Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives
Abstract
:1. Introduction
2. General Principles and the Fundamental Components of LFIAs
3. Literature Review Methodology
4. Applications
4.1. Clinical Applications
4.2. Food Safety Applications
4.3. Veterinary Applications
4.4. Environmental Applications
4.5. Other Applications
5. Multiplex LFIAs
6. LFIA Applications Trends
7. Evergreen and New Challenges
8. Future Perspectives
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Emerging Point-of-care Technologies for Food Safety Analysis. Sensors 2019, 19, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlermroj, R.; Phuengwas, S.; Makornwattana, M.; Sooksimuang, T.; Sahasithiwat, S.; Panchan, W.; Sukbangnop, W.; Elliott, C.T.; Karoonuthaisiri, N. Development of a microarray lateral flow strip test using a luminescent organic compound for multiplex detection of five mycotoxins. Talanta 2021, 233, 122540. [Google Scholar] [CrossRef] [PubMed]
- Campbell, V.R.; Carson, M.S.; Lao, A.; Maran, K.; Yang, E.J.; Kamei, D.T. Point-of-Need Diagnostics for Foodborne Pathogen Screening. SLAS Technol. 2020, 26, 55–79. [Google Scholar]
- Soh, J.H.; Chan, H.-M.; Ying, J.Y. Strategies for developing sensitive and specific nanoparticle-based lateral flow assays as point-of-care diagnostic device. Nano Today 2020, 30, 100831. [Google Scholar] [CrossRef]
- Hansen, S.; Abd El Wahed, A. Point-Of-Care or Point-Of-Need Diagnostic Tests: Time to Change Outbreak Investigation and Pathogen Detection. Trop. Med. Infect. Dis. 2020, 5, 151. [Google Scholar] [CrossRef]
- Makarona, E.; Petrou, P.; Kakabakos, S.; Misiakos, K.; Raptis, I. Point-of-Need bioanalytics based on planar optical interferometry. Biotechnol. Adv. 2016, 34, 209–233. [Google Scholar] [CrossRef] [PubMed]
- Weihs, F.; Anderson, A.; Trowell, S.; Caron, K. Resonance Energy Transfer-Based Biosensors for Point-of-Need Diagnosis—Progress and Perspectives. Sensors 2021, 21, 660. [Google Scholar] [CrossRef]
- Parolo, C.; Merkoçi, A. Paper-based nanobiosensors for diagnostics. Chem. Soc. Rev. 2013, 42, 450–457. [Google Scholar] [CrossRef]
- Van Emon, J.M. Immunoassay and Other Bioanalytical Techniques, 1st ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kettler, H.; White, K.; Hawkes, S.J.; UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Mapping the Landscape of Diagnostics for Sexually Transmitted Infections: Key Findings and Recommendations. 2004. Available online: https://apps.who.int/iris/handle/10665/68990 (accessed on 17 May 2021).
- Global Lateral Flow Assay Market Size by Type, by Technique, by Application, by End-user, by Geography and Forecast. Available online: https://www.verifiedmarketresearch.com/product/lateral-flow-assay-market/ (accessed on 17 May 2021).
- Li, J.; Macdonald, J. Multiplexed lateral flow biosensors: Technological advances for radically improving point-of-care diagnoses. Biosens. Bioelectron. 2016, 83, 177–192. [Google Scholar] [CrossRef]
- Eltzov, E.; Guttel, S.; Kei, A.L.Y.; Sinawang, P.D.; Ionescu, R.E.; Marks, R.S. Lateral Flow Immunoassays—From Paper Strip to Smartphone Technology. Electroanalysis 2015, 27, 2116–2130. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. TrAC-Trend. Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Mak, W.C.; Beni, V.; Turner, A.P.F. Lateral-flow technology: From visual to instrumental. Trends Anal. Chem. 2016, 79, 297–305. [Google Scholar] [CrossRef]
- Bishop, J.D.; Hsieh, H.V.; Gasperino, J.D.J.; Weigl, B.H. Sensitivity enhancement in lateral flow assays: A systems perspective. Lab Chip 2019, 19, 2486–2499. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.; Song, S.; Park, S.; Joo, C. Recent advances in high-sensitivity detection methods for paper-based lateral-low assay. Biosens. Bioelectron. 2020, 152, 112015. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Shibata, H.; Suzuki, K.; Citterio, D. Toward practical application of paper-based microfluidics for medical diagnostics: State-of-the-art and challenges. Lab. Chip. 2017, 17, 1206. [Google Scholar] [CrossRef] [PubMed]
- Espejo, A.P.; Akgun, Y.; Al Mana, A.F.; Tjendra, Y.; Millan, N.C.; Gomez-Fernandez, C.; Cray, C. Review of Current Advances in Serologic Testing for COVID-19. Am. J. Clin. Pathol. 2020, 154, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Ruhan, A.; Wang, H.; Wang, W.; Tan, W. Summary of the Detection Kits for SARS-CoV-2 Approved by the National Medical Products Administration of China and Their Application for Diagnosis of COVID-19. Virol. Sin. 2020, 35, 699–712. [Google Scholar]
- Zhu, N.; Woong, P.K. Advances in Viral Diagnostic Technologies for Combating COVID-19 and Future Pandemics. SLAS Technol. 2020, 25, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; Ferrante di Ruffano, L.; et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020, 6, CD013652. [Google Scholar]
- Dinnes, J.; Deeks, J.J.; Adriano, A.; Berhane, S.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; Beese, S.; et al. Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2020, 8, CD013705. [Google Scholar]
- Vashist, S.K. In Vitro Diagnostic Assays for COVID-19: Recent Advances and Emerging Trends. Diagnostics 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekonnen, D.; Mengist, H.M.; Derbie, A.; Nibret, E.; Munshea, A.; He, H.; Li, B.; Jin, T. Diagnostic accuracy of serological tests and kinetics of severe acute respiratory syndrome coronavirus 2 antibody: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 31, e2181. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Mehta, V.N.; Koduru, J.R.; Basu, H.; Singhal, R.K.; Murthy, Z.V.P.; Park, T.-J. An overview of molecular biology and nanotechnology based analytical methods for the detection of SARS-CoV-2: Promising biotools for the rapid diagnosis of COVID-19. Analyst 2021, 146, 1489–1513. [Google Scholar] [CrossRef]
- Antiochia, R. Paper-Based Biosensors: Frontiers in Point-of-Care Detection of COVID-19 Disease. Biosensors 2021, 11, 110. [Google Scholar] [CrossRef]
- Ernst, E.; Wolfe, P.; Stahura, C.; Edwards, K.A. Technical considerations to development of serological tests for SARS-CoV-2. Talanta 2021, 224, 121883. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, T.; Wang, W.; Wen, Y.; Li, K.; Qian, L.; Zhang, X.; Liu, G. Lateral flow biosensors based on the use of micro- and nanomaterials: A review on recent developments. Microchim. Acta 2020, 187, 70. [Google Scholar] [CrossRef]
- Jauset-Rubio, M.; El-Shahawi, M.S.; Bashammakh, A.S.; Alyoubi, A.O.; O’Sullivan, C. Advances in aptamers-based lateral flow assays. Trends Anal. Chem. 2017, 97, 385–398. [Google Scholar] [CrossRef]
- Reid, R.; Chatterjee, B.; Das, S.J.; Ghosh, S.; Sharma, T.K. Application of aptamers as molecular recognition elements in lateral flow assays. Anal. Biochem. 2020, 593, 113574. [Google Scholar] [CrossRef]
- Anfossi, L.; Di Nardo, F.; Cavalera, S.; Giovannoli, C.; Baggiani, C. Multiplex Lateral Flow Immunoassay: An Overview of Strategies towards High-throughput Point-of-Need Testing. Biosensors 2019, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Tian, S.; Zhao, W.; Liu, K.; Ma, X.; Guo, J. Multiplexed detection of biomarkers in lateral-flow immunoassays. Analyst 2020, 145, 2828–2840. [Google Scholar] [CrossRef]
- Mahmoudi, T.; de la Guardia, M.; Shirdel, B.; Mokhtarzadeh, A.; Baradan, B. Recent advancements in structural improvements of lateral flow assays towards point-of-care testing. Trends Anal. Chem. 2019, 116, 13–30. [Google Scholar] [CrossRef]
- Anfossi, L.; Di Nardo, F.; Cavalera, S.; Giovannoli, C.; Spano, G.; Speranskaya, E.S.; Goryacheva, I.Y.; Baggiani, C. A lateral flow immunoassay for straightforward determination of fumonisin mycotoxins based on the quenching of the fluorescence of CdSe/ZnS quantum dots by gold and silver nanoparticles. Microchim. Acta 2018, 185, 94. [Google Scholar] [CrossRef]
- Urusov, A.E.; Zherdev, A.V.; Dzantiev, B.B. Towards Lateral Flow Quantitative Assays: Detection Approaches. Biosensors 2019, 9, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Aguilar, Z.P.; Xu, H.; Lai, W.; Xiong, Y. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens. Bioelectron. 2016, 75, 166–180. [Google Scholar] [CrossRef]
- O’Farrell, B. Lateral Flow Technology for Field-Based Applications—Basics and Advanced Developments. Top. Companion Anim. Med. 2015, 30, 139–147. [Google Scholar] [CrossRef]
- Parolo, C.; Sena-Torralba, A.; Bergua, J.F.; Calucho, E.; Fuentes-Chust, C.; Hu, L.; Rivas, L.; Alvarez-Diduk, R.; Nguyen, E.P.; Cinti, S.; et al. Tutorial: Design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 2020, 15, 3788–3816. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Pai, N.P.; Vadnais, C.; Denkinger, C.; Engel, N.; Pai, M. Point-of-Care Testing for Infectious Diseases: Diversity, Complexity, and Barriers in Low- And Middle-Income Countries. PLoS Med. 2012, 9, e1001306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Farrell, B. Lateral Flow Immunoassay Systems: Evolution from the Current State of the Art to the Next Generation of Highly Sensitive, Quantitative Rapid Assays. In The Immunoassay Handbook. Theory and Applications of Ligand Binding, ELISA and Related Techniques, 4th ed.; Wild, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 89–107. [Google Scholar]
- World Health Organization. First WHO Model List of Essential In Vitro Diagnostics; WHO Technical Report Series, No. 1017; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. The Selection and Use of Essential In Vitro Diagnostics: Report of the Second Meeting of the WHO Strategic Advisory Group of Experts on In Vitro Diagnostics, 2019 (Including the Second WHO Model List of Essential In Vitro Diagnostics); WHO Technical Report Series, No. 1022; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- WHO. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. Available online: http://apps.who.int/iris/bitstream/10665/199350/1/9789241565165_eng.pdf (accessed on 17 May 2021).
