Assessment of the Use of Multi-Channel Organic Electrodes to Record ENG on Small Nerves: Application to Phrenic Nerve Burst Detection
Abstract
1. Introduction
2. Methods
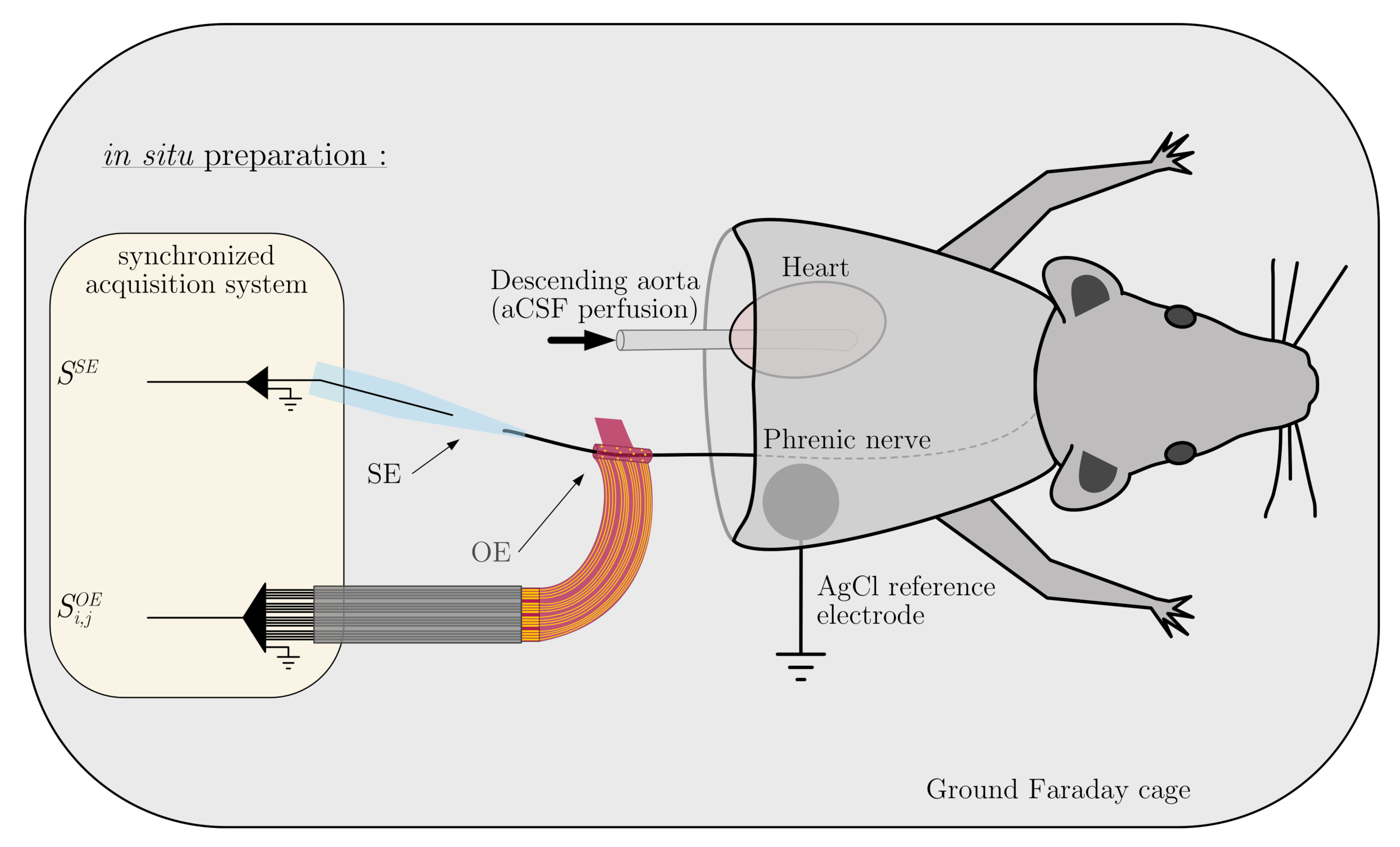
2.1. In Situ Preparation
2.2. Organic Electrode for ENG Recording
2.3. Electrochemical Impedance Spectroscopy
2.4. Nerve Recordings
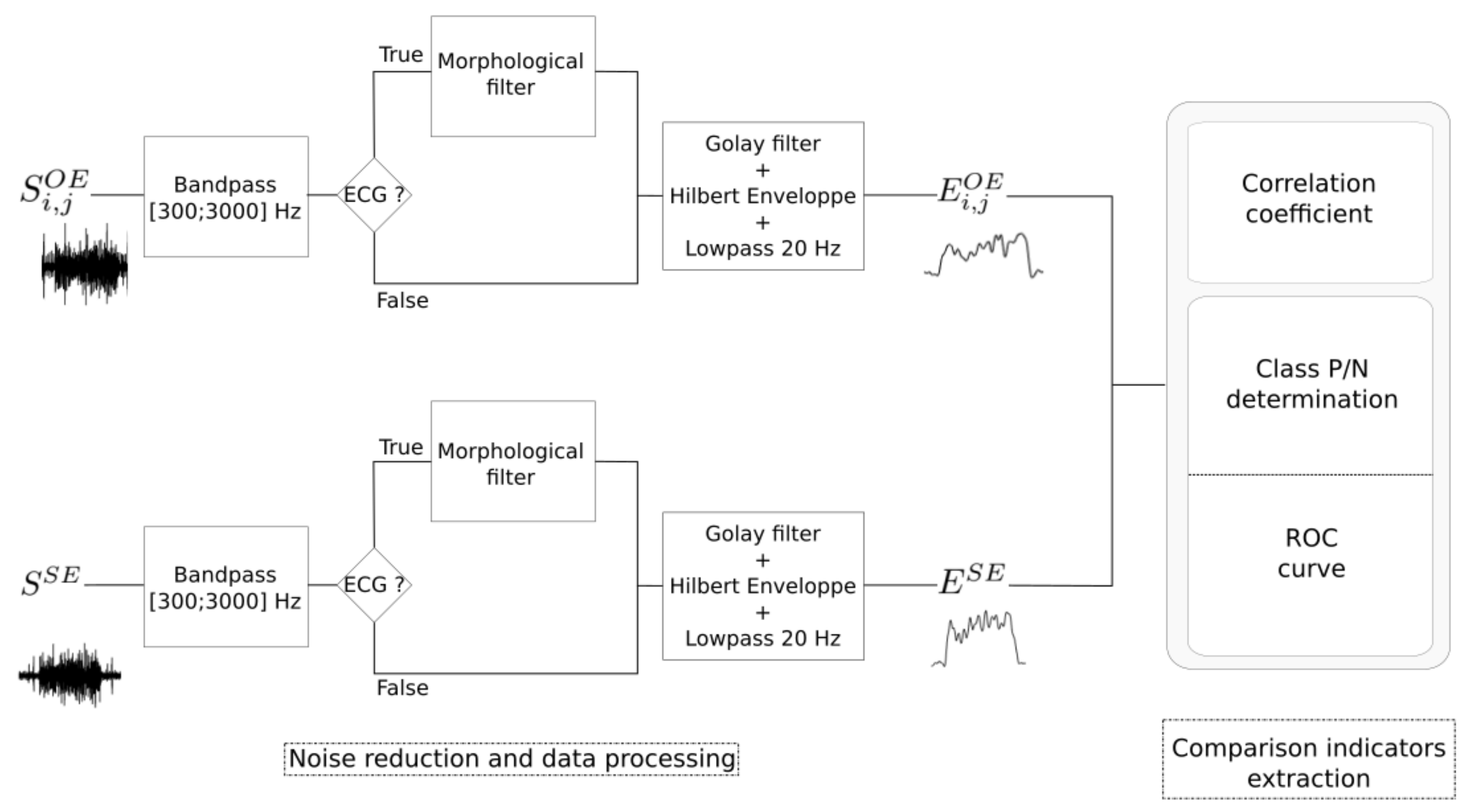
2.5. Data Processing
2.5.1. Noise Reduction and Envelope Detection
2.5.2. Performance Indicators
3. Results
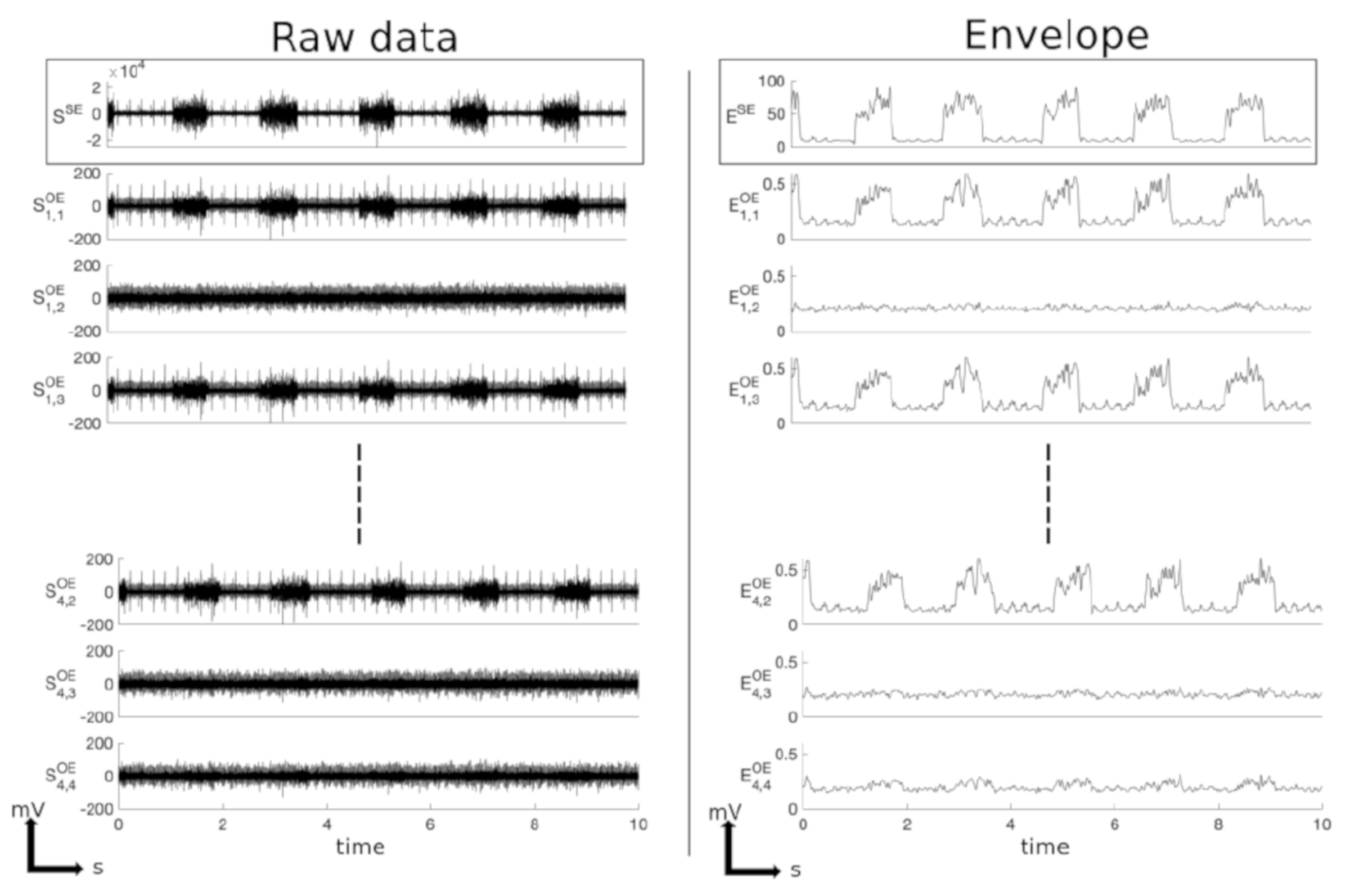
3.1. Example of Data Processing and Analysis
3.2. Global Results
4. Discussion
4.1. Electrophysiological Relevance of PEDOT:PSS
4.2. Suction Electrode for ENG Recording
4.3. Acute ENG Recordings with OE
4.4. Towards a Better Selectivity to Record and Stimulate with OE?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ENG | Electroneurogram |
| OE | Organic electrode |
| pENG | Phrenic electroneurogram |
| PHR | Phrenic nerve |
| ROC | Receiver operating characteristic |
| SE | Suction electrode |
References
- Farrand, A.Q.; Helke, K.L.; Gregory, R.A.; Gooz, M.; Hinson, V.K.; Boger, H.A. Vagus nerve stimulation improves locomotion and neuronalpopulations in a model of Parkinson’s disease. Brain Stimul. 2017, 10, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.A.; Schrader, L.M.; DeGiorgio, C.M.; Miller, P.R.; Maremont, E.R.; Leuchter, A.F. Trigeminal nerve stimulation in major depressive disorder: Acute outcomes in an open pilot study. Epilepsy Behav. 2013, 28, 221–226. [Google Scholar] [CrossRef]
- Milby, A.H.; Halpern, C.H.; Baltuch, G.H. Vagus Nerve Stimulation for Epilepsy and Depression. Neurotherapeutics 2008, 5, 75–85. [Google Scholar] [CrossRef]
- Pop, J.; Murray, D.; Markovic, D.; DeGiorgio, C.M. Acute and long-term safety of external trigeminal nerve stimulation fordrug-resistant epilepsy. Epilepsy Behav. 2011, 22, 574–576. [Google Scholar] [CrossRef]
- Gilja, V.; Nuyujukian, P.; Chestek, C.A.; Cunningham, J.P.; Yu, B.M.; Fan, J.M.; Churchland, M.M.; Kaufman, M.T.; Kao, J.C.; Ryu, S.I.; et al. A high-performance neural prosthesis enabled by control algorithm design. Nat. Neurosci. 2012, 15, 1752–1757. [Google Scholar] [CrossRef]
- Gustafson, K.J.; Pinault, G.C.; Neville, J.J.; Syed, I.; Davis, J.A.; Jean-Claude, J., Jr.; Triolo, R.J. Fascicular anatomy of human femoral nerve: Implications for neural prostheses using nerve cuff electrodes. J. Rehabil. Res. Dev. 2009, 46, 973–984. [Google Scholar] [CrossRef]
