, A.; Romano-Rodríguez, A.; Morante, J.R.; Sangaletti, L.; Sberveglieri, G. Influence of the completion of oxidation on the long-term response of RGTO SnO2 gas sensors. Sens. Actuators B Chem. 2000, 66, 40–42. [Google Scholar] [CrossRef]
- Gołaszewska, K.; Kamińska, E.; Pustelny, T.; Struk, P.; Piotrowski, T.; Piotrowska, A.; Ekielski, M.; Kruszka, R.; Wzorek, M.; Borysiewicz, M.; et al. Planar Optical Waveguides for Application in Optoelectronic Gas Sensors. Acta Phys. Pol. 2008, 114, A223–A230. [Google Scholar] [CrossRef]
- Hunt, C.P.; Anthony, M.T.; Seah, M.P. AES and XPS depth profiling certified reference material. Surf. Interface Anal. 1984, 6, 92–93. [Google Scholar] [CrossRef]
- Davis, L.E.; MacDonald, N.C.; Palmberg, P.W.; Riach, G.E.; Weber, R.E. Handbook of Auger Electron Spectroscopy, 2nd ed.; Physical Electronics Division, Perkin-Elmer Corp.: Eden Prairie, MI, USA, 1976. [Google Scholar]
- Shirley, D.A. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- ICDD-JCPDS-International Centre for Diffraction Data. PCPDFWIN Version 2.1, 2000 (JCPDS, SnO2: 41-1445; Au: 04-0784; Al2O3: 46-1212). Available online: https://en.wikipedia.org/wiki/International_Centre_for_Diffraction_Data (accessed on 2 April 2021).
- Yawale, S.P.; Yawale, S.S.; Lamdhade, G.T. Tin oxide and zinc oxide based doped humidity sensors. Sens. Actuator A Phys. 2007, 135, 388–393. [Google Scholar] [CrossRef]
- Izydorczyk, W.; Pisarek, M.; Żak, J. Structure, surface morphology and sensing properties of the SnO₂ thin films research. Elektron. Konstr. Technol. Zastos. 2010, 6, 50–53. (In Polish) [Google Scholar]