- Anfossi, L. Immunoassays|Food applications. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–30. [Google Scholar]
- General Standard for Contaminants and Toxins in Food and Feed (CODEX STAN 193-1995). Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (accessed on 27 July 2021).
- Di Nardo, F.; Anfossi, L. Chapter Eight—Commercial biosensors for detection of food additives, contaminants, and pathogens. In Commercial Biosensors and Their Applications. Clinical, Food, and Beyond, 1st ed.; Sezgintürk, M.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 183–215. [Google Scholar]
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxinf detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [Google Scholar] [CrossRef]
- Veterinary Services. Available online: https://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/VS-FINAL-EN.pdf (accessed on 17 May 2021).
- Available online: https://www.oie.int/en/who-we-are/mission/ (accessed on 17 May 2021).
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E.J. Risk factors for human disease emergence. Philos. Trans. R. Soc. B. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Cummins, B.M.; Ligler, F.S.; Walker, G.M. Point-of-care diagnostic for niche applications. Biotechnol. Adv. 2016, 34, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Flatland, B.; Freeman, K.P.; Vap, L.M.; Harr, K.E. ASVCP guidelines: Quality assurance for point-of-care testing in veterinary medicine. Vet. Clin. Pathol. 2013, 42, 405–423. [Google Scholar] [CrossRef]
- Buller, H.; Adam, K.; Bard, A.; Bruce, A.; Chan, K.W.; Hinchliffe, S.; Morgans, L.; Rees, G.; Reyher, K.K. Veterinary Diagnostic Practice and the Use of Rapid Tests in Antimicrobial Stewardship on UK Livestock Farms. Front. Vet. Sci. 2020, 7, 765–777. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance. Available online: https://apps.who.int/iris/handle/10665/193736 (accessed on 17 May 2021).
- Global Framework for Development & Stewardship to Combat Antimicrobial Resistance: Draft Roadmap. Available online: https://www.who.int/publications/m/item/global-framework-for-development-stewardship-to-combat-antimicrobial-resistance-draft-roadmap (accessed on 17 May 2021).
- Amiard-Triquet, C. Introduction. In Aquatic Ecotoxicology, 1st ed.; Amiard-Triquet, C., Amiard, J.-C., Moneyrac, C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 1–23. [Google Scholar]
- Chapman, J.; Truong, V.K.; Elbourne, A.; Gangadoo, S.; Cheeseman, S.; Rajapaksha, P.; Latham, K.; Crawford, R.J.; Cozzolino, D. Combining Chemometrics and Sensors: Toward New Applications in Monitoring and Environmental Analysis. Chem. Rev. 2020, 120, 6048–6069. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Jayawardane, B.M.; Kolev, S.D.; McKelvie, I.D. Developments of microfluidic paper-based analytical devices (μPADs) for water analysis: A review. Talanta 2018, 177, 176–190. [Google Scholar] [CrossRef]
- Marquez, S.; Liu, J.; Morales-Narváez, E. Paper-based analytical devices in environmental applications and their integration with portable technologies. Curr. Opin. Environ. Sci. Health 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Artiola, J.F.; Pepper, I.L.; Brusseau, M.L. Monitoring and characterization of the environment. In Environmental Monitoring and Characterization; Artiola, J.F., Pepper, I.L., Brusseau, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2004; pp. 1–9. [Google Scholar]
- Amiard, J.-C.; Amiard-Triquet, C. Quality Standard Setting and Environmental Monitoring. In Aquatic Ecotoxicology, 1st ed.; Amiard-Triquet, C., Amiard, J.-C., Moneyrac, C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 51–76. [Google Scholar]
- Liu, B.; Zhuang, J.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Bendicho, C.; Pavlović, D.M.; Martín-Esteban, A.; Díaz-Álvarez, M.; Pan, Y.; Cooper, J.; Yang, Z.; Safarik, I.; Pospiskova, K.; et al. Miniaturized analytical methods for determination of environmental contaminants of emerging concern—A review. Anal. Chim. Acta 2021, 1158, 238108. [Google Scholar] [CrossRef]
- Cuprys, A.; Suralikerimath, N.; Pachapur, V.L.; Hegde, K.; Brar, S.K. Recent advances in nanomaterial-based sensors as tool for environmental monitoring. In Tools, Techniques and Protocols for Monitoring Environmental Contaminants; Brar, S.K., Hegde, K., Pachapur, V.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 391–403. [Google Scholar]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van der Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Daverey, A.; Dutta, K.; Sarkar, A. An overview of analytical methodologies for environmental monitoring. In Tools, Techniques and Protocols for Monitoring Environmental Contaminants; Brar, S.K., Hegde, K., Pachapur, V.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–17. [Google Scholar]
- Communication from the Commission to the Council and the European Parliament. A European One Health Action Plan against Antimicrobial Resistance (AMR). COM/2017/0339 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52017DC0339 (accessed on 17 May 2021).
- Sollweck, K.; Streich, P.; Elsner, M.; Seidel, M. A Chip-Based Colony Fusion Recombinase Polymerase Amplification Assay for Monitoring of Antimicrobial Resistance Genes and Their Carrying Species in Surface Water. ACS EST Water 2021, 1, 584–594. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Zheng, H.; Zhou, Y.; Wang, B.; Hu, Z. Development of a gold-based immunochromatographic strip assay for the detection of ancient silk. Anal. Methods 2015, 7, 7824–7830. [Google Scholar] [CrossRef]
- Sciutto, G.; Zangheri, M.; Anfossi, L.; Guardigli, M.; Prati, S.; Mirasoli, M.; Di Nardo, F.; Baggiani, C.; Mazzeo, R.; Roda, A. Miniaturized Biosensors to Preserve and Monitor Cultural Heritage: From Medical to Conservation Diagnosis. Angew. Chem. 2018, 57, 7385–7389. [Google Scholar] [CrossRef] [Green Version]
- Di Nardo, F.; Baggiani, C.; Giovannoli, C.; Spano, G.; Anfossi, L. Multicolor immunochromatographic strip test based on gold nanoparticles for the determination of aflatoxin B1 and fumonisins. Microchim. Acta 2017, 184, 1295–1304. [Google Scholar] [CrossRef]
- Zangheri, M.; Di Nardo, F.; Anfossi, L.; Giovannoli, C.; Baggiani, C.; Roda, A.; Mirasoli, M. Multiplex chemiluminescent biosensor for type B-fumonisins and aflatoxin B1 quantitative detection in maize flour. Analyst 2014, 140, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Xing, C.; Liu, L.; Song, S.; Feng, M.; Kuang, H.; Xu, C. Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosens. Bioelectron. 2015, 66, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Carrio, A.; Sampedro, C.; Sanchez-Lopez, J.L.; Pimienta, M.; Campoy, P. Automated Low-Cost Smartphone-Based Lateral Flow Saliva Test Reader for Drugs-of-Abuse Detection. Sensors 2015, 15, 29569–29593. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, H.; Zhang, P.; Sun, C.; Wang, X.; Wang, X.; Yang, R.; Wang, C.; Zhou, L. Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Sci. Rep. 2016, 6, 21342. [Google Scholar] [CrossRef] [PubMed]
- Schenk, F.; Weber, P.; Vogler, J.; Hecht, L.; Dietzel, A.; Gauglitz, G. Development of a paper-based lateral flow immunoassay for simultaneous detection of lipopolysaccharides of Salmonella serovars. Anal. Bioanal. Chem. 2018, 410, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Purrà, M.; Carré-Camps, M.; De Puig, H.; Bosch, I.; Gehrke, L.; Hamad-Schifferli, K. Surface-enhanced raman spectroscopy-based sandwich immunoassays for multiplexed detection of zika and dengue viral biomarkers. ACS Infect. Dis. 2017, 3, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, L.; Liu, B.; Su, E.; Chen, H.-Y.; Gu, Z.; Zhao, X. Quantitative detection of multiplex cardiac biomarkers with encoded SERS nanotags on a single T line in lateral flow assay. Sens. Actuators B Chem. 2018, 277, 502–509. [Google Scholar] [CrossRef]
- Di Nardo, F.; Alladio, E.; Baggiani, C.; Cavalera, S.; Giovannoli, C.; Spano, G.; Anfossi, L. Colour-encoded lateral flow immunoassay for the simultaneous detection of aflatoxin B1 and type-B fumonisins in a single Test line. Talanta 2019, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cao, X.; Finkelstein, J.L.; Cárdenas, W.B.; Erickson, D.; Mehta, S. A two-colour multiplexed lateral flow immunoassay system to differentially detect human malaria species on a single test line. Malar. J. 2019, 18, 313. [Google Scholar] [CrossRef] [PubMed]