- Chakravarthy, K.; Chaudhry, H.; Williams, K.; Christo, P.J. Review of the Uses of Vagal Nerve Stimulation in Chronic Pain Management. Curr. Pain Headache Rep. 2015, 19, 54. [Google Scholar] [CrossRef]
- Ojeda, D.; Rolle, V.L.; Romero-Ugalde, H.M.; Gallet, C.; Bonnet, J.L.; Henry, C.; Bel, A.; Mabo, P.; Carrault, G.; Hernández, A.I. Sensitivity Analysis of Vagus Nerve Stimulation Parameters on Acute Cardiac Autonomic Responses: Chronotropic, Inotropic and Dromotropic Effects. PLoS ONE 2016, 11, e0163734. [Google Scholar] [CrossRef]
- Guiraud, D.; Andreu, D.; Bonnet, S.; Carrault, G.; Couderc, P.; Hagège, A.; Henry, C.; Hernandez, A.; Karam, N.; Rolle, V.L.; et al. Vagus nerve stimulation: State of the art of stimulation and recording strategies to address autonomic function neuromodulation. J. Neural Eng. 2016, 13, 041002. [Google Scholar] [CrossRef][Green Version]
- Romero Ugalde, H.; Rolle, V.; Bonnet, J.L.; Henry, C.; Mabo, P.; Carrault, G.; Hernandez, A. Closed-Loop Vagus Nerve Stimulation Based on State Transition Models. IEEE Trans. Biomed. Eng. 2017, 65, 1630–1638. [Google Scholar] [CrossRef]
- Gallet, C.; Bonnet, S.; Le Rolle, V.; Laporte, L.; Bonnet, J.; Karam, N.; Hagège, A.; Malbert, C.; Mabo, P.; Maubert, S.; et al. Characteristics of the right cervical vagal activity during baseline and Valsalva-like manoeuvre. In Proceedings of the 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpellier, France, 22–24 April 2015; pp. 988–991. [Google Scholar] [CrossRef]
- Tyler, D.; Durand, D. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 2002, 10, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Christie, B.; Freeberg, M.; Memberg, W.; Pinault, G.; Hoyen, H.; Tyler, D.; Triolo, R. Long-term stability of stimulating spiral nerve cuff electrodes on human peripheral nerves. J. Neuroeng. Rehabil. 2017, 14, 70. [Google Scholar] [CrossRef]
- Boretius, T.; Badia, J.; Pascual-Font, A.; Schuettler, M.; Navarro, X.; Yoshida, K.; Stieglitz, T. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens. Bioelectron. 2010, 26, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Ottestad, E.; Orlovich, D.S. History of Peripheral Nerve Stimulation—Update for the 21st Century. Pain Med. 2020, 21, S3–S5. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.; Roche, A.; Chakrabarty, S. Peripheral nerve bionic interface: A review of electrodes. Int. J. Intell. Robot. Appl. 2019, 3, 11–18. [Google Scholar] [CrossRef]
