Influence of Atmosphere on Calibration of Radiation Thermometers
Abstract
1. Introduction
1.1. Significance of the Research
1.2. Existing Works and Research
2. Materials and Methods
2.1. Calculation of Moist Air–Gas Ratios
2.2. Dry Air Composition
2.3. Spectral Transmissivity Calculation
2.4. Compiling of the MATLAB Model
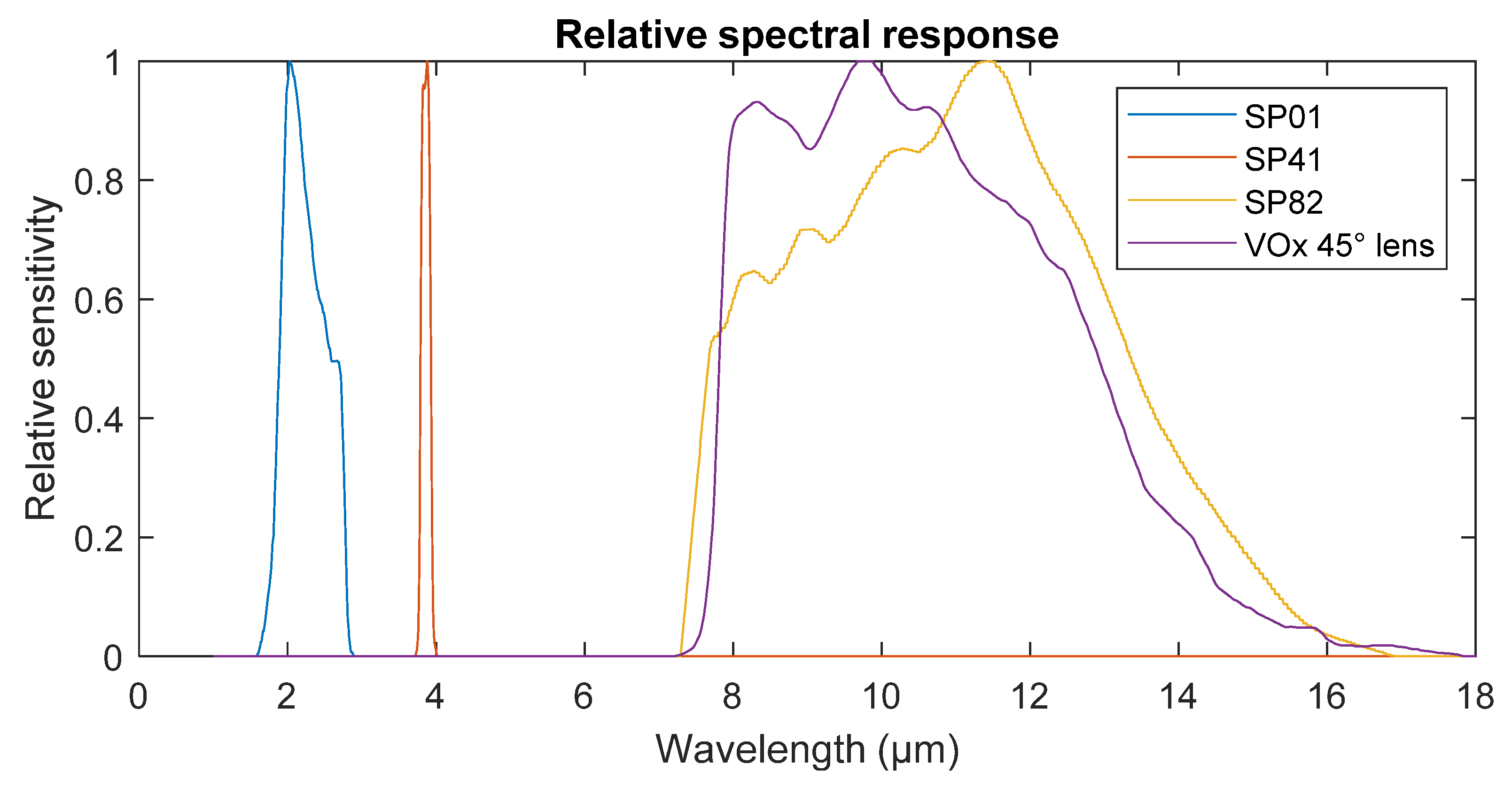
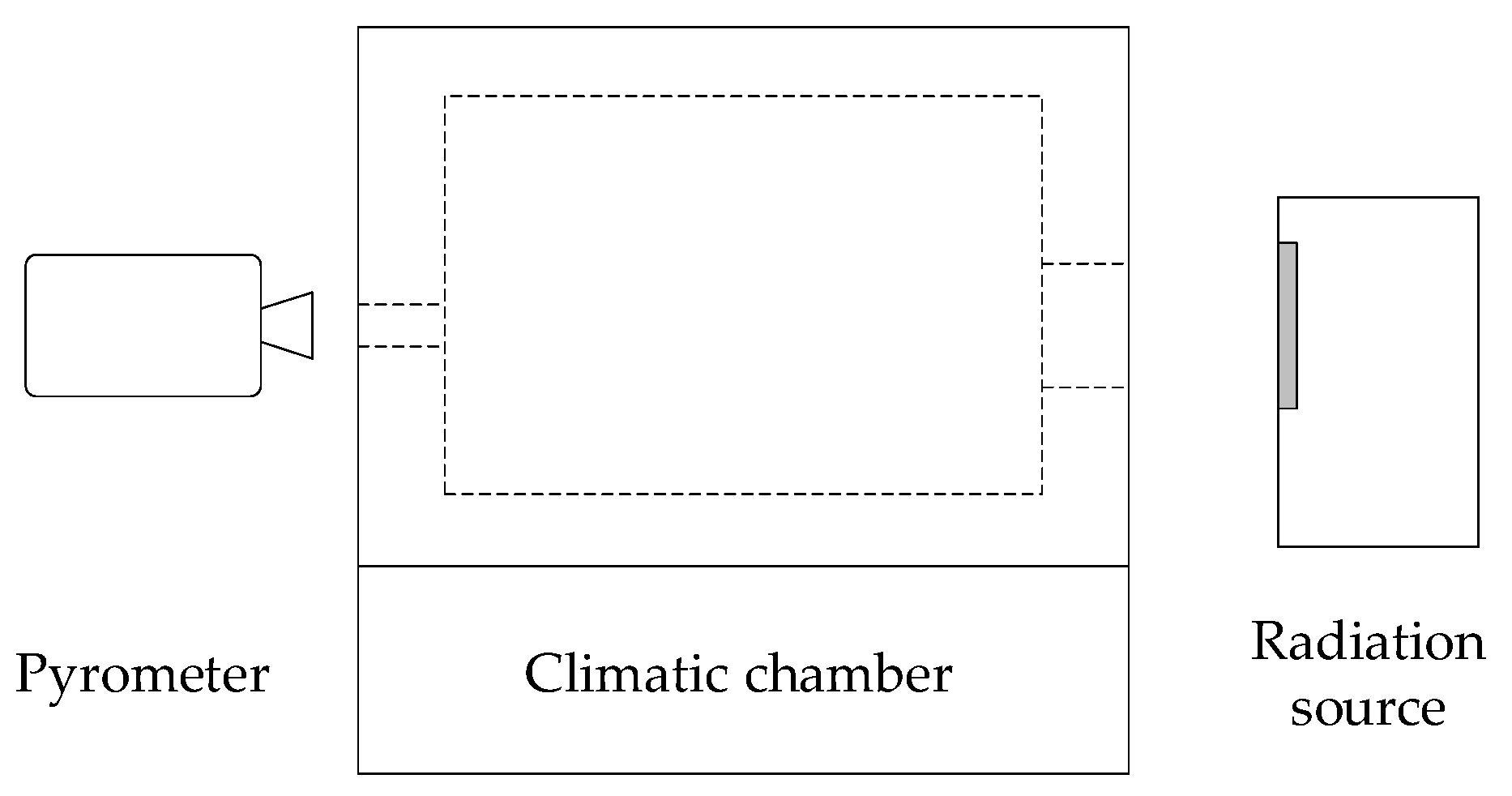
2.5. Comparison with Experimental Results
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elder, T.; Strong, J. The infrared transmission of atmospheric windows. J. Frankl. Inst. 1953, 255, 189–208. [Google Scholar] [CrossRef]
- Wyatt, P.J.; Stull, V.R.; Plass, G.N. The Infrared Transmittance of Water Vapor. Appl. Opt. 1964, 3, 229–241. [Google Scholar] [CrossRef]
- Burch, D.E.; Singleton, E.B.; France, W.L.; Williams, D. Infrared Absorption by Carbon Dioxide, Water Vapor, and Minor Atmospheric Constituents; The Ohio State University Research Foundation: Cambridge, MA, USA, 1960. [Google Scholar]
- Zhang, H.; Wang, C.; Li, X.; Sun, B.; Jiang, D. Design and Implementation of an Infrared Radiant Source for Humidity Testing. Sensors 2018, 18, 3088. [Google Scholar] [CrossRef] [PubMed]
- Kneizys, F.X.; Shettle, E.P.; Abreu, L.W.; Chetwynd, J.H.; Anderson, G.P.; Gallery, W.O.; Selby, J.E.A.; Clough, S.A. Users Guide to LOWTRAN 7; U.S. Government Printing Office: Hanscom, MA, USA, 1988. [Google Scholar]
- Kneizys, F.X.; Abreu, L.W.; Shettle, E.P.; Chetwynd, J.H.; Anderson, G.P.; Berk, A.; Bernstein, L.S.; Robertson, D.C.; Acharya, P.; Rothman, L.S.; et al. The MODTRAN 2/3 Report and LOWTRAN 7 MODEL; Ontar Corp.: North Andover, MA, USA, 1996. [Google Scholar]