- Cavalera, S.; Di Nardo, F.; Forte, L.; Marinoni, F.; Chiarello, M.; Baggiani, C.; Anfossi, L. Switching from Multiplex to Multimodal Colorimetric Lateral Flow Immunosensor. Sensors 2020, 20, 6609. [Google Scholar] [CrossRef] [PubMed]
- Couturier, C.; Wada, A.; Louis, K.; Mistretta, M.; Beitz, B.; Povoguiu, M.; Ripaux, M.; Mignon, C.; Werle, B.; Lugari, A.; et al. Characterization and analytical validation of a new antigenic rapid diagnostic test for Ebola virus disease detection. PLoS Negl. Trop. Dis. 2020, 14, 0007965. [Google Scholar] [CrossRef] [Green Version]
- DeMers, H.L.; He, S.; Pandit, S.G.; Hannah, E.E.; Zhang, Z.; Yan, F.; Green, H.R.; Reyes, D.F.; HauI, D.; McLarty, M.E.; et al. Development of an antigen detection assay for early point-of-care diagnosis of Zaire ebolavirus. PLoS Negl. Trop. Dis. 2020, 14, e0008817. [Google Scholar] [CrossRef] [PubMed]
- Wonderly, B.; Jones, S.; Gatton, M.L.; Barber, J.; Killip, M.; Hudson, C.; Carter, L.; Brooks, T.; Simpson, A.J.H.; Semper, A.; et al. Comparative performance of four rapid Ebola antigen-detection lateral flow immunoassays during the 2014–2016 Ebola epidemic in West Africa. PLoS ONE 2019, 14, e0212113. [Google Scholar]
- Martiskainen, I.; Juntunen, E.; Salminen, T.; Vuorenpää, K.; Bayoumy, S.; Vuorinen, T.; Khanna, N.; Pettersson, K.; Batra, G.; Talha, S.M. Double-Antigen Lateral Flow Immunoassay for the Detection of Anti-HIV-1 and -2 Antibodies Using Upconverting Nanoparticle Reporters. Sensors 2021, 21, 330. [Google Scholar] [CrossRef]
- Xu, M.; Lu, F.; Lyu, C.; Wu, Q.; Zhang, J.; Tian, P.; Xue, L.; Xu, T.; Wang, D. Broad-range and effective detection of human noroviruses by colloidal gold immunochromatographic assay based on the shell domain of the major capsid protein. BMC Microbiol. 2021, 21, 22. [Google Scholar] [CrossRef]
- Yoo, S.J.; Shim, H.S.; Yoon, S.; Moon, H.-W. Evaluation of high-throughput digital lateral flow immunoassays for the detection of influenza A/B viruses from clinical swab samples. J. Med. Virol. 2020, 92, 1040–1046. [Google Scholar] [CrossRef]
- Suzuki, K.; Huits, R.; Phadungsombat, J.; Tuekprakhon, A.; Nakayama, E.E.; van den Berg, R.; Barbè, B.; Cnops, L.; Rahim, R.; Hasan, A.; et al. Promising application of monoclonal antibody against chikungunya virus E1-antigen across genotypes in immunochromatographic rapid diagnostic tests. Virol. J. 2020, 17, 90. [Google Scholar] [CrossRef]
- Xiong, Y.; Luo, Y.; Li, H.; Wu, W.; Ruan, X.; Mu, X. Rapid visual detection of dengue virus by combining reverse transcription recombinase-aided amplification with lateral-flow dipstick assay. Int. J. Infect. Dis. 2020, 95, 406–412. [Google Scholar] [CrossRef]
- Goux, H.J.; Raja, B.; Kourentzi, K.; Trabuco, J.R.C.; Vu, B.V.; Paterson, A.S.; Kirkpatrick, A.; Townsend, B.; Lee, M.; Truong, V.T.T.; et al. Evaluation of a nanophosphor lateral-flow assay for self-testing for herpes simplex virus type 2 seropositivity. PLoS ONE 2019, 14, e0225365. [Google Scholar] [CrossRef]
- Wen, T.; Huang, C.; Shi, F.J.; Zeng, X.Y.; Lu, T.; Ding, S.N.; Jiao, Y.J. Development of a lateral flow immunoassay strip for rapid detection of IgG antibody against SARS-CoV-2 virus. Analyst 2020, 145, 5345–5352. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Zhai, X.; Li, Y.; Lin, L.; Zhao, H.; Bian, L.; Li, P.; Yu, L.; Wu, Y.; et al. Rapid and Sensitive Detection of anti-SARS-CoV-2 IgG, Using Lanthanide-Doped Nanoparticles-Based Lateral Flow Immunoassay. Anal. Chem. 2020, 92, 7226–7231. [Google Scholar] [CrossRef]
- Yu, S.; Nimse, S.B.; Kim, J.; Song, K.S.; Kim, T. Development of a Lateral Flow Strip Membrane Assay for Rapid and Sensitive Detection of the SARS-CoV-2. Anal. Chem. 2020, 92, 14139–14144. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Sui, Z.; Huang, Z.; Xie, J.; Wen, K.; Zhang, Y.; Huang, W.; Mi, W.; Peng, K.; Dai, X.; et al. Point-of-care test system for detection of immunoglobulin-G and -M against nucleocapsid protein and spike glycoprotein of SARS-CoV-2. Sens. Actuators B Chem. 2021, 331, 129415. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, J.H.; Kim, M.J.; Park, S.C.; Choi, M.; Lee, W.; Ku, K.B.; Kim, B.-T.; Park, E.C.; Kim, V.; et al. Development of a SARS-CoV-2-specific biosensor for antigen detection using scFv-Fc fusion proteins. Biosens. Bioelectron. 2021, 175, 112868. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Cavalera, S.; Di Nardo, F.; Calabria, D.; Rosati, S.; Simoni, P.; Colitti, B.; Baggiani, C.; Roda, M.; Anfossi, L. Dual lateral flow optical/chemiluminescence immunosensors for the rapid detection of salivary and serum IgA in patients with COVID-19 disease. Biosens. Bioelectron. 2021, 172, 112765. [Google Scholar] [CrossRef]
- Cavalera, S.; Colitti, B.; Rosati, S.; Ferrara, G.; Bertolotti, L.; Nogarol, C.; Guiotto, C.; Cagnazzo, C.; Denina, M.; Fagioli, F.; et al. A multi-target lateral flow immunoassay enabling the specific and sensitive detection of total antibodies to SARS-CoV-2. Talanta 2021, 223, 121737. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, J.; Cao, J.; Ma, J.; Li, X.; Shiet, F. Ultrasensitive dual-color rapid lateral flow immunoassay via gold nanoparticles with two different morphologies for the serodiagnosis of human brucellosis. Anal. Bioanal. Chem. 2019, 411, 8033–8042. [Google Scholar] [CrossRef]
- Han, Y.; Dai, W.; Meng, F.; Gan, X.; Liu, M.; Deng, X.; Li, Y.; Wang, G. Diagnosis of Helicobacter pylori infection in the elderly using an immunochromatographic assay-based stool antigen test. Microbiol. Open 2020, 9, e1102. [Google Scholar] [CrossRef]
- Romero Herrero, D.; Soler-Palacin, P.; Burgos Cibrian, J.; Falcó Ferrer, V.; Anton Pagarolas, A.; Martin-Gomez, M.T. Detection of Streptococcus pneumoniae antigen in pleural fluid: Usefulness of an immunofluorescence-based lateral flow assay for the diagnosis of pneumococcal pneumonia. Diagn Microbiol. Infect Dis. 2020, 98, 115162. [Google Scholar] [CrossRef]
- Salminen, T.; Mehdi, F.; Rohila, D.; Kumar, M.; Talha, S.M.; Prakash, J.A.J.; Khanna, N.; Pettersson, K.; Batra, G. Ultrasensitive and Robust Point-of-Care Immunoassay for the Detection of Plasmodium falciparum Malaria. Anal. Chem. 2020, 92, 15766–15772. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, J.; Kim, K.; Jeon, J.; Lee, S.; Ko, J.; Lee, J.; Kang, M.; Chung, D.R.; Choo, J. Quantitative Serodiagnosis of Scrub Typhus Using Surface-Enhanced Raman Scattering-Based Lateral Flow Assay Platforms. Anal. Chem. 2019, 91, 12275–12282. [Google Scholar] [CrossRef]
- Choi, J.Y.; Hii, K.C.; Bailey, E.S.; Chuang, J.Y.; Tang, W.Y.; Yuen Wong, E.K.; Ti, T.; Pau, K.S.; Berita, A.; Saihidi, I.; et al. Burkholderia pseudomallei Detection among Hospitalized Patients, Sarawak. Am. J. Trop. Med. Hyg. 2020, 102, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Tomás, A.L.; de Almeida, M.P.; Cardoso, F.; Pinto, M.; Pereira, E.; Franco, R.; Matos, O. Development of a Gold Nanoparticle-Based Lateral-Flow Immunoassay for Pneumocystis pneumonia Serological Diagnosis at Point-of-Care. Front. Microbiol. 2020, 10, 2917. [Google Scholar] [CrossRef]
- Boonroumkaew, P.; Sadaow, L.; Sanpool, O.; Rodpai, R.; Thanchomnang, T.; Phupiewkham, W.; Intapan, P.M.; Maleewong, W. Effectiveness of Strongyloides Recombinant IgG Immunoreactive Antigen in Detecting IgG and IgG4 Subclass Antibodies for Diagnosis of Human Strongyloidiasis Using Rapid Immunochromatographic Tests. Diagnostics 2020, 10, 615. [Google Scholar] [CrossRef]
- Sadaow, L.; Sanpool, O.; Rodpai, R.; Boonroumkaew, P.; Maleewong, W.; Intapan, P.M. Development of immunochromatographic device as a point-of-care tool for serodiagnosis of human strongyloidiasis cases. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Matsui, H.; Higashide, M.; Hanaki, H. Evaluation of a rapid immunochromatographic test for the detection of Candida species from oropharyngeal samples. J. Microbiol. Methods 2020, 179, 106090. [Google Scholar] [CrossRef]
- Cáceres, D.H.; Gómez, B.L.; Tobón, A.M.; Chiller, T.M.; Lindsley, M.D. Evaluation of a Histoplasma antigen lateral flow assay for the rapid diagnosis of progressive disseminated histoplasmosis in Colombian patients with AIDS. Mycoses 2020, 63, 139–144. [Google Scholar] [CrossRef]
- Ybañez, R.H.D.; Nishikawa, Y. Serological detection of T. gondii infection in humans using an immunochromatographic assay based on dense granule protein 7. Parasitol. Int. 2020, 76, 102089. [Google Scholar] [CrossRef] [PubMed]