- Sahin, M.; Haxhiu, M.A.; Durand, D.M.; Dreshaj, I.A. Spiral nerve cuff electrode for recordings of respiratory output. J. Appl. Physiol. 1997, 83, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, S.; Dellon, A.; Hudson, A.; Hunter, D. Chronic nerve compression; an experimental model in the rat. Ann. Plast. Surg. 1984, 13, 112–120. [Google Scholar] [CrossRef]
- Mortimer, J.T.; Bhadra, N. Peripheral Nerve And Muscle Stimulation. In Neuroprosthetics: Theory and Practice; World Scientific: Singapore, 2004; pp. 638–682. [Google Scholar] [CrossRef]
- Romero, E.; Denef, J.F.; Delbeke, J.; Robert, A.; Veraart, C. Neural morphological effects of long-term implantation of the self-sizing spiral cuff nerve electrode. Med. Biol. Eng. Comput. 2001, 39, 90–100. [Google Scholar] [CrossRef]
- Cooreman, P.; Thoelen, R.; Manca, J.; vandeVen, M.; Vermeeren, V.; Michiels, L.; Ameloot, M.; Wagner, P. Impedimetric immunosensors based on the conjugated polymer PPV. Biosens. Bioelectron. 2005, 20, 2151–2156. [Google Scholar] [CrossRef]
- Bettinger, C. Recent advances in materials and flexible electronics for peripheral nerve interfaces. Bioelectron. Med. 2018, 4, 6. [Google Scholar] [CrossRef]
- Yoo, P.B.; Sahin, M.; Durand, D.M. Selective stimulation of the canine hypoglossal nerve using a multi-contact cuff electrode. Ann. Biomed. Eng. 2004, 32, 511–519. [Google Scholar] [CrossRef]
- Khodagholy, D.; Doublet, T.; Gurfinkel, M.; Quilichini, P.; Ismailova, E.; Leleux, P.; Hervé, T.; Sanaur, S.; Bernard, C.; Malliaras, G. Highly Conformable Conducting Polymer Electrodes for In Vivo Recordings. Adv. Mater. 2011, 23, H268–H272. [Google Scholar] [CrossRef] [PubMed]
- Berggren, M.; Richter-Dahlfors, A. Organic Bioelectronics. Adv. Mater. 2007, 19, 3201–3213. [Google Scholar] [CrossRef]
- Williamson, A.; Rivnay, J.; Kergoat, L.; Jonsson, A.; Inal, S.; Uguz, I.; Ferro, M.; Ivanov, A.; Sjöström, T.A.; Simon, D.T.; et al. Controlling Epileptiform Activity with Organic Electronic Ion Pumps. Adv. Mater. 2015, 27, 3138–3144. [Google Scholar] [CrossRef]
- Kergoat, L.; Dieuset, G.; Le Rolle, V.; Malliaras, G.; Martin, B.; Bernard, C.; Hernandez, A. PEDOT:PSS electrodes for acute experimental evaluation of vagus nerve stimulation on rodents. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 4760–4763. [Google Scholar] [CrossRef]
- Decataldo, F.; Cramer, T.; Martelli, D.; Gualandi, I.; Korim, W.; Yao, S.; Tessarolo, M.; Murgia, M.; Scavetta, E.; Amici, R.; et al. Stretchable Low Impedance Electrodes for Bioelectronic Recording from Small Peripheral Nerves. Sci. Rep. 2019, 9, 10598. [Google Scholar] [CrossRef]