- Stefanov, P.; Atanasova, G.; Manolov, E.; Raicheva, Z.; Lazarova, V. Preparation and Characterization of SnO2 Films for Sensing Applications. J. Phys. Conf. Ser. 2008, 100, 082046. [Google Scholar] [CrossRef]
- Bittencourt, C.; Llobet, E.; Silva, M.A.P.; Landers, R.; Nieto, L.; Vicaro, K.O.; Sueiras, J.E.; Calderer, J.; Correig, X. Influence of the deposition method on the morphology and elemental composition of SnO2 films for gas sensing: Atomic force and X-ray photoemission spectroscopy analysis. Sens. Actuators B Chem. 2003, 92, 67–72. [Google Scholar] [CrossRef]
- Ottaviano, L.; Kwoka, M.; Bisti, F.; Parisse, P.; Grossi, V.; Santucci, S.; Szuber, J. Local surface morphology and chemistry of SnO2 thin films deposited by rheotaxial growth and thermal oxidation method for gas sensor application. Thin Solid Films 2009, 517, 6161–6169. [Google Scholar] [CrossRef]
- Faglia, G.; Comini, E.; Sberveglieri, G.; Rella, R.; Siciliano, P.; Vasanelli, L. Square and collinear four probe array and Hall measurements on metal oxide thin film gas sensors. Sens. Actuators B Chem. 1998, 53, 69–75. [Google Scholar] [CrossRef]
- Childs, K.D.; Carlson, B.A.; Vanier, L.A.; Moulder, J.F.; Paul, D.F.; Stickle, W.F.; Watson, D.G. Handbook of Auger Electron Spectroscopy, 3rd ed.; Hedberg, C.L., Ed.; Physical Electronics, Inc.: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Buono-Core, G.E.; Cabello, G.A.; Espinoza, H.; Klahn, A.H.; Tejos, M.; Hill, R.H. Photochemical deposition of Pd-loaded and Pt-loaded tin oxide thin films. J. Chil. Chem. Soc. 2006, 51, 950–956. [Google Scholar] [CrossRef] [Green Version]