- Chen, X.; Wei, H. A Combined Atmospheric Radiative Transfer Model (CART): A Review and Applications. J. Opt. Soc. Korea 2010, 14, 190–198. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hill, C.; Kochanov, R.V.; Tan, Y.; Bernath, P.F.; Birk, M.; Boudon, V.; Campargue, A.; Chance, K.V.; et al. The HITRAN2016 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 3–69. [Google Scholar] [CrossRef]
- Karman, T.; Gordon, I.E.; van der Avoird, A.; Baranov, Y.I.; Boulet, C.; Drouin, B.J.; Groenenboom, G.C.; Gustafsson, M.; Hartmann, J.-M.; Kurucz, R.L.; et al. Update of the HITRAN collision-induced absorption section. Icarus 2019, 328, 160–175. [Google Scholar] [CrossRef]
- Hartmann, J.M.; Boulet, C.; Toon, G.C. Collision-induced absorption by N2 near 2.16 μm: Calculations, model and consequences for atmospheric remote sensing. J. Geophys. Res. Atmos. 2019, 122, 2419–2428. [Google Scholar] [CrossRef]
- Picard, A.; Davis, R.; Gläser, M.; Fujii, K. Revised formula for the density of moist air (CIPM-2007). Metrologia 2008, 45, 149–155. [Google Scholar] [CrossRef]
- Hardy, B. ITS-90 formulations for vapor pressure, frostpoint temperature, dewpoint temperature, and enhancement factors in the range −100 to +100 C. In Proceedings of the Third International Symposium on Humidity & Moisture, Teddington, London, UK, 6 April 1998. [Google Scholar]
- Krogh, A. The Composition of the Atmosphere; Royal Danish Academy of Sciences and Letters: Copenhagen, Denmark, 1919; Volume 12. [Google Scholar]