- Rodger, D.; Fong, A.; Li, W.; Ameri, H.; Ahuja, A.; Gutierrez, C.; Lavrov, I.; Zhong, H.; Menon, P.; Meng, E.; et al. Flexible parylene-based multielectrode array technology for high-density neural stimulation and recording. Sens. Actuators B Chem. 2008, 132, 449–460. [Google Scholar] [CrossRef]
- Takeuchi, S.; Ziegler, D.; Yoshida, Y.; Mabuchi, K.; Suzuki, T. Parylene flexible neural probes integrated with microfluidic channels. Lab Chip 2005, 5, 519–523. [Google Scholar] [CrossRef]
- Lee, D.; Kim, S.; Kwon, E.; Park, C.; Jun, S.; Choi, B.; Kim, S. Comparison of in vivo biocompatibilities between parylene-C and polydimethylsiloxane for implantable microelectronic devices. Bull. Mater. Sci. 2013, 36, 1127–1132. [Google Scholar] [CrossRef]
- Dutschmann, M.; Kron, M.; Mörschel, M.; Gestreau, C. Activation of Orexin B receptors in the pontine Kölliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir. Physiol. Neurobiol. 2007, 159, 232–235. [Google Scholar] [CrossRef]
- Wang, S.; Benamer, N.; Zanella, S.; Kumar, N.N.; Shi, Y.; Bévengut, M.; Penton, D.; Guyenet, P.G.; Lesage, F.; Gestreau, C.; et al. TASK-2 Channels Contribute to pH Sensitivity of Retrotrapezoid Nucleus Chemoreceptor Neurons. J. Neurosci. 2013, 33, 16033–16044. [Google Scholar] [CrossRef]
- Paton, J.F. A working heart-brainstem preparation of the mouse. J. Neurosci. Methods 1996, 65, 63–68. [Google Scholar] [CrossRef]
- Khodagholy, D.; Doublet, T.; Quilichini, P.; Gurfinkel, M.; Leleux, P.; Ghestem, A.; Ismailova, E.; Hervé, T.; Sanaur, S.; Bernard, C.; et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 2013, 4, 1575. [Google Scholar] [CrossRef]
- Donahue, M.J.; Kaszas, A.; Turi, G.F.; Rózsa, B.; Slézia, A.; Vanzetta, I.; Katona, G.; Bernard, C.; Malliaras, G.G.; Williamson, A. Multimodal Characterization of Neural Networks Using Highly Transparent Electrode Arrays. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Morel, G.L. Feasibility and Advantages of Real-Time Monitoring of Diaphragmatic Ventilatory Fatigue in Anesthesiology and in Intensive Care Unit. Ph.D. Thesis, Université Jean Monnet Saint-Etienne, Saint-Etienne, France, 2014. [Google Scholar]
- Raspopovic, S.; Carpaneto, J.; Udina, E.; Navarro, X.; Micera, S. On the identification of sensory information from mixed nerves by using single-channel cuff electrodes. J. Neuroeng. Rehabil. 2010, 7, 17. [Google Scholar] [CrossRef]
- Gestreau, C.; Dutschmann, M.; Obled, S.; Bianchi, A. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir. Physiol. Neurobiol. 2005, 147, 159–176. [Google Scholar] [CrossRef]