- Mercier, T.; Dunbar, A.; de Kort, E.; Schauwvlieghe, A.; Reynders, M.; Guldentops, E.; Blijlevens, N.M.A.; Vonk, A.G.; Rijnders, B.; Verweij, P.E.; et al. Lateral flow assays for diagnosing invasive pulmonary aspergillosis in adult hematology patients: A comparative multicenter study. Med. Mycol. 2020, 58, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Hunter, E.S.; Page, I.D.; Richardson, M.D.; Denning, D.W. Evaluation of the LDBio Aspergillus ICT lateral flow assay for serodiagnosis of allergic bronchopulmonary aspergillosis. PLoS ONE 2020, 15, e0238855. [Google Scholar] [CrossRef] [PubMed]
- Sadaow, L.; Yamasaki, H.; Morishima, Y.; Sanpool, O.; Rodpai, R.; Janwan, P.; Boonroumkaew, P.; Maleewong, W.; Intapan, P.M. Effectiveness of Fasciola gigantica excretory-secretory and recombinant cathepsin L antigens for rapid diagnosis of human fascioliasis using immunochromatographic devices. Parasitol. Res. 2020, 119, 3691–3698. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.D.; Silva, Â.A.O.; Santos, E.F.; Leony, L.M.; Freitas, N.E.M.; Daltro, R.T.; Ferreira, A.G.P.; Diniz, R.L.; Bernardo, A.R.A.O.; Krieger, M.A.; et al. Development of a New Lateral Flow Assay Based on IBMP-8.1 and IBMP-8.4 Chimeric Antigens to Diagnose Chagas Disease. BioMed. Res. Int. 2020, 2020, 1803515. [Google Scholar] [CrossRef]
- Somboonpatarakun, C.; Intapan, P.M.; Sadaow, L.; Rodpai, R.; Sanpool, O.; Maleewong, W. Development of an Immunochromatographic Device to Detect Antibodies for Rapid Diagnosis of Human Angiostrongyliasis. Parasitology 2020, 147, 194–198. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Z.; Liang, W.; Zhang, Y.; Song, W.; Song, J.; Xue, K.; Wang, M.; Sun, W.; Gu, J.; et al. A novel immunochromatographic strips assay for rapid and simple detection of systemic lupus erythematosus. Sci. Rep. 2020, 10, 14178. [Google Scholar] [CrossRef]
- Huang, D.; Ying, H.; Jiang, D.; Liu, F.; Tian, Y.; Du, C.; Zhang, L.; Pu, X. Rapid and sensitive detection of interleukin-6 in serum via time-resolved lateral flow immunoassay. Anal. Biochem. 2020, 588, 113468. [Google Scholar] [CrossRef] [PubMed]
- Ki, H.; Jang, H.; Oh, J.; Han, G.-R.; Lee, H.; Kim, S.; Kim, M.-G. Simultaneous Detection of Serum Glucose and Glycated Albumin on a Paper-Based Sensor for Acute Hyperglycemia and Diabetes Mellitus. Anal. Chem. 2020, 92, 11530–11534. [Google Scholar] [CrossRef]
- Ki, H.; Oh, J.; Hana, G.-R.; Kim, M.-G. Glycation ratio determination through simultaneous detection of human serum albumin and glycated albumin on an advanced lateral flow immunoassay sensor. Lab Chip 2020, 20, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, D.P.; Gangula, A.; Ghoshdastidar, S.; Kannan, R.; Upendran, A. Diabetic Retinopathy Screening Using a Gold Nanoparticle-Based Paper Strip Assay for the At-Home Detection of the Urinary Biomarker 8-Hydroxy-2′-Deoxyguanosine. Am. J. Ophthalmol. 2020, 213, 306–319. [Google Scholar] [CrossRef]
- Nelson, A.C.; Motum, P.I.; Emeto, T.I. Evaluation of an immunochromatographic test for alpha thalassaemia screening in a multi-ethnic population. Int. J. Lab. Hematol. 2019, 41, 397–403. [Google Scholar] [CrossRef]
- Choi, E.-S.; Hasan Al Faruque, H.; Kim, J.-H.; Cho, J.-H.; Park, K.M.; Kim, E. Immunochromatographic assay to detect α-tubulin in urine for the diagnosis of kidney injury. J. Clin. Lab. Anal. 2020, 34, e23015. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Li, X.; Fan, Y.; Yang, C.; Lv, X. Simultaneous detection of gastric cancer screening biomarkers plasma pepsinogen I/II using fluorescent immunochromatographic strip coupled with a miniature analytical device. Sens. Actuators B Chem. 2019, 286, 272–281. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Musselwhite, L.W.; de Paula Pantano, N.; Vazquez, F.L.; Smith, J.S.; Schweizer, J.; Belmares, M.; Possati-Resende, J.C.; de Andrade Vieira, M.; Longatto-Filho, A.; et al. Detection of HPV E6 oncoprotein from urine via a novel immunochromatographic assay. PLoS ONE 2020, 15, e0232105. [Google Scholar] [CrossRef] [Green Version]
- Bayoumy, S.; Hyytiä, H.; Leivo, J.; Talha, S.M.; Huhtinen, K.; Poutanen, M.; Hynninen, J.; Perheentupa, A.; Lamminmäki, U.; Gidwani, K.; et al. Glycovariant-based lateral flow immunoassay to detect ovarian cancer–associated serum CA125. Commun. Biol. 2020, 3, 460. [Google Scholar] [CrossRef]
- Lei, Q.; Zhao, L.; Ye, S.; Sun, Y.; Xie, F.; Zhang, H.; Zhou, F.; Wu, S. Rapid and quantitative detection of urinary Cyfra21-1 using fluorescent nanosphere-based immunochromatographic test strip for diagnosis and prognostic monitoring of bladder cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4266–4272. [Google Scholar] [CrossRef] [Green Version]
- Di Nardo, F.; Occhipinti, S.; Gontero, P.; Cavalera, S.; Chiarello, M.; Baggiani, C.; Anfossi, L. Detection of urinary prostate specific antigen by a lateral flow biosensor predicting repeat prostate biopsy outcome. Sens. Actuators B Chem. 2020, 325, 128812. [Google Scholar] [CrossRef]
- Rey, E.G.; Finkelstein, J.L.; Erickson, D. Fluorescence lateral flow competitive protein binding assay for the assessment of serum folate concentrations. PLoS ONE 2019, 14, e0217403. [Google Scholar] [CrossRef]
- Di Nardo, F.; Cavalera, S.; Baggiani, C.; Giovannoli, C.; Anfossi, L. Direct vs Mediated Coupling of Antibodies to Gold Nanoparticles: The Case of Salivary Cortisol Detection by Lateral Flow Immunoassay. ACS Appl. Mater. Interfaces 2019, 11, 32758–32768. [Google Scholar] [CrossRef]
- Thakur, R.; Akram, F.; Rastogi, V.; Mitra, A.; Nawani, R.; Av, V.; Dubey, S.K.; Shakher, C. Development of Smartphone-Based Lateral Flow Device for the Quantification of LH and E3G Hormones. IEEE Sens. J. 2020, 20, 14491–14500. [Google Scholar] [CrossRef]
- Zangheri, M.; Mirasoli, M.; Guardigli, M.; Di Nardo, F.; Anfossi, L.; Baggiani, C.; Simoni, P.; Benassai, M.; Roda, A. Chemiluminescence-based biosensor for monitoring astronauts’ health status during space missions: Results from the International Space Station. Biosens. Bioelectron. 2019, 129, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; He, Q.; Chen, Y.; Shen, D.; Xiao, H.; Eremin, S.A.; Cui, X.; Zhao, S. Platinum nanoflowers with peroxidase-like property in a dual immunoassay for dehydroepiandrosterone. Microchim. Acta 2020, 187, 592. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; He, Q.; Pan, J.; Ding, S.; Xiao, H.; Cui, X.; Zhao, S. A Pt–Ir nanocube amplified lateral flow immunoassay for dehydroepiandrosterone. Analyst 2021, 146, 2726–2733. [Google Scholar] [CrossRef]
- Znoyko, S.L.; Orlov, A.V.; Bragina, V.A.; Nikitin, M.P.; Nikitin, P.I. Nanomagnetic lateral flow assay for high-precision quantification of diagnostically relevant concentrations of serum TSH. Talanta 2020, 216, 120961. [Google Scholar] [CrossRef] [PubMed]
- Byzova, N.A.; Vengerov, Y.Y.; Voloshchuk, S.G.; Zherdev, A.V.; Dzantiev, B.B. Development of a Lateral Flow Highway: Ultra-Rapid Multitracking Immunosensor for Cardiac Markers. Sensors 2019, 19, 5494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, C.; Fishlock, S.J.; Steele, D.N.; Puttaswamy, S.V.; Lubarsky, G.; Raj, S.; Mclaughlin, J. A Point-of-Care Measurement of NT-proBNP for Heart Failure Patients. IEEE Access. 2020, 8, 138973–138983. [Google Scholar] [CrossRef]
- Han, G.-R.; Kim, M.-G. Highly Sensitive Chemiluminescence-Based Lateral Flow Immunoassay for Cardiac Troponin I Detection in Human Serum. Sensors 2020, 20, 2593. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yang, H.; Pan, J.; Cui, X.; Shen, D.; Eremin, S.A.; Fang, Y.; Zhao, S. Lateral Flow Immunosensor for Ferritin Based on Dual Signal-Amplified Strategy by Rhodium Nanoparticles. ACS Appl. Bio Mater. 2020, 3, 8849–8856. [Google Scholar] [CrossRef]
- Gandhi, M.; Wang, G.; King, R.; Rodrigues, W.C.; Vincent, M.; Glidden, D.V.; Cressey, T.R.; Bacchetti, P.; Spinelli, M.A.; Okochi, H.; et al. Development and validation of the first point-of-care assay to objectively monitor adherence to HIV treatment and prevention in real-time in routine settings. AIDS 2020, 34, 255–260. [Google Scholar] [CrossRef]
- Cavalera, S.; Agulló, C.; Mercader, J.V.; Di Nardo, F.; Chiarello, M.; Anfossi, L.; Baggiani, C.; D’Avolio, A.; Abad-Somovilla, A.; Abad-Fuentes, A. Monoclonal antibodies with subnanomolar affinity to tenofovir for monitoring adherence to antiretroviral therapies: From hapten synthesis to prototype development. J. Mater. Chem. B 2020, 8, 10439–10449. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, R.; Wang, S.; Yang, X.; Bai, Z.; Li, X.; Rong, Z.; Shen, B.; Wang, S. Magnetic quantum dot based lateral flow assay biosensor for multiplex and sensitive detection of protein toxins in food samples. Biosens. Bioelectron. 2019, 146, 111754. [Google Scholar] [CrossRef]