- Hellmich, W.; Braunmühl, C.B.; Müller, G.; Sberveglieri, G.; Berti, M.; Perego, C. The Kinetics of Formation of Gas-Sensitive RGTO-SnO2 Films. Thin Solid Films 1995, 26, 237–331. [Google Scholar] [CrossRef]
- Morar, J.F.; Himpsel, F.J.; Hollinger, G.; Jordan, J.L.; Huges, G.; McFeely, F.R. C 1s excitation studies of diamond (111). I. Surface core levels. Phys. Rev. B 1986, 33, 1340. [Google Scholar] [CrossRef]
- Desimoni, E.; Casella, G.I.; Salvi, A.M.; Cataldi, T.R.I.; Morone, A. XPS investigation of ultra-high-vacuum storage effects on carbon fibre surfaces. Carbon 1992, 30, 527–531. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray photoelectron Spectroscopy; Chastain, J., King, R.C., Jr., Eds.; Physical Electronics, Inc.: Chanhassen, MN, USA, 1995. [Google Scholar]
- Ansell, R.O.; Dickinson, T.; Povey, A.F.; Sherwood, P.M.A. Quantitative use of the angular variation technique in studies of tin by X-ray photoelectron spectroscopy. J. Electron Spectros. Relat. Phenomena 1977, 11, 301–313. [Google Scholar] [CrossRef]
- Yatsimirskii, V.K.; Maksimovich, N.P.; Telegeeva, A.G.; Nikitina, N.V.; Boldyreva, N.A. Semiconductor sensors based on SnO2 with Pt additives and their catalytic activity in oxidation of methane. Theor. Exp. Chem. 2005, 41, 187–191. [Google Scholar] [CrossRef]
- Badrinarayanan, S.; Mandale, A.B.; Gunjikar, V.G.; Sinha, A.P.B. Mechanism of high-temperature oxidation of tin selenide. J. Mater. Sci. 1986, 21, 3333–3338. [Google Scholar] [CrossRef]