- Bergé-Laval, V.; Gestreau, C. Quipazine Elicits Swallowing in the Arterially Perfused Rat Preparation: A Role for Medullary Raphe Nuclei? Int. J. Mol. Sci. 2020, 21, 5120. [Google Scholar] [CrossRef]
- Proctor, C.M.; Rivnay, J.; Malliaras, G.G. Understanding volumetric capacitance in conducting polymers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1433–1436. [Google Scholar] [CrossRef]
- Dijk, G.; Ruigrok, H.J.; O’Connor, R.P. Influence of PEDOT:PSS Coating Thickness on the Performance of Stimulation Electrodes. Adv. Mater. Interfaces 2020, 7, 2000675. [Google Scholar] [CrossRef]
- Rivnay, J.; Wang, H.; Fenno, L.; Deisseroth, K.; Malliaras, G.G. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 2017, 3, e1601649. [Google Scholar] [CrossRef]
- Venkatraman, S.; Hendricks, J.; King, Z.A.; Sereno, A.J.; Richardson-Burns, S.; Martin, D.; Carmena, J.M. In Vitro and In Vivo Evaluation of PEDOT Microelectrodes for Neural Stimulation and Recording. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 307–316. [Google Scholar] [CrossRef]
- Hashimoto, K.; Sugiyama, Y.; Fuse, S.; Umezaki, T.; Oku, Y.; Dutschmann, M.; Hirano, S. Activity of swallowing-related neurons in the medulla in the perfused brainstem preparation in rats: Swallowing-Related Neurons in Perfused Rats. Laryngoscope 2018, 129, E72–E79. [Google Scholar] [CrossRef]
- Fuse, S.; Sugiyama, Y.; Hashimoto, K.; Umezaki, T.; Oku, Y.; Dutschmann, M.; Hirano, S. Laryngeal afferent modulation of swallowing interneurons in the dorsal medulla in perfused rats. Laryngoscope 2020, 130, 1885–1893. [Google Scholar] [CrossRef]
- Baekey, D.; Morris, K.; Gestreau, C.; Li, Z.; Lindsey, B.; Shannon, R. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. J. Physiol. 2001, 534, 565–581. [Google Scholar] [CrossRef]
- Ott, M.; Nuding, S.; Segers, L.; O’Connor, R.; Morris, K.; Lindsey, B. Central chemoreceptor modulation of breathing via multipath tuning in medullary ventrolateral respiratory column circuits. J. Neurophysiol. 2012, 107, 603–617. [Google Scholar] [CrossRef]
- Parker, J.L.; Cameron, T. Technology for Peripheral Nerve Stimulation. In Progress in Neurological Surgery; S. Karger AG: Basel, Switzerland, 2016; pp. 1–19. [Google Scholar] [CrossRef]
- Rodrigues, A.; Ferreira, R.; Salgado, H.; Fazan, V. Morphometric analysis of the phrenic nerve in male and female Wistar-Kyoto (WKY) and spontaneously hypertensive rats (SHR). Braz. J. Med. Biol. Res. 2011, 44, 583–591. [Google Scholar] [CrossRef]