- Paneth, F.A. The chemical composition of the atmosphere. Q. J. R. Meteorol. Soc. 1937, 63, 433–443. [Google Scholar] [CrossRef]
- Giacomo, P. Equation for the Determination of the Density of Moist Air. Metrologia 1982, 18, 33–40. [Google Scholar] [CrossRef]
- Keeling, R.F. Measuring correlations between atmospheric oxygen and carbon dioxide mole fractions: A preliminary study in urban air. J. Atmos. Chem. 1988, 7, 153–176. [Google Scholar] [CrossRef]
- Gatley, D.P.; Herrmann, S.; Kretzschmar, H.-J. A Twenty-First Century Molar Mass for Dry Air. HVAC&R Res. 2008, 14, 655–662. [Google Scholar] [CrossRef]
- Ginzburg, A.S.; Vinogradova, A.A.; Fedorova, E.I.; Nikitich, E.V.; Karpov, A.V. Content of oxygen in the atmosphere over large cities and respiratory problems. Izv. Atmos. Ocean. Phys. 2014, 50, 782–792. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.S.; Lee, J.B.; Esler, M.B.; Davis, R.S.; Wielgosz, R.I. A redetermination of the argon content of air for buoyancy corrections in mass standard comparisons. Metrologia 2004, 41, 387–395. [Google Scholar] [CrossRef]
- Schlatter, T. Atmospheric Composition and Vertical Structure. Environ. Impact Manuf. 2009, 6, 1–54. [Google Scholar]
- Dlugokencky, E. NOAA/GML. 4 May 2021. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/global.html (accessed on 26 March 2021).
- United States Environmental Protection Agency. Status and Trends of Key Air Pollutants. 2019. Available online: https://www.epa.gov/air-trends (accessed on 6 May 2021).