- Ibrahim, Z.; Othaman, Z.; Abd Karim, M.M.; Holland, D. X-Ray, Photoemission Spectroscopy (XPS) analysis on platinum doped stannic oxide ceramic. Solid State Technol. 2007, 15, 65–73. [Google Scholar]
- Egdell, R.G.; Eriksen, S.; Flavell, W.R. A spectroscopic study of electron and ion beam reduction of SnO2(110). Surf. Sci. 1987, 192, 265–274. [Google Scholar] [CrossRef]
- Chen, S.; Pan, B.; Zeng, L.; Luo, S.; Wang, X.; Su, W. La2Sn2O7 enhanced photocatalytic CO2 reduction with H2O by deposition of Au co-catalyst. RSC Adv. 2017, 7, 14186–14191. [Google Scholar] [CrossRef] [Green Version]
- Izydorczyk, W. Numerical Analysis of Oxygen Adsorption on SnO2 Surface Using Slab Geometry. Adv. Condens. Matter Phys. 2014, 2014, 957067. [Google Scholar] [CrossRef] [Green Version]
- Rantala, T.S.; Lantto, V. Some effects of mobile donors on electron trapping at semiconductor surfaces. Surf. Sci. 1996, 352, 765–770. [Google Scholar] [CrossRef]
- Sanjines, R.; Levy, F.; Demarne, V.; Grisel, A. Some aspects of the interaction of oxygen with polycrystalline SnOx thin films. Sens. Actuators B Chem. 1990, 1, 176–182. [Google Scholar] [CrossRef]
- Becker, T.; Ahlers, S.; Bosh-v.Braunmuhl, C.; Müller, G.; Kiesewetter, O. Gas sensing properties of thin- and thick-film tin-oxide materials. Sens. Actuators B Chem. 2001, 77, 55–61. [Google Scholar] [CrossRef]




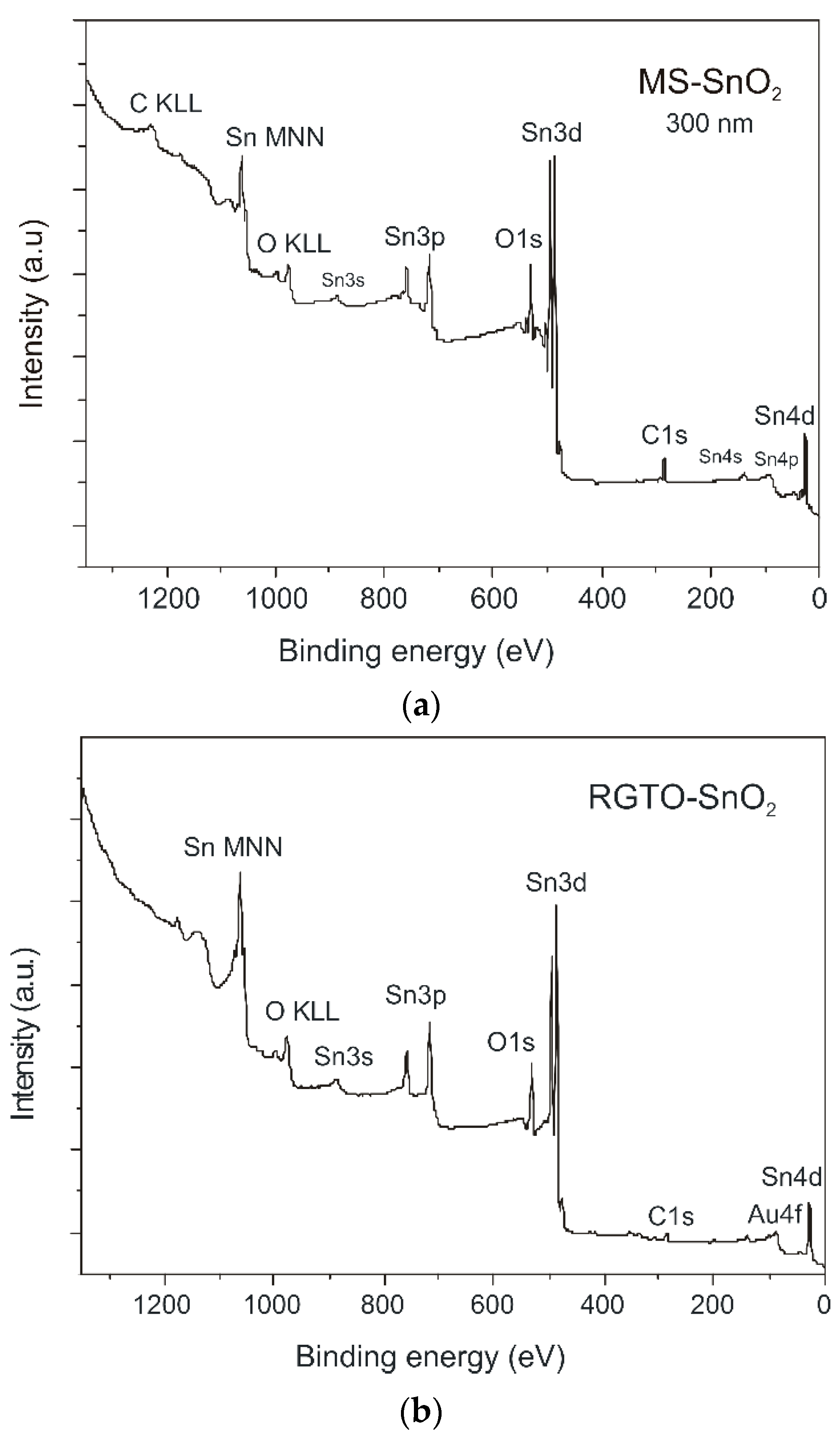
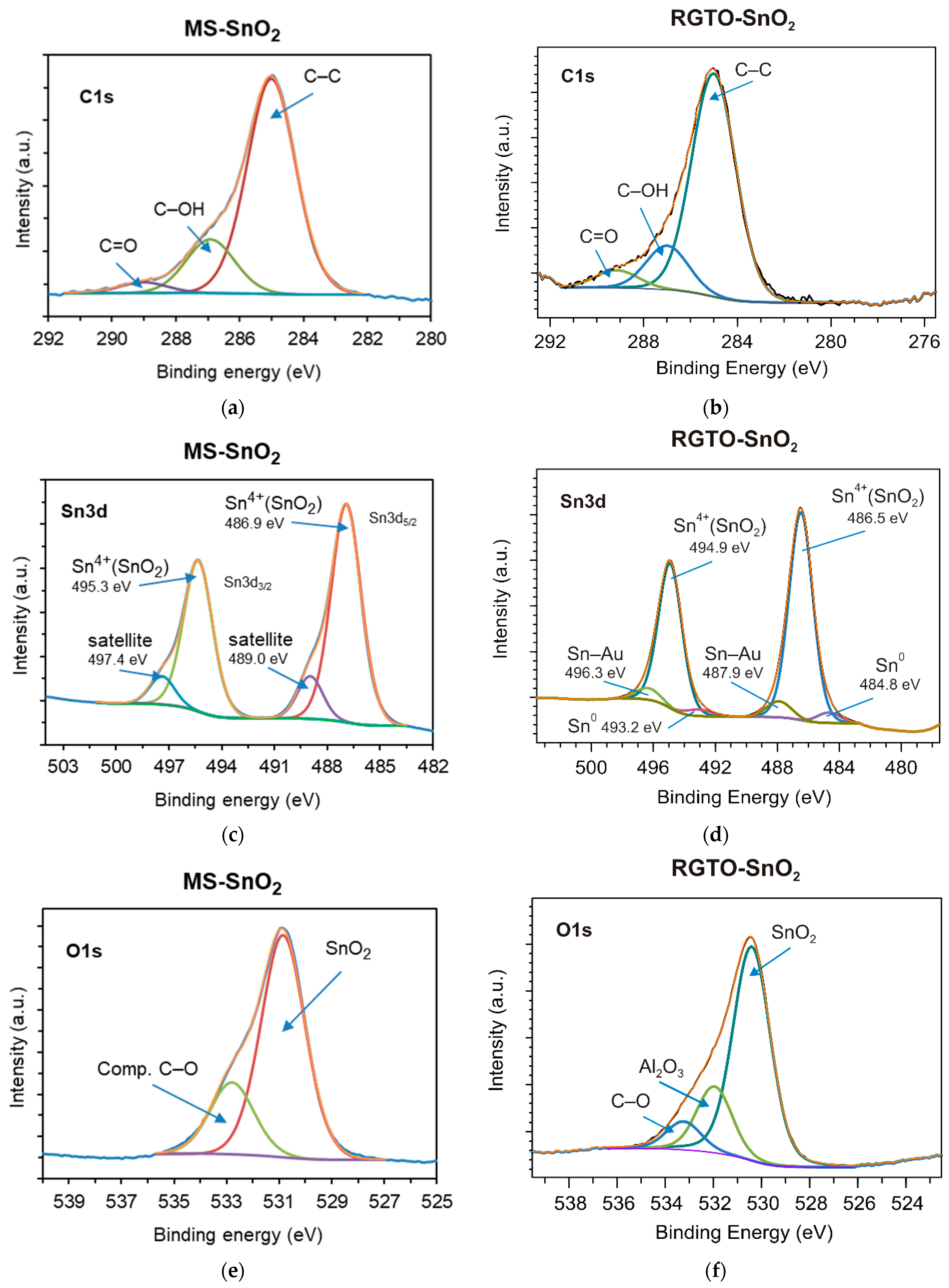
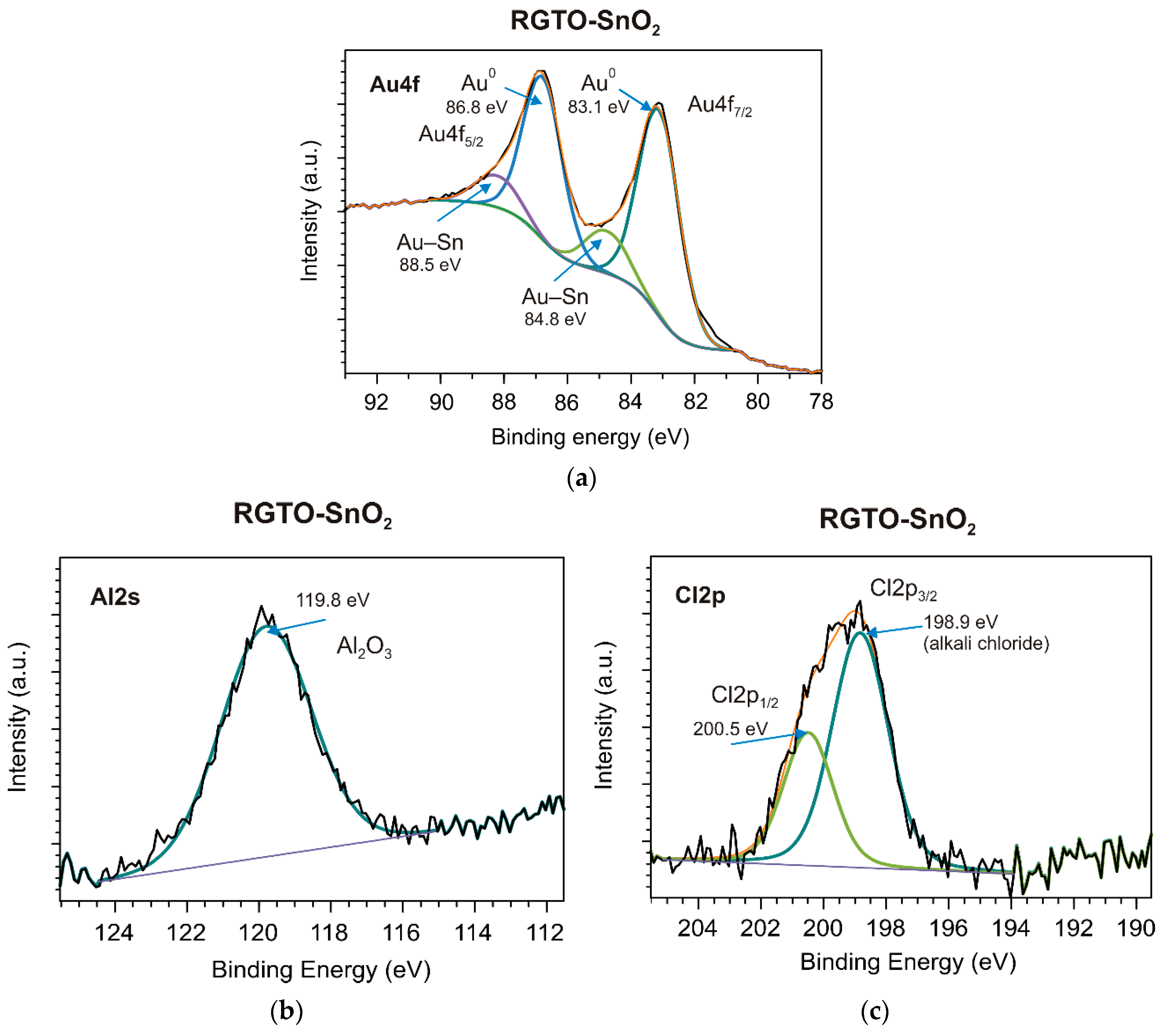


| Layer | Sn3d5 (eV) | C1s (eV) | O1s (eV) | Al2s (eV) | Au4f7 (eV) | Cl2p3 (eV) | Chemical State | Chemical Composition at (%) |