- Green, R.; Lovell, N.; Wallace, G.; Poole-Warren, L. Conducting polymers for neural interfaces: Challenges in developing an effective long-term implant. Biomaterials 2008, 29, 3393–3399. [Google Scholar] [CrossRef]
- Williamson, A.; Ferro, M.; Leleux, P.; Ismailova, E.; Kaszas, A.; Doublet, T.; Quilichini, P.; Rivnay, J.; Rózsa, B.; Katona, G.; et al. Localized Neuron Stimulation with Organic Electrochemical Transistors on Delaminating Depth Probes. Adv. Mater. 2015, 27, 4405–4410. [Google Scholar] [CrossRef]
- Jonsson, A.; Inal, S.; Uguz, L.; Williamson, A.; Kergoat, L.; Rivnay, J.; Khodagholy, D.; Berggren, M.; Bernard, C.; Malliaras, G.; et al. Bioelectronic neural pixel: Chemical stimulation and electrical sensing at the same site. Proc. Natl. Acad. Sci. USA 2016, 113, 9440–9445. [Google Scholar] [CrossRef]
- Rivnay, J.; Leleux, P.; Ferro, M.; Sessolo, M.; Williamson, A.; Koutsouras, D.; Khodagholy, D.; Ramuz, M.; Strakosas, X.; Owens, R.; et al. High-performance transistors for bioelectronics through tuning of channel thickness. Sci. Adv. 2015, 1, e1400251. [Google Scholar] [CrossRef]
- Khodagholy, D.; Gelinas, J.; Thesen, T.; Doyle, W.; Devinsky, O.; Malliaras, G.; Buzsáki, G. NeuroGrid: Recording action potentials from the surface of the brain. Nat. Neurosci. 2014, 18, 310–315. [Google Scholar] [CrossRef]
- Bautista, T.; Dutschmann, M. Ponto-medullary nuclei involved in the generation of sequential pharyngeal swallowing and concomitant protective laryngeal adduction in situ. J. Physiol. 2014, 592, 2605–2623. [Google Scholar] [CrossRef]
- Chen, S.P.; Ay, I.; Morais, A.; Qin, T.; Zheng, Y.; Sadhegian, H.; Oka, F.; Simon, B.; Eikermann-Haerter, K.; Ayata, C. Vagus nerve stimulation inhibits cortical spreading depression. Pain 2015, 157, 797–805. [Google Scholar] [CrossRef]
- Yagi, M.; Morishita, K.; Ueno, A.; Nakamura, H.; Akabori, H.; Senda, A.; Kojima, M.; Aiboshi, J.; Costantini, T.; Coimbra, R.; et al. Electrical stimulation of the vagus nerve improves intestinal blood flow after trauma and hemorrhagic shock. Surgery 2019, 167, 638–645. [Google Scholar] [CrossRef]
- Bucksot, J.E.; Wells, A.J.; Rahebi, K.C.; Sivaji, V.; Romero-Ortega, M.; Kilgard, M.P.; Rennaker, R.L., II; Hays, S.A. Flat electrode contacts for vagus nerve stimulation. PLoS ONE 2019, 14, e0215191. [Google Scholar] [CrossRef]
- Dijk, G.; Rutz, A.L.; Malliaras, G.G. Stability of PEDOT:PSS-Coated Gold Electrodes in Cell Culture Conditions. Adv. Mater. Technol. 2020, 5, 1900662. [Google Scholar] [CrossRef]