- Williams, D.R. NASA Earth Fact Sheet. NASA Goddart Space Flight Center. 25 November 2020. Available online: https://nssdc.gsfc.nasa.gov/planetary/factsheet/earthfact.html (accessed on 26 March 2021).
- Sedunov, J.S.; Avdjushin, S.I.; Borisenkov, E.P.; Volkovickij, O.A.; Petrov, N.N.; Rejtenbah, R.G.; Smirnov, V.I.; Chernikov, A.A. Spravochnik (Spravochnye Dannye, Modeli) [The Atmosphere: A Handbook (Reference Data and Models)]; Gidrometeoizdat: Sankt Petersburg, Russia, 1991. [Google Scholar]
- COESA. U.S. Standard Atmosphere, 1962; U.S. Government Printing Office: Washington, DC, USA, 1962. [Google Scholar]
- DeVore, P.T.S. Load HITRAN 2004+ Data. 23 May 2014. Available online: https://www.mathworks.com/matlabcentral/fileexchange/45819-load-hitran-2004-data (accessed on 26 March 2021).
- De Bievre, P.; Gallet, M.; Holden, N.E.; Barnes, I.L. Isotopic Abundances and Atomic Weights of the Elements. J. Phys. Chem. Ref. Data 1984, 13, 809–891. [Google Scholar] [CrossRef]