- Wu, K.H.; Huang, W.C.; Shyu, R.H.; Chang, S.C. Silver nanoparticle-base lateral flow immunoassay for rapid detection of Staphylococcal enterotoxin B in milk and honey. J. Inorg. Biochem. 2020, 210, 11116. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Liu, L.; Kuang, H.; Xu, L.; Xu, C. A gold nanoparticle-based lateral flow immunosensor for ultrasensitive detection of tetrodotoxin. Analyst 2020, 145, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Bever, C.S.; Adams, C.A.; Hnasko, R.M.; Cheng, L.W.; Stanker, L.H. Lateral flow immunoassay (LFIA) for the detection of lethal amatoxins from mushrooms. PLoS ONE 2020, 15, e0231781. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, G.; Fang, B.; Xiong, Q.; Duan, H.; Lai, W. Lateral Flow Immunoassay Based on Polydopamine-Coated Gold Nanoparticles for the Sensitive Detection of Zearalenone in Maize. ACS Appl. Mater. Interfaces 2019, 11, 31283–31290. [Google Scholar] [CrossRef]
- Pan, M.; Ma, T.; Yang, J.; Li, S.; Liu, S.; Wang, S. Development of Lateral Flow Immunochromatographic Assays Using Colloidal Au Sphere and Nanorods as Signal Marker for the Determination of Zearalenone in Cereals. Foods 2020, 9, 281. [Google Scholar] [CrossRef] [Green Version]
- Qie, Z.; Yan, W.; Gao, Z.; Meng, W.; Xiao, R.; Wang, S. Ovalbumin antibody-based fluorometric immunochromatographic lateral flow assay using CdSe/ZnS quantum dot beads as label for determination of T-2 toxin. Microchim. Acta 2019, 186, 816. [Google Scholar] [CrossRef]
- Li, R.; Meng, C.; Wen, Y.; Fu, W.; He, P. Fluorometric lateral flow immunoassay for simultaneous determination of three mycotoxins (aflatoxin B1, zearalenone and deoxynivalenol) using quantum dot microbeads. Microchim. Acta 2019, 186, 748. [Google Scholar] [CrossRef]
- Huang, X.; Huang, X.; Xie, J.; Li, X.; Huang, Z. Rapid simultaneous detection of fumonisin B1 and deoxynivalenol in grain by immunochromatographic test strip. Anal. Biochem. 2020, 606, 113878. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Wang, J.; Hua, Q.; Wu, J.; Shen, X.; Sun, Y.; Lei, H. Three lateral flow immunochromatographic assays based on different nanoparticle probes for on-site detection of tylosin and tilmicosin in milk and pork. Sens. Actuators B Chem. 2019, 301, 127059. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Bao, H.; Xing, K.; Liu, J.; Xia, J.; Lai, W.; Peng, J. Immunochromatographic assay based on time-resolved fluorescent nanobeads for the rapid detection of sulfamethazine in egg, honey, and pork. J. Sci. Food Agric. 2020, 101, 684–692. [Google Scholar] [CrossRef]
- Byzova, N.A.; Serchenya, T.S.; Vashkevich, I.I.; Zherdev, A.V.; Sviridov, O.V.; Dzantiev, B.B. Lateral flow immunoassay for rapid qualitative and quantitative control of the veterinary drug bacitracin in milk. Microchem. J. 2020, 156, 104884. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, X.; Liu, L.; Xu, L.; Kuang, H.; Xu, C. Rapid and sensitive detection of diclazuril in chicken samples using a gold nanoparticle-based lateral-flow strip. Food Chem. 2020, 312, 126116. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Zvereva, E.A.; Popravko, D.S.; Zherdev, A.V.; Xu, C.; Dzantiev, B.B. An immunochromatographic test system for the determination of lincomycin in foodstuffs of animal origin. J. Chromatogr. B, 2020, 1141, 122014. [Google Scholar] [CrossRef]
- Guo, L.; Wu, X.; Cui, G.; Song, S.; Kuang, H.; Xu, C. Colloidal Gold Immunochromatographic Assay for Rapid Detection of Carbadox and Cyadox in Chicken Breast. ACS Omega 2020, 5, 1422–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, G.; Hu, X.; Yang, J.; Sun, Y.; Kwee, S.; Tang, L.; Xing, G.; Xing, Y.; Zhang, G. Colloidal gold-based immunochromatographic strip assay for the rapid detection of bacitracin zinc in milk. Food Chem. 2020, 327, 126879. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Zvereva, E.A.; Zherdev, A.V.; Godjevargova, T.; Xu, C.; Dzantiev, B.B. Development of a double immunochromatographic test system for simultaneous determination of lincomycin and tylosin antibiotics in foodstuffs. Food Chem. 2020, 318, 126510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, F.; Sun, Y.; Mi, T.; Wang, L.; Li, Q.; Li, J.; Ma, W.; Liu, W.; Zuo, J.; et al. Development of a highly sensitive lateral flow immunoassay based on receptor-antibody-amorphous carbon nanoparticles to detect 22 β-lactams in milk. Sens. Actuators B Chem. 2020, 321, 128458. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Guo, L.; Xu, X.; Wu, A.; Kuang, H.; Sun, L.; Song, S.; Xu, C. Sensitive Lateral Flow Immunoassay for the Residues of Imidocarb in Milk and Beef Samples. ACS Omega 2021, 6, 2559–2569. [Google Scholar] [CrossRef]
- Li, Y.; Jin, G.; Liu, L.; Kuang, H.; Xiao, J.; Xu, C. A portable fluorescent microsphere-based lateral flow immunosensor for the simultaneous detection of colistin and bacitracin in milk. Analyst 2021, 145, 7884–7892. [Google Scholar] [CrossRef]
- Zhao, M.; Yao, X.; Liu, S.; Zhang, H.; Wang, L.; Yin, X.; Su, L.; Xu, B.; Wang, J.; Lan, Q.; et al. Antibiotic and mammal IgG based lateral flow assay for simple and sensitive detection of Staphylococcus aureus. Food Chem. 2021, 339, 127955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Long, M.; Lv, C.; Xin, S.; Han, X.; Jiang, W. Lanthanide-labeled fluorescent-nanoparticle immunochromatographic strips enable rapid and quantitative detection of Escherichia coli O157:H7 in food samples. Food Control 2020, 109, 106894. [Google Scholar] [CrossRef]
- Liu, C.; Fang, S.; Tian, Y.; Ma, J.; Wang, Z.; Xu, D.; Li, Y.; Hou, D.; Liu, Q. Rapid detection of Escherichia coli O157:H7 in milk, bread, and jelly by lac dye coloration-based bidirectional lateral flow immunoassay strip. J. Food Saf. 2021, 41, e12862. [Google Scholar] [CrossRef]
- Zhuang, L.; Gong, J.; Ji, Y.; Tian, P.; Kong, F.; Bai, H.; Gu, N.; Zhang, Y. Lateral flow fluorescent immunoassay based on isothermal amplification for rapid quantitative detection of: Salmonella spp. Analyst 2020, 145, 2367–2377. [Google Scholar] [CrossRef]
- Ilhan, H.; Tayyarcan, E.K.; Caglayan, M.J.; Boyaci, I.K.; Saglam, N.; Tamer, U. Replacement of antibodies with bacteriophages in lateral flow assay of Salmonella enteritidis. Biosens. Bioelectron. 2021, 189, 113383. [Google Scholar] [CrossRef]
- He, D.; Wu, Z.; Cui, B.; Xu, E.; Jin, Z. Establishment of a dual mode immunochromatographic assay for Campylobacter jejuni detection. Food Chem. 2019, 289, 708–713. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Y.; Liu, C.; Tian, Y.; Fang, S.; Yang, H.; Li, B.; Liu, Q. Development and comparison of immunochromatographic strips with four nanomaterial labels: Colloidal gold, new colloidal gold, multi-branched gold nanoflowers and Luminol-reduced Au nanoparticles for visual detection of Vibrio parahaemolyticus in seafood. Aquaculture 2021, 539, 736563. [Google Scholar] [CrossRef]
- Anfossi, L.; Di Nardo, F.; Russo, A.; Cavalera, S.; Giovannoli, C.; Spano, G.; Baumgartner, S.; Lauter, K.; Baggiani, C. Silver and gold nanoparticles as multi-chromatic lateral flow assay probes for the detection of food allergens. Anal. Bioanal. Chem. 2019, 411, 1905–1913. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Z.; Lin, H.; Siddanakoppalu, P.N.; Zhou, J.; Chen, G.; Yu, Z. Quantum-dot-based lateral flow immunoassay for the rapid detection of crustacean major allergen tropomyosin. Food Control 2019, 106, 106714. [Google Scholar] [CrossRef]
- Galan-Malo, P.; Pellicer, S.; Pérez, M.D.; Sánchez, L.; Razquin, P.; Mata, L. Development of a novel duplex lateral flow test for simultaneous detection of casein and β-lactoglobulin in food. Food Chem. 2019, 293, 41–48. [Google Scholar] [CrossRef]
- Yin, H.-Y.; Fang, T.J.; Li, Y.-T.; Fung, Y.-F.; Tsai, W.-C.; Dai, H.-Y.; Wen, H.-W. Rapidly detecting major peanut allergen-Ara h2 in edible oils using a new immunomagnetic nanoparticle-based lateral flow assay. Food Chem. 2019, 271, 505–515. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Zhao, Y.; Xu, N.; Peng, L.; Wang, Y.; Wei, X. Novel monoclonal antibody-sandwich immunochromatographic assay based on Fe3O4/Au nanoparticles for rapid detection of fish allergen parvalbumin. Food Res. Int. 2021, 142, 110102. [Google Scholar] [CrossRef] [PubMed]