- Oldroyd, P.; Malliaras, G. Achieving long-term stability of thin-film electrodes for neurostimulation. Acta Biomater. 2021, in press. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef]
- Lecomte, A.; Degache, A.; Descamps, E.; Dahan, L.; Bergaud, C. In vitro and in vivo biostability assessment of chronically-implanted Parylene C neural sensors. Sens. Actuators B Chem. 2017, 251, 1001–1008. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, G.; Cai, Z.; Jiao, B.; Zhao, Y.; Li, S.; Luo, A. Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci. Biobehav. Rev. 2021, 127, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Senova, S.; Rabu, C.; Beaumont, S.; Michel, V.; Palfi, S.; Mallet, L.; Domenech, P. Stimulation du nerf vague dans le traitement de la dépression. La Presse Médicale 2019, 48, 1507–1519. [Google Scholar] [CrossRef]
- Urits, I.; Schwartz, R.; Smoots, D.; Koop, L.; Veeravelli, S.; Orhurhu, V.; Cornett, E.; Manchikanti, L.; Kaye, A.; Imani, F.; et al. Peripheral Neuromodulation for the Management of Headache. Anesthesiol. Pain Med. 2020, in press. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Mantilla, C.B.; Sieck, G.C. Breathing: Motor Control of Diaphragm Muscle. Physiology 2018, 33, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Gottschall, J. The diaphragm of the rat and its innervation. Muscle fiber composition; perikarya and axons of efferent and afferent neurons. Anat. Embryol. 2004, 161, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Ueno, A.; Katayama, N.; Karashima, A.; Nakao, M. Improvement of Diameter Selectivity in Nerve Recruitment Using Multi-cuff Electrodes. Adv. Biomed. Eng. 2012, 1, 36–42. [Google Scholar] [CrossRef]
- Fontanesi, L.B.; Fazan, F.S.; Dias, F.J.; Schiavoni, M.C.L.; Marques Jr, W.; Fazan, V.P.S. Sensory and Motor Conduction Velocity in Spontaneously Hypertensive Rats: Sex and Aging Investigation. Front. Syst. Neurosci. 2019, 13, 62. [Google Scholar] [CrossRef]
- Donaldson, N.; Rieger, R.; Schuettler, M.; Taylor, J. Noise and selectivity of velocity-selective multi-electrode nerve cuffs. Med. Biol. Eng. Comput. 2008, 46, 1005–1018. [Google Scholar] [CrossRef]



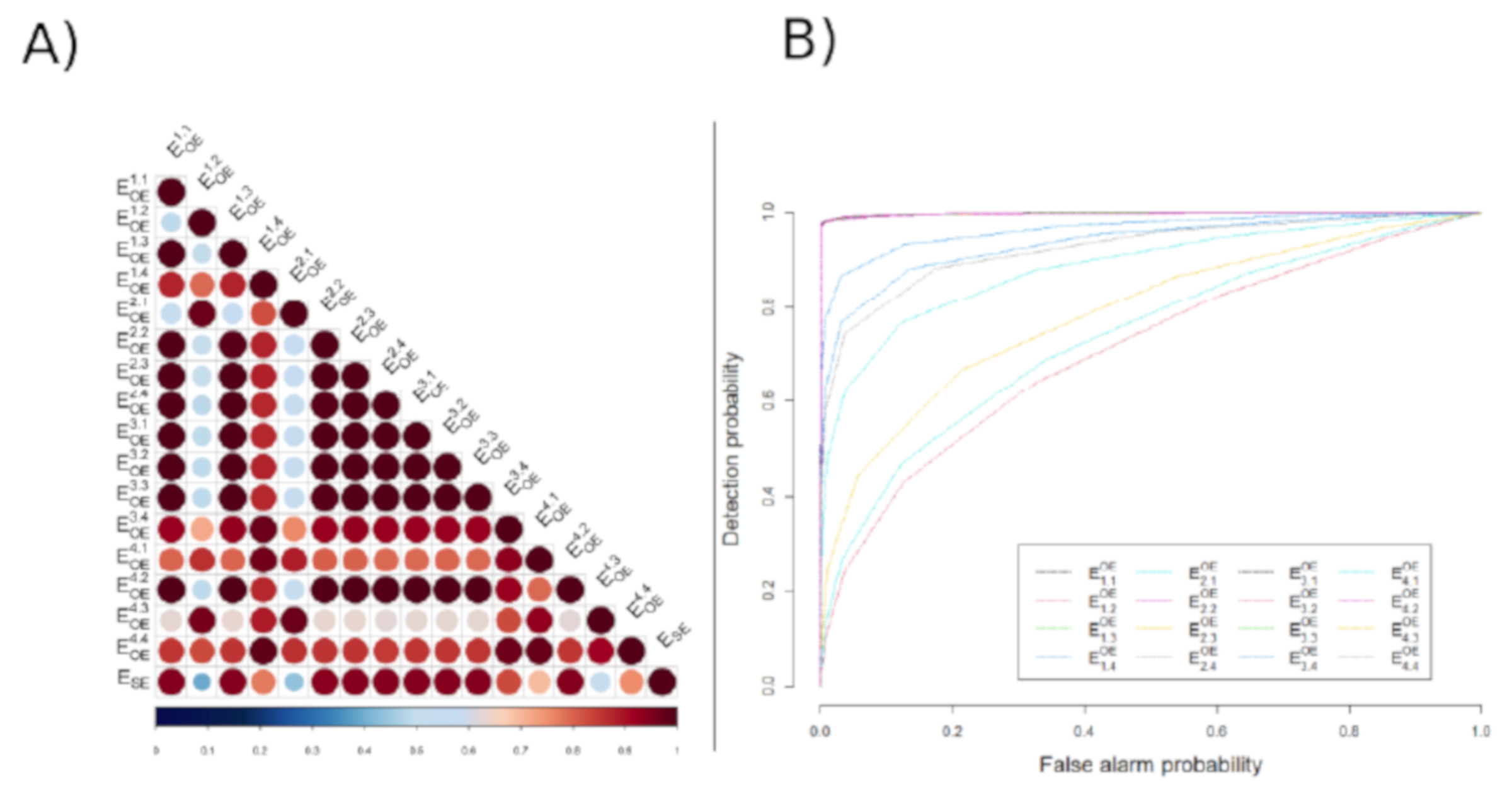
| Channels | 1,1 | 1,2 | 1,3 | 1,4 | 2,1 | 2,2 | 2,3 | 2,4 |
| Correlation | 0.934 | 0.391 | 0.933 | 0.768 | 0.439 | 0.927 | 0.932 | 0.933 |
| AUC | 0.994 | 0.708 | 0.994 | 0.929 | 0.734 | 0.992 | 0.994 | 0.993 |
| SNR (dB) | 8.705 | 0.678 | 8.574 | 2.278 | 0.745 | 7.188 | 8.396 | 8.758 |
| 3,1 | 3,2 | 3,3 | 3,4 | 4,1 | 4,2 | 4,3 | 4,4 | Mean ± STD |