|---|---|---|---|---|---|---|---|---|
| (a) SnO2/ SiO2 | 486.9 - - - | - 285.0 286.9 289.0 | 530.9 - 532.8 - | - - - - | - - - - | - - - - | SnO2 C–C comp. (C, O, H) organic comp. (C, O) | O (46.4) C (32.3) Sn (21.3) |
| (b) SnO2/ Al2O3 | 486.5 - - - - - - - - 484.8 487.9 | - 285.0 - 287.0 289.1 - - - - - - | 530.4 - 532.0 533.2 - - - - - - - | - - - - - 119.8 - - - - - | - - - - - - - 83.1 84.8 - - | - - - - - - 198.9 - - - - | SnO2 C–C metallic oxide (Al, O) comp. (C, O, H) organic comp. (C, O) Al with O (Al2O3) alkali chloride Au Au with Sn Sn0 Sn–Au | O (44.9) Sn (25.3) C (21.5) Al (5.2) Au (1.6) Cl (1.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izydorczyk, W.; Izydorczyk, J. Structure, Surface Morphology, Chemical Composition, and Sensing Properties of SnO2 Thin Films in an Oxidizing Atmosphere. Sensors 2021, 21, 5741. https://doi.org/10.3390/s21175741
Izydorczyk W, Izydorczyk J. Structure, Surface Morphology, Chemical Composition, and Sensing Properties of SnO2 Thin Films in an Oxidizing Atmosphere. Sensors. 2021; 21(17):5741. https://doi.org/10.3390/s21175741
Chicago/Turabian StyleIzydorczyk, Weronika, and Jacek Izydorczyk. 2021. "Structure, Surface Morphology, Chemical Composition, and Sensing Properties of SnO2 Thin Films in an Oxidizing Atmosphere" Sensors 21, no. 17: 5741. https://doi.org/10.3390/s21175741
APA StyleIzydorczyk, W., & Izydorczyk, J. (2021). Structure, Surface Morphology, Chemical Composition, and Sensing Properties of SnO2 Thin Films in an Oxidizing Atmosphere. Sensors, 21(17), 5741. https://doi.org/10.3390/s21175741