- Picard, A.; Fang, H.; Glaser, M. Discrepancies in air density determination between the thermodynamic formula and a gravimetric method: Evidence for a new value of the mole fraction of argon in air. Metrologia 2004, 41, 396–400. [Google Scholar] [CrossRef]
- Heitronics Infrarot Messtechnik GmbH. Infrared Radiation Pyrometer KT19 II Operation Instructions; Heitronics Infrarot Messtechnik GmbH: Wiesbaden, Germany, 2002. [Google Scholar]
- Rohatgi, A. WebPlotDigitizer: Version 4.4. 2020. Available online: https://automeris.io/WebPlotDigitizer/ (accessed on 17 May 2021).
- Teledyne FLIR, LLC. What Is the Typical Spectral Response for Certain Camera/Lens Combination? Available online: https://www.flir.com/support-center/Instruments/what-is-the-typical-spectral-response-for-certain-cameralens-combination/ (accessed on 26 March 2021).
- Omejc, J. Vpliv Relativne Vlažnosti na Kazanje Sevalnih Termometrov [Influence of relative humidity on radiation thermometer measurements]. Diploma Thesis, Univerza v Ljubljani, Ljubljana, Slovenia, 2020. [Google Scholar]
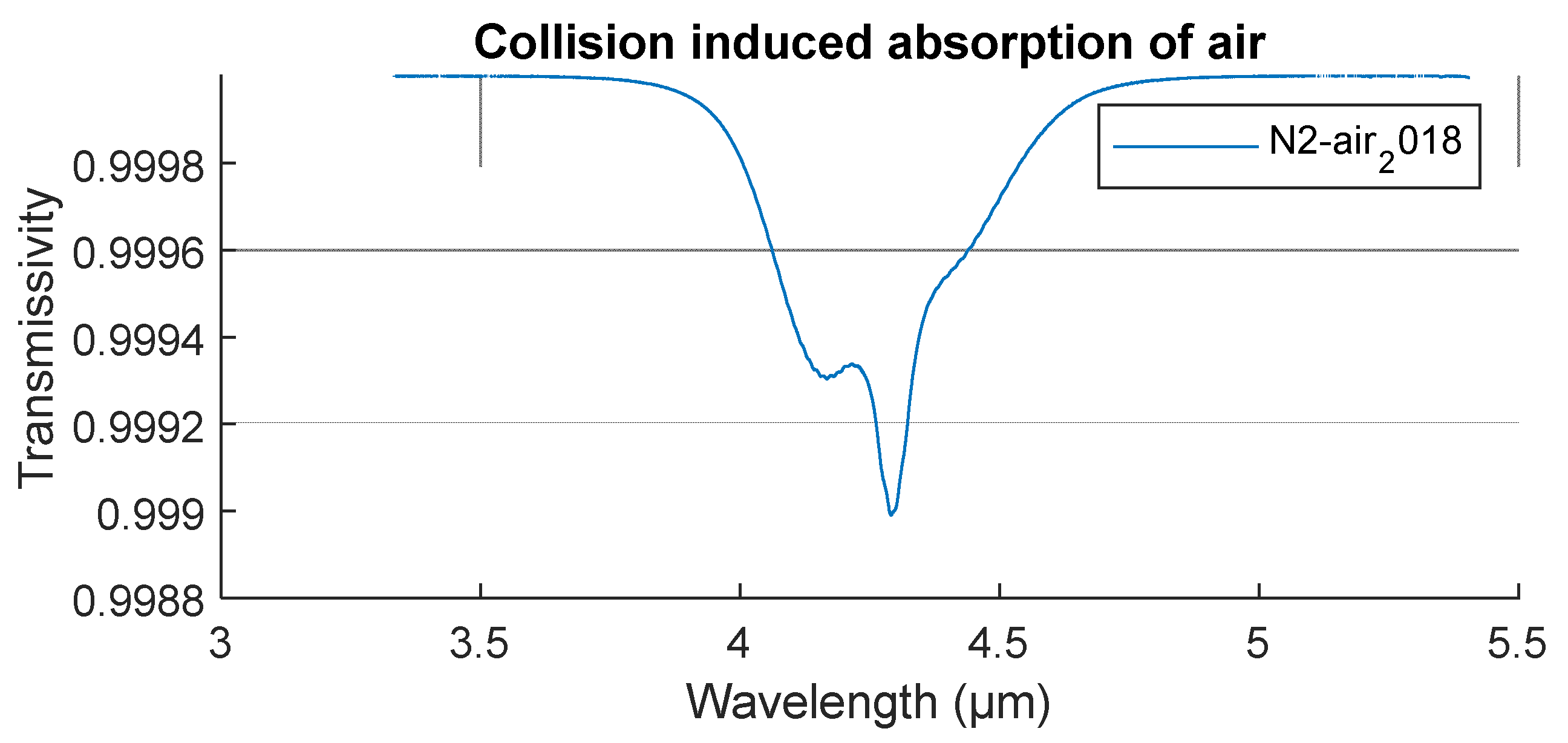
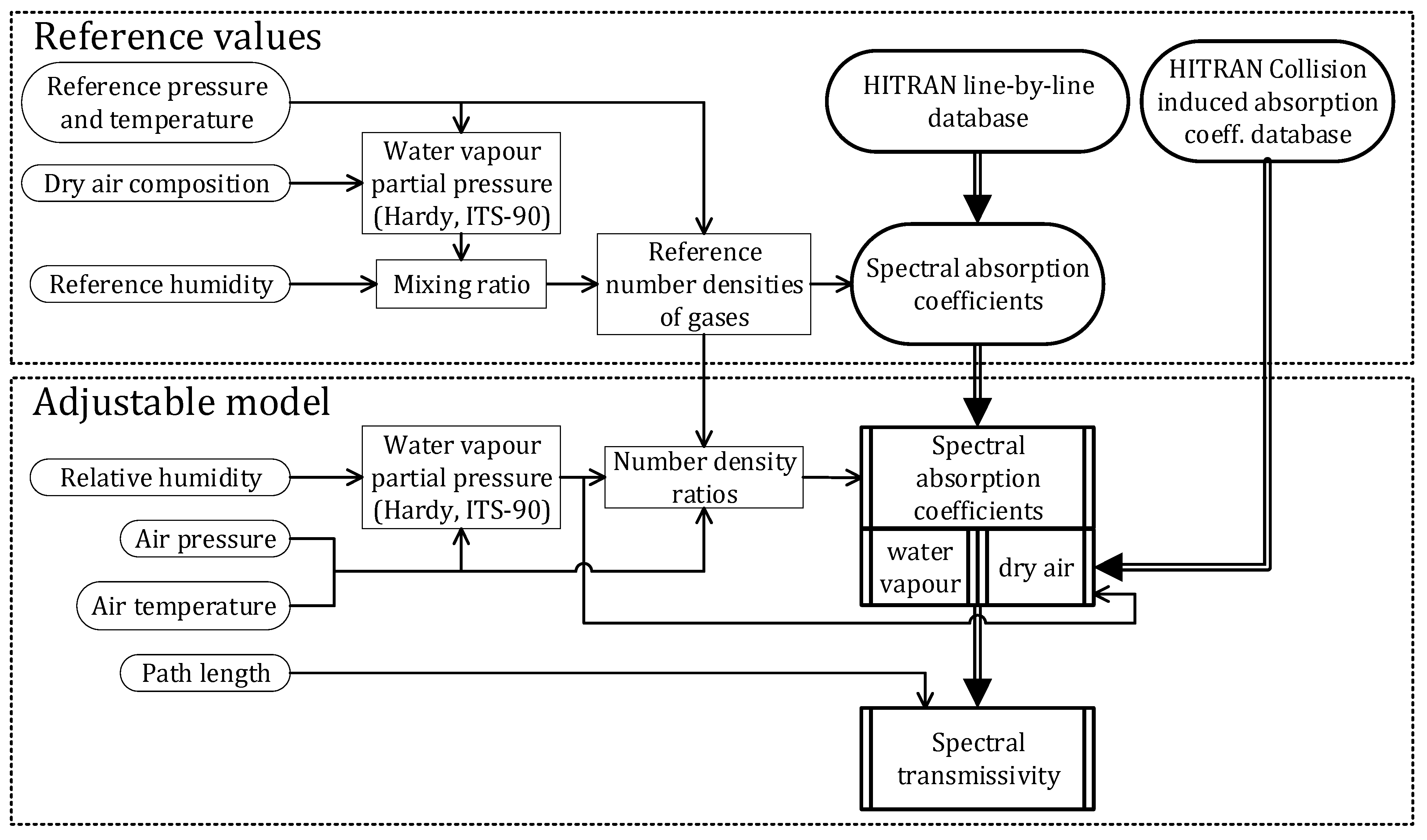