- Hnasko, R.M.; Jackson, E.S.; Lin, A.V.; Haff, R.P.; McGarvey, J.A. A rapid and sensitive lateral flow immunoassay (LFIA) for the detection of gluten in foods. Food Chem. 2021, 355, 129514. [Google Scholar]
- Zhang, J.; Portela, S.B.; Horrell, J.B.; Leung, A.; Weitmann, D.R.; Artiuch, J.B.; Wilson, S.M.; Cipriani, M.; Slakey, L.K.; Burt, A.M.; et al. An integrated, accurate, rapid, and economical handheld consumer gluten detector. Food Chem. 2019, 275, 446–456. [Google Scholar] [CrossRef]
- Xi, J.; Yu, Q. The development of lateral flow immunoassay strip tests based on surface enhanced Raman spectroscopy coupled with gold nanoparticles for the rapid detection of soybean allergen β-conglycinin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 241, 118640. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Liu, Z.; Wang, J.; Hua, Q.; Liang, J.; Shen, X.; Xu, Z.; Lei, H.; Sun, Y. Latex microsphere immunochromatography for quantitative detection of dexamethasone in milk and pork. Food Chem. 2021, 345, 128607. [Google Scholar] [CrossRef]
- Yao, X.; Wang, Z.; Zhao, M.; Liu, S.; Su, L.; Dou, L.; Li, T.; Wang, J.; Zhang, D. Graphite-like carbon nitride-laden gold nanoparticles as signal amplification label for highly sensitive lateral flow immunoassay of 17β-estradiol. Food Chem. 2021, 347, 129001. [Google Scholar] [CrossRef]
- Ge, W.; Suryoprabowo, S.; Kuang, H.; Liu, L.; Song, S. Rapid detection of triazophos in cucumber using lateral flow immunochromatographic assay. Food Agric. Immunol. 2020, 31, 1051–1060. [Google Scholar] [CrossRef]
- Cevallos-Cedeño, R.E.; Agulló, C.; Abad-Fuentes, A.; Abad-Somovilla, A.; Mercader, J.V. Enzyme and lateral flow monoclonal antibody-based immunoassays to simultaneously determine spirotetramat and spirotetramat-enol in foodstuffs. Sci Rep. 2021, 11, 1809. [Google Scholar] [CrossRef]
- Wu, K.-H.; Huang, W.-C.; Chang, S.-C.; Kao, C.-H.; Shyu, R.-H. Colloidal silver-based lateral flow immunoassay for rapid detection of melamine in milk and animal feed. Mater. Chem. Phys. 2019, 231, 121–130. [Google Scholar] [CrossRef]
- Chen, Q.; Qie, M.; Peng, X.; Chend, Y.; Wang, Y. Immunochromatographic assay for melamine based on luminescent quantum dot beads as signaling probes. RSC Adv. 2020, 10, 3307. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Verma, A.; Shinde, N.; Mann, B.; Gandhi, K.; Wichers, J.H.; van Amerongen, A. Adulteration of cow’s milk with buffalo’s milk detected by an on-site carbon nanoparticles-based lateral flow immunoassay. Food Chem. 2021, 351, 129311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, B.; Xiang, L.; Xiong, C.; Shi, Y.; Wu, L.; Meng, X.; Dong, G.; Xie, Y.; Sun, W. A novel onsite and visual molecular technique to authenticate saffron (Crocus sativus) and its adulterants based on recombinase polymerase amplification. Food Control 2019, 100, 117–121. [Google Scholar] [CrossRef]
- Qin, P.; Qiao, D.; Xu, J.; Song, Q.; Yao, L.; Lu, J.; Chen, W. Rapid visual sensing and quantitative identification of duck meat in adulterated beef with a lateral flow strip platform. Food Chem. 2019, 294, 224–230. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Liao, G.; Shang, Y.; Ge, C.; Chen, R.; Wang, Y.; Xu, W. Species-specific TM-LAMP and Trident-like lateral flow biosensor for on-site authenticity detection of horse and donkey meat. Sens. Actuators B Chem. 2019, 301, 127039. [Google Scholar] [CrossRef]
- Magiati, M.; Myridaki, V.M.; Christopoulos, T.K.; Kalogianni, D.P. Lateral flow test for meat authentication with visual detection. Food Chem. 2019, 274, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, E.A.; Byzova, N.A.; Hendrickson, O.D.; Popravko, D.S.; Belichenko, K.A.; Dzantiev, B.B.; Zherdev, A.V. Immunochromatographic Detection of Myoglobin as a Specific Biomarker of Porcine Muscle Tissues in Meat Products. Appl. Sci. 2020, 10, 7437. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Hua, M.Z.; Liu, J.; Zhang, H.; Hu, Y.; Chen, Y.; Lu, X.; Zheng, W. Development of a species-specific PCR coupled with lateral flow immunoassay for the identification of goose ingredient in foods. Food Control 2020, 114, 107240. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Zvereva, E.A.; Vostrikova, N.L.; Chernukha, I.M.; Dzantiev, B.B.; Zherdev, A.V. Lateral flow immunoassay for sensitive detection of undeclared chicken meat in meat products. Food Chem. 2021, 344, 128598. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Xiao, M.; Wei, S.; Yang, H.; Yin, R. Polymerase chain reaction with lateral flow sensor assay for the identification of horse meat in raw and processed meat products. Food Chem. 2021, 345, 128840. [Google Scholar] [CrossRef]
- Zhang, S.-W.; Sun, Y.-Y.; Sun, Y.-M.; Wang, H.; Li, Z.-F.; Xu, Z.-L. Visual upconversion nanoparticle-based immunochromatographic assay for the semi-quantitative detection of sibutramine. Anal. Bioanal. Chem. 2020, 412, 8135–8144. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, X.; Wang, L.; Guo, L.; Liu, L.; Kuang, H.; Xu, C. A fluorescence based immunochromatographic sensor for monitoring chlorpheniramine and its comparison with a gold nanoparticle-based lateral-flow strip. Analyst 2021, 146, 3589. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Zhang, J.; Li, N.; Chen, T.; Wang, L.; Zhang, F.; Mi, L.; Zhang, J.; Wang, S.; Wang, Y.; et al. Rapid and sensitive recombinase polymerase amplification combined with lateral flow strip for detecting African swine fever virus. Front. Microbiol. 2019, 10, 1004. [Google Scholar] [CrossRef]
- Wang, X.; Ji, P.; Fan, H.; Dang, L.; Wan, W.; Liu, S.; Li, Y.; Yu, W.; Li, X.; Ma, X.; et al. CRISPR/Cas12a technology combined with immunochromatographic strips for portable detection of African swine fever virus. Commun. Biol. 2020, 3, 62. [Google Scholar] [CrossRef] [Green Version]
- Tenzin, T.; Lhamo, K.; Rai, P.B.; Tshering, D.; Jamtsho, P.; Namgyal, J.; Wangdi, T.; Letho, S.; Rai, T.; Jamtsho, S.; et al. Evaluation of a rapid immunochromatographic test kit to the gold standard fluorescent antibody test for diagnosis of rabies in animals in Bhutan. BMC Vet. Res. 2020, 16, 183. [Google Scholar] [CrossRef]
- Kimitsuki, K.; Saito, N.; Yamada, K.; Park, C.-H.; Inoue, S.; Suzuki, M.; Saito-Obata, M.; Kamiya, Y.; Manalo, D.L.; Demetria, C.S.; et al. Evaluation of the diagnostic accuracy of lateral flow devices as a tool to diagnose rabies in post-mortem animals. PLoS Negl. Trop. Dis. 2020, 14, e0008844. [Google Scholar] [CrossRef]
- Liu, J.; Gao, R.; Shi, H.; Cong, G.; Chen, J.; Zhang, X.; Shi, D.; Cao, L.; Wang, X.; Zhang, J.; et al. Development of a rapid immunochromatographic strip test for the detection of porcine epidemic diarrhea virus specific SIgA in colostrum. J. Virol. Methods 2020, 279, 113855. [Google Scholar] [CrossRef]
- Xu, F.; Jin, Z.; Zou, S.; Chen, C.; Song, Q.; Deng, S.; Xiao, W.; Zhang, X.; Jia, A.; Tang, Y. EuNPs-mAb fluorescent probe based immunochromatographic strip for rapid and sensitive detection of porcine epidemic diarrhea virus. Talanta 2020, 214, 120865. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, F.; Tang, T.; Wang, M.; Yu, X.; Wang, R.; Li, Y.; Xu, Y.; Tang, L.; Wang, L.; et al. Development of a Colloidal Gold Immunochromatographic Strip Assay for Rapid Detection of Bovine Rotavirus. Viral Immunol. 2019, 32, 393–401. [Google Scholar] [CrossRef]
- Wang, H.; Guan, J.; Liu, X.; Shi, Y.; Wu, Q.; Luo, M.; Zhu, Y.; Wang, Z.; Wang, L.; Pan, Y. Rapid detection of avian leukosis virus using a fluorescent microsphere immunochromatographic test strip assay. Poult. Sci. 2019, 98, 6492–6496. [Google Scholar] [CrossRef]
- Liu, I.-L.; Lin, Y.-C.; Lin, Y.-C.; Jian, C.-Z.; Cheng, I.-C.; Chen, H.-W. A Novel Immunochromatographic Strip for Antigen Detection of Avian Infectious Bronchitis Virus. Int. J. Mol. Sci. 2019, 20, 2216. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Li, Y.; Jin, X.; Xu, Q.; Cheng, F.; Wang, X. Immunosensor-based rapid quantitative detection of Newcastle disease virus antibodies using innovative gold immunochromatographic assay. J. Appl. Microbiol. 2020, 129, 1751–1757. [Google Scholar] [CrossRef]
- Hou, F.; Bai, M.; Zhang, Y.; Liu, H.; Sun, S.; Guo, H. Fluorescent immunochromatographic assay for quantitative detection of the foot-and-mouth disease virus serotype O antibody. Microchem. J. 2020, 155, 104690. [Google Scholar] [CrossRef]