| 0.934 | 0.934 | 0.933 | 0.821 | 0.686 | 0.935 | 0.523 | 0.748 | 0.798 ± 0.191 |
| 0.994 | 0.994 | 0.994 | 0.958 | 0.879 | 0.994 | 0.783 | 0.918 | 0.925 ± 0.072 |
| 8.811 | 8.701 | 8.861 | 2.974 | 1.611 | 8.874 | 0.980 | 2.063 | 5.510 ± 3.61 |
| Rat | Chan. | Corr. Mean | Corr. Interval | Corr. std | AUC Mean | AUC Interval | AUC std |
|---|---|---|---|---|---|---|---|
| 1 | 16 | 0.798 | [0.391;0.935] | 0.191 | 0.925 | [0.708;0.994] | 0.072 |
| 2 | 3 | 0.872 | [0.858;0.879] | 0.012 | 0.998 | [0.998;0.999] | |
| 3 | 8 | 0.661 | [0.047;0.997] | 0.175 | 0.860 | [0.557;0.967] | 0.3553 |
| 4 | 8 | 0.398 | [0.097;0.960] | 0.275 | 0.816 | [0.628;0.999] | 0.1207 |
| 5 | 8 | 0.656 | [0.166;0.940] | 0.297 | 0.908 | [0.648;0.999] | 0.1600 |
| 6 | 8 | 0.549 | [0.451;0.945] | 0.169 | 0.825 | [0.758;0.999] | 0.0769 |
| 7 | 16 | 0.582 | [0.505;0.702] | 0.052 | 0.867 | [0.819;0.943] | 0.0337 |
| 8 | 8 | 0.954 | [0.915;0.995] | 0.042 | 0.999 | [0.996;1] | |
| 9 | 3 | 0.972 | [0.969;0.973] | 0.003 | 0.999 | [0.998;0.998] | |
| Mean | 0.736 | [0.610;0.923] | 0.135 | 0.900 | [0.852;0.989] | 0.091 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdeew, Y.; Bergé-Laval, V.; Le Rolle, V.; Dieuset, G.; Moreau, D.; Kergoat, L.; Martin, B.; Bernard, C.; Gestreau, C.; Hernández, A. Assessment of the Use of Multi-Channel Organic Electrodes to Record ENG on Small Nerves: Application to Phrenic Nerve Burst Detection. Sensors 2021, 21, 5594. https://doi.org/10.3390/s21165594
Avdeew Y, Bergé-Laval V, Le Rolle V, Dieuset G, Moreau D, Kergoat L, Martin B, Bernard C, Gestreau C, Hernández A. Assessment of the Use of Multi-Channel Organic Electrodes to Record ENG on Small Nerves: Application to Phrenic Nerve Burst Detection. Sensors. 2021; 21(16):5594. https://doi.org/10.3390/s21165594
Chicago/Turabian StyleAvdeew, Yvan, Victor Bergé-Laval, Virginie Le Rolle, Gabriel Dieuset, David Moreau, Loïg Kergoat, Benoît Martin, Christophe Bernard, Christian Gestreau, and Alfredo Hernández. 2021. "Assessment of the Use of Multi-Channel Organic Electrodes to Record ENG on Small Nerves: Application to Phrenic Nerve Burst Detection" Sensors 21, no. 16: 5594. https://doi.org/10.3390/s21165594
APA StyleAvdeew, Y., Bergé-Laval, V., Le Rolle, V., Dieuset, G., Moreau, D., Kergoat, L., Martin, B., Bernard, C., Gestreau, C., & Hernández, A. (2021). Assessment of the Use of Multi-Channel Organic Electrodes to Record ENG on Small Nerves: Application to Phrenic Nerve Burst Detection. Sensors, 21(16), 5594. https://doi.org/10.3390/s21165594