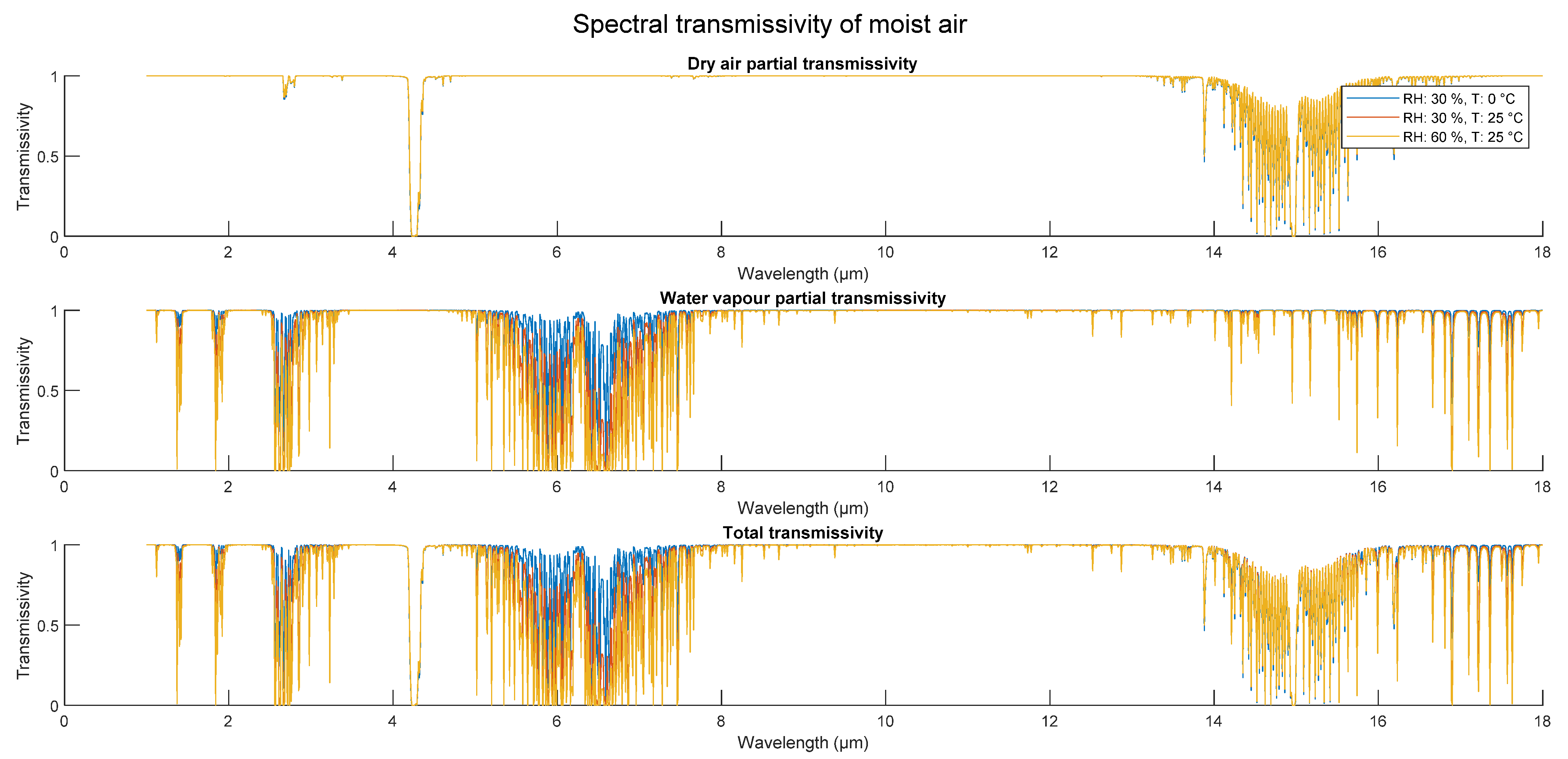
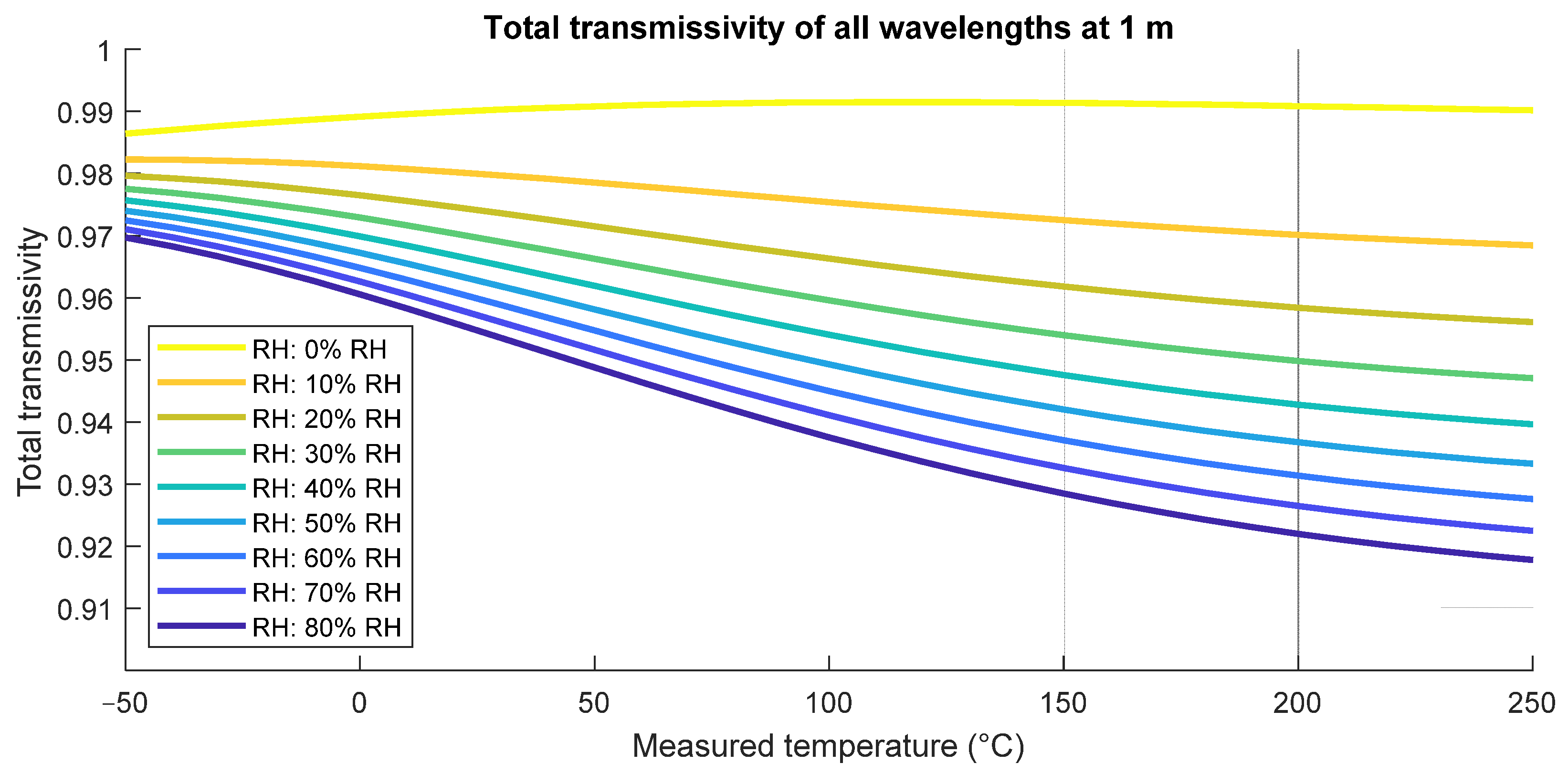


| Citation | [21] | [22] | [17] | [11] | [19] | [16] | [15] | [23] | [20] | [24], in [18] | [25] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | NOAA | US EPA trends | Gatley et al., | Picard CIPM07 | Park et al. | Keeling | Giacomo CIPM 81 | NASA Factsheet | Schlatter | Atmosfera—Gidrometeoizdat | US Std Atm 62 |
| Year | 2020 | 2019 | 2004–8 | 2007 | 2004 | 1970–86 | 1981 | 2020 | 2009 | 1991 | 1954 |
| Fraction | Mole | Mole | Mole | Mole | Mole | Mole | Mole | Volume | Volume | Volume | Volume |
| N2 | 780,818 | 780,848 | 781,010 | 780,670 | 781,010 | 780,800 | 780,840 | 780,840 | 780,840 | ||
| O2 | 209,435 | 209,390 | 209,390 | 209,460 | 209,390 | 209,500 | 209,460 | 209,460 | 209,476 | ||
| H2O | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Ar | 9332 | 9332 | 9170 | 9340 | 9170 | 9340 | 9340 | 9340 | 9340 | ||
| CO2 | 412.4 | 385 | 400 | 400 | 339 | 400 | 410 | 384 | 394.45 | 314 | |
| Ne | 18.2 | 18.2 | 18.2 | 18.18 | 18.2 | 18.18 | 18.18 | 18.18 | 18.18 | ||