- Yang, M.; Mudabuka, B.; Quizon, K.; Nfon, C. Generation of monoclonal antibodies against foot-and-mouth disease virus SAT 2 and the development of a lateral flow strip test for virus detection. Transbound. Emerg. Dis. 2019, 66, 1158–1166. [Google Scholar] [CrossRef]
- Wang, S.; Wen, Y.; An, T.; Duan, G.; Sun, M.; Ge, J.; Li, X.; Yang, K.; Cai, X. Development of an Immunochromatographic Strip for Rapid Detection of Canine Adenovirus. Front. Microbiol. 2019, 10, 2882. [Google Scholar] [CrossRef]
- Manasa, M.; Revathi, P.; Prudhvi Chand, M.; Maroudam, V.; Navaneetha, P.; Dhinakar Raj, G.; Kavi Kishor, P.B.; De, B.; Rathnagiri, P. Protein-G-based lateral flow assay for rapid serodiagnosis of brucellosis in domesticated animals. J. Immunoassay Immunochem. 2019, 40, 149–158. [Google Scholar] [CrossRef]
- Syahruni, S.; Hartati, Y.W.; Yusuf, M.; Kusumawardani, S.; Wibawan, I.Y.T.; Arnafia, W.; Sibit, G.; Subroto, T. Development of lateral flow assay based on anti-IBDV IgY for the rapid detection of Gumboro disease in poultry. J. Virol. Methods 2021, 291, 114065. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Kiku, Y.; Sugawara, K.; Yabusaki, N.; Oono, K.; Fujii, K.; Suzuki, T.; Maehana, K.; Hayashi, T. Rapid Staphylococcus aureus Detection From Clinical Mastitis Milk by Colloidal Gold Nanoparticle-Based Immunochromatographic Strips. Front. Vet. Sci. 2020, 6, 504. [Google Scholar] [CrossRef]
- Alhussien, M.N.; Dang, A.K. Sensitive and rapid lateral-flow assay for early detection of subclinical mammary infection in dairy cows. Sci. Rep. 2020, 10, 11161. [Google Scholar] [CrossRef]
- Griffioen, K.; Cornelissen, J.; Heuvelink, A.; Adusei, D.; Mevius, D.; van der Wal, F.J. Development and evaluation of 4 loop-mediated isothermal amplification assays to detect mastitis-causing bacteria in bovine milk samples. J. Dairy Sci. 2020, 103, 8407–8420. [Google Scholar] [CrossRef]
- Ashforda, R.T.; Anderson, P.; Waring, L.; Davé, D.; Smith, F.; Delahay, R.J.; Gormley, E.; Chambers, M.A.; Sawyer, J.; Leselliera, S. Evaluation of the Dual Path Platform (DPP) VetTB assay for the detection of Mycobacterium bovis infection in badgers. Prev. Vet. Med. 2020, 180, 105005. [Google Scholar] [CrossRef] [PubMed]
- Fresco-Taboada, A.; Risalde, M.; Gortázar, C.; Tapia, I.; González, I.; Venteo, Á.; Sanz, A.; Rueda, P. A lateral flow assay for the rapid diagnosis of Mycobacterium bovis infection in wild boar. Transbound. Emerg. Dis. 2019, 66, 2175–2179. [Google Scholar] [CrossRef] [PubMed]
- Serhan, W.S.; Khan, R.A.; Gasim, E.F.; Alketbi, M.S.; De Massis, F.; Calistri, P.; Giovannini, A.; Al Hosani, M.A.; Al Jaberi, S.A.; Al Mansoori, A.M.; et al. Performance of an Immunochromatographic Test (ICT) in Comparison to Some Commonly Used Serological Tests for the Diagnosis of Brucellosis in Dromedary Camels (Camelus dromedarius). Microorganisms 2019, 7, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart Tayebwa, D.; Magdy Beshbishy, A.; Batiha, G.E.-S.; Komugisha, M.; Joseph, B.; Vudriko, P.; Yahia, R.; Alkazmi, L.; Hetta, H.F.; Yokoyama, N.; et al. Assessing the Immunochromatographic Test Strip for Serological Detection of Bovine Babesiosis in Uganda. Microorganisms 2020, 8, 1110. [Google Scholar] [CrossRef]
- Ganzinelli, S.; Benitez, D.; Gantuya, S.; Guswanto, A.; Florin-Christensen, M.; Schnittger, L.; Igarashi, I. Highly sensitive nested PCR and rapid immunochromatographic detection of Babesia bovis and Babesia bigemina infection in a cattle herd with acute clinical and fatal cases in Argentina. Transbound. Emerg. Dis. 2020, 67, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Yadav, S.C.; Kumar, S.; Dilbaghi, N. Development of membrane-based flow-through assay for detection of trypanosomosis in equines. J. Parasit. Dis. 2020, 44, 99–104. [Google Scholar] [CrossRef]
- Xifeng, W.; Mengfan, Q.; Kai, Z.; Guowu, Z.; Jing, L.; Lixia, W.; Jun, Q.; Qingling, M.; Shasha, G.; Yunfu, H.; et al. Development and evaluation of a colloidal gold immunochromatographic assay based on recombinant protein CatL1D for serodiagnosis of sheep fasciolosis. J. Helminthol. 2019, 94, e98. [Google Scholar] [CrossRef]
- Garcia, V.S.; Guerrero, S.; Gugliotta, L.; Gonzalez, V.D.G. A lateral flow immunoassay based on colored latex particles for detection of canine visceral leishmaniasis. Acta Trop. 2020, 212, 105643. [Google Scholar] [CrossRef]
- Karimi Kakh, M.; Golchin, M.; Kazemi Arababadi, M.; Daneshvar, H. Application of the Leishmania infantum 21-kDa recombinant protein for the development of an immunochromatographic test. Parasite Immunol. 2020, 42, e12770. [Google Scholar] [CrossRef] [PubMed]
- Ybañez, R.H.D.; Kyan, H.; Nishikawa, Y. Detection of antibodies against Toxoplasma gondii in cats using an immunochromatographic test based on GRA7 antigen. J. Vet. Med. Sci. 2020, 82, 441–445. [Google Scholar] [CrossRef] [Green Version]
- Oertly, M.; Gerber, V.; Anhold, H.; Chan, D.-S.; Pusterla, N. The Accuracy of Serum Amyloid A in Determining Early Inflammation in Horses After Long-Distance Transportation by Air. J. Equine Vet. Sci. 2021, 97, 103337. [Google Scholar] [CrossRef] [PubMed]
- Masello, M.; Lu, Z.; Erickson, D.; Gavalchin, J.; Giordano, J.O. A lateral flow-based portable platform for determination of reproductive status of cattle. J. Dairy Sci. 2020, 103, 4743–4753. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Sun, W.; Chen, L.; Zhou, H.; Fan, Y.; Diao, X.; Wang, B.; Zhao, H. Simultaneous and rapid detection of carbofuran and 3-hydroxy-carbofuran in water samples and pesticide preparations using lateral-flow immunochromatographic assay. Food Agric. Immunol. 2020, 31, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, L.; Kuang, H.; Xu, C. Preparing monoclonal antibodies and developing immunochromatographic strips for paraquat determination in water. Food Chem. 2020, 311, 125897. [Google Scholar] [CrossRef]
- Ruan, X.; Wang, X.Y.; Kwon, E.Y.; Wang, L.; Cheng, N.; Niu, X.; Ding, S.; Van Wie, B.J.; Lin, Y.; Du, D. Nanomaterial-enhanced 3D-printed sensor platform for simultaneous detection of atrazine and acetochlor. Biosens. Bioelectron. 2021, 184, 113238. [Google Scholar] [CrossRef]
- Cheng, N.; Shi, Q.; Zhu, C.; Li, S.; Lin, Y.; Du, D. Pt–Ni(OH)2 nanosheets amplified two-way lateral flow immunoassays with smartphone readout for quantification of pesticides. Biosens. Bioelectron. 2019, 142, 111498. [Google Scholar] [CrossRef]
- Hassan, A.H.A.; Bergua, J.F.; Morales-Narváez, E.; Mekoçi, A. Validity of a single antibody-based lateral flow immunoassay depending on graphene oxide for highly sensitive determination of E. coli O157:H7 in minced beef and river water. Food Chem. 2019, 297, 124965. [Google Scholar] [CrossRef]
- Rames, E.K.; Macdonald, J. Rapid assessment of viral water quality using a novel recombinase polymerase amplification test for human adenovirus. Appl. Microbiol. Biotechnol. 2019, 103, 8115–8125. [Google Scholar] [CrossRef]
- Prentice, K.W.; Depalma, L.; Ramage, J.G.; Sarwar, J.; Parameswaran, N.; Petersen, J.; Yockey, B.; Young, J.; Joshi, M.; Thirunavvukarasu, N.; et al. Comprehensive Laboratory Evaluation of a Lateral Flow Assay for the Detection of Yersinia pestis. Health Secur. 2019, 17, 439–453. [Google Scholar] [CrossRef] [Green Version]
- Berlina, A.N.; Komova, N.S.; Zherdev, A.V.; Dzantiev, B.B. Combination of phenylboronic acid and oligocytosine for selective and specific detection of lead(ii) by lateral flow test strip. Anal. Chim. Acta. 2021, 1155, 338318. [Google Scholar] [CrossRef]
- Schwenke, K.U.; Spiehl, D.; Krauße, M.; Riedler, L.; Ruppenthal, A.; Villforth, K.; Meckel, T.; Biesalski, M.; Rupprecht, D.; Schwall, G. Analysis of free chlorine in aqueous solution at very low concentration with lateral flow tests. Sci. Rep. 2019, 9, 17212. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.; Wang, Y.; Zhou, J.; Li, C. Establishment and application of hyperbranched rolling circle amplification coupled with lateral flow dipstick for the sensitive detection of Karenia mikimotoi. Harmful Algae 2019, 84, 151–160. [Google Scholar] [CrossRef]
- Fu, M.; Chen, G.; Zhang, C.; Wang, Y.; Sun, R.; Zhou, J. Rapid and sensitive detection method for Karlodinium veneficum by recombinase polymerase amplification coupled with lateral flow dipstick. Harmful Algae 2019, 84, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, E.A.; Hendrickson, O.D.; Zherdev, A.V.; Dzantiev, B.B. Immunochromatographic tests for the detection of microcystin-LR toxin in water and fish samples. Anal. Methods 2020, 12, 392–400. [Google Scholar] [CrossRef]