| He | 5.2 | 5.2 | 5.2 | 5.24 | 5.2 | 5.24 | 5.24 | 5.24 | 5.24 | ||
| CH4 | 1.8923 | 1.5 | 1.5 | 1.5 | 1.7 | 1.5 | 1.7 | 1.774 | 1.79 | 2 | |
| Kr | 1.1 | 1.1 | 1.1 | 1.14 | 1.1 | 1.14 | 1.14 | 1.14 | 1.14 | ||
| H2 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.55 | 0.56 | 0.55 | 0.5 | ||
| N2O | 0.3336 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | - | 0.32 | 0.325 | 0.5 | |
| CO | 1.08 | 0.2 | 0.2 | 0.2 | 0.025 | 0.2 | - | - | 0.1 | - | |
| Xe | 0.1 | 0.1 | 0.1 | 0.1 | 0.09 | 0.09 | 0.087 | ||||
| O3 | 0.064 | 0 to 0.1 | 0.01 to 0.1 | 0 to 0.07 | 0.02 to 0.07 |
| Compound | Name | Content in Dry Air (ppm) | Reference Content |
|---|---|---|---|
| N2 | Nitrogen | 780,818 | 774,352 |
| O2 | Oxygen | 209,407.6 | 207,673 |
| H2O | Water | 0 | 8391 |
| Ar | Argon | 9332 | |
| CO2 | Carbon Dioxide | 412.4 | 408.98 |
| Ne | Neon | 18.2 | |
| He | Helium | 5.2 | |
| CH4 | Methane | 1.8923 | 1.876 |
| Kr | Krypton | 1.1 | |
| H2 | Hydrogen | 0.5 | 0.496 |
| N2O | Nitrous Oxide | 0.3336 | 0.331 |
| CO | Carbon Monoxide | 1.08 | 1.071 |
| Xe | Xenon | 0.1 | |
| O3 | Ozone | 0.064 | 0.063 |
| Symbol | Value | Quantity |
|---|---|---|
| 30% | Relative humidity of air | |
| 296 K | Air temperature | |
| 1013.25 hPa | Air pressure | |
| 2.48∙1019 cm−3 | Number density of air * | |
| Number density of a molecular species | ||
| 0.1 nm | Spectral resolution | |
| 10 m | Path length (used only in plots) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlačnik, V.; Pušnik, I. Influence of Atmosphere on Calibration of Radiation Thermometers. Sensors 2021, 21, 5509. https://doi.org/10.3390/s21165509
Mlačnik V, Pušnik I. Influence of Atmosphere on Calibration of Radiation Thermometers. Sensors. 2021; 21(16):5509. https://doi.org/10.3390/s21165509
Chicago/Turabian StyleMlačnik, Vid, and Igor Pušnik. 2021. "Influence of Atmosphere on Calibration of Radiation Thermometers" Sensors 21, no. 16: 5509. https://doi.org/10.3390/s21165509
APA StyleMlačnik, V., & Pušnik, I. (2021). Influence of Atmosphere on Calibration of Radiation Thermometers. Sensors, 21(16), 5509. https://doi.org/10.3390/s21165509