- Yan, X.; Persaud, K.C. The Optimization of a Lateral Flow Immunoassay for Detection of Aflatoxin B1 in Potable Water Samples. IEEE Sens. J. 2019, 19, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Dzantiev, B.B.; Nadezhda, A.; Taranova, N.A.; Semeykina, A.A.; Zherdev, A.V. Lateral flow immunoassay for bisphenol A: Development of test strips and their application for ecological monitoring. J. Phys. Conf. Ser. 2019, 1172, 012088. [Google Scholar] [CrossRef]
- Ren, S.; Li, Q.; Wang, J.; Fan, B.; Bai, J.; Peng, Y.; Li, S.; Han, D.; Wu, J.; Wang, J.; et al. Development of a fast and ultrasensitive black phosphorus-based colorimetric/photothermal dual-readout immunochromatography for determination of norfloxacin in tap water and river water. J. Hazard. Mater. 2021, 402, 123781. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, X.; Li, D.; Hu, J.; Wan, D.; Zhang, Z.; Hammock, B.D. Development of nanobody-based flow-through dot ELISA and lateral-flow immunoassay for rapid detection of 3-phenoxybenzoic acid. Anal. Methods 2021, 13, 1757–1765. [Google Scholar] [CrossRef]
- Selvarajan, R.; Kanichelvam, P.S.; Balasubramanian, V.; Subramanian, S.S. A rapid and sensitive lateral flow immunoassay (LFIA) test for the on-site detection of banana bract mosaic virus in banana plants. J. Virol. Methods 2020, 284, 113929. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.K.; Kokane, S.B.; Gowda, S. Development of a reverse transcription recombinase polymerase based isothermal amplification coupled with lateral flow immunochromatographic assay (CTV-RT-RPA-LFICA) for rapid detection of Citrus tristeza virus. Sci. Rep. 2020, 10, 20593. [Google Scholar] [CrossRef]
- Ni, P.; Liu, L.; Hao, C.; Xu, X.; Song, S.; Kuang, H.; Xu, C. Rapid and Sensitive Immunochromatographic Method-Based Monoclonal Antibody for the Quantitative Detection of Metalaxyl in Tobacco. ACS Omega 2020, 5, 18168–18175. [Google Scholar] [CrossRef] [PubMed]
- Razo, S.C.; Safenkova, I.V.; Drenova, N.V.; Kharchenko, A.A.; Tsymbal, Y.S.; Varitsev, Y.A.; Zherdev, A.V.; Pakina, E.N.; Dzantiev, B.B. New lateral flow immunoassay for on-site detection of Erwinia amylovora and its application on various organs of infected plants. Physiol. Mol. Plant Pathol. 2021, 114, 101637. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Shmyglya, I.V.; Zherdev, A.V.; Dzantiev, B.B.; Safenkova, I.V. The Challenge for Rapid Detection of High-Structured Circular RNA: Assay of Potato Spindle Tuber Viroid Based on Recombinase Polymerase Amplification and Lateral Flow Tests. Plants 2020, 9, 1369. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Safenkova, I.V.; Drenova, N.V.; Zherdev, A.V.; Dzantiev, B.B. Development of lateral flow assay combined with recombinase polymerase amplification for highly sensitive detection of Dickeya solani. Mol. Cell. Probes 2020, 53, 101622. [Google Scholar] [CrossRef]
- Lei, X.; Xu, X.; Liu, L.; Kuang, H.; Xu, L.; Hao, C.; Xu, C. Rapid quantitative determination of fentanyl in human urine and serum using a gold-based immunochromatographic strip sensor. J. Mater. Chem. B 2020, 8, 8573–8584. [Google Scholar] [CrossRef]
- Angelini, D.J.; Biggs, T.D.; Prugh, A.M.; Smith, J.A.; Hanburger, J.A.; Llano, B.; Avelar, R.; Ellis, A.; Lusk, B.; Malik Naanaa, A.; et al. The use of lateral flow immunoassays for the detection of fentanyl in seized drug samples and postmortem urine. J. Forensic Sci. 2021, 66, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Angelini, D.J.; Biggs, T.D.; Prugh, A.M.; Smith, J.A.; Hanburger, J.A.; Llano, B.; Avelar, R.; Ellis, A.; Lusk, B.; Naanaa, A.; et al. Detection of fentanyl and derivatives using a lateral flow immunoassay. Forensic Chem. 2021, 23, 100309. [Google Scholar] [CrossRef]
- Guteneva, N.V.; Znoyko, S.L.; Orlov, A.V.; Nikitin, M.P.; Nikitin, P.I. Rapid lateral flow assays based on the quantification of magnetic nanoparticle labels for multiplexed immunodetection of small molecules: Application to the determination of drugs of abuse. Microchim. Acta 2019, 186, 62. [Google Scholar] [CrossRef]
- Thapa, D.; Samadi, N.; Patel, N.; Tabatabaei, N. Thermographic detection and quantification of THC in oral fluid at unprecedented low concentrations. Biomed. Opt. Express 2020, 24, 2178–2190. [Google Scholar] [CrossRef]
- Chand, R.; Mittal, N.; Srinivasan, S.; Rajabzadeh, A.R. Upconverting nanoparticle clustering based rapid quantitative detection of tetrahydrocannabinol (THC) on lateral-flow immunoassay. Analyst 2021, 146, 574–580. [Google Scholar] [CrossRef]
- Samadi, N.; Thapa, D.; Salimi, M.; Parkhimchyk, A.; Tabatabaei, N. Low-Cost Active Thermography using Cellphone Infrared Cameras: From Early Detection of Dental Caries to Quantification of THC in Oral Fluid. Sci. Rep. 2020, 10, 7857. [Google Scholar] [CrossRef]
- Ciesielski, A.L.; Wagner, J.R.; Alexander-Scott, M.; Smith, J.; Snawder, J. Surface Contamination Generated by “One-Pot” Methamphetamine Production. ACS Chem. Health Saf. 2021, 28, 49–54. [Google Scholar] [CrossRef]
- Kishbaugh, J.M.; Cielski, S.; Fotusky, A.; Lighthart, S.; Maguire, K.; Quarino, L.; Conte, J. Detection of prostate specific antigen and salivary amylase in vaginal swabs using SERATEC® immunochromatographic assays. Forensic Sci. Int. 2019, 304, 109899. [Google Scholar] [CrossRef]
- Murahashi, M.; Makinodan, M.; Yui, M.; Hibi, T.; Kobayashi, M. Immunochromatographic detection of human hemoglobin from deteriorated bloodstains due to methamphetamine contamination, aging, and heating. Anal. Bioanal. Chem. 2020, 412, 5799–5809. [Google Scholar] [CrossRef] [PubMed]
- Nuntawong, P.; Ochi, A.; Chaingam, J.; Tanaka, H.; Sakamoto, S.; Morimoto, S. The colloidal gold nanoparticle-based lateral flow immunoassay for fast and simple detection of plant-derived doping agent, higenamine. Drug Test. Anal. 2021, 13, 762–769. [Google Scholar] [CrossRef]
- Šuláková, A.; Fojtíková, L.; Holubová, B.; Bártová, K.; Lapčík, O.; Kuchař, M. Two immunoassays for the detection of 2C-B and related hallucinogenic phenethylamines. J. Pharmacol. Toxicol. Methods 2019, 95, 36–46. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, L.; Wang, Z.; Xu, X.; Song, S.; Xu, L.; Kuang, H.; Li, A.; Xu, C. Immunoassays for the rapid detection of pantothenic acid in pharmaceutical and food products. Food Chem. 2021, 348, 129114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Nan, T.-G.; Xin, J.; Zhan, Z.-L.; Kang, L.-P.; Yuan, Y.; Wang, B.-M.; Huang, L.-Q. Development of a colloidal gold-based lateral flow dipstick immunoassay for rapid detection of chlorogenic acid and luteoloside in flos Lonicerae japonicae. J. Pharm. Biomed. Anal 2019, 170, 83–88. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.; Yu, M.; Zhao, H. The application of a lateral flow immunographic assay to rapidly test for dexamethasone in commercial facial masks. Anal. Bioanal. Chem. 2019, 411, 5703–5710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, X.; Tan, G.; Chen, X.; Wang, M.; Wang, B.; Cui, L. Development of a lateral flow dipstick for simultaneous and semi-quantitative analysis of dihydroartemisinin and piperaquine in an artemisinin combination therapy. Drug Test. Anal. 2019, 11, 1444–1452. [Google Scholar] [CrossRef]
- Kitisripanya, T.; Sermpradit, W.; Sakamoto, S.; Tanaka, H.; Putalun, W. An estimated quantitative lateral flow immunoassay for determination of artesunate using monoclonal antibody. Biomed. Chromatogr. 2020, 34, e4718. [Google Scholar] [CrossRef]
- Qian, J.; He, Q.; Liu, L.; Wang, M.; Wang, B.; Cui, L. Rapid quantification of artemisinin derivatives in antimalarial drugs with dipstick immunoassays. J. Pharm. Biomed. Anal. 2020, 191, 113605. [Google Scholar] [CrossRef]
- Novikova, A.S.; Ponomaryova, T.S.; Goryacheva, I.Y. Fluorescent AgInS/ZnS quantum dots microplate and lateral flow immunoassays for folic acid determination in juice samples. Microchim. Acta 2020, 187, 427. [Google Scholar] [CrossRef]
- Achilihu, H.; Feng, J.; Wang, L.; Bernert, J.Y. Tobacco Use Classification by Inexpensive Urinary Cotinine Immunoassay Test Strips. J. Anal. Toxicol. 2019, 43, 149–153. [Google Scholar] [CrossRef]
- Calabria, D.; Calabretta, M.M.; Zangheri, M.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Michelini, E.; Di Nardo, F.; Anfossi, L.; Baggiani, C.; et al. Recent Advancements in Enzyme-Based Lateral Flow Immunoassays. Sensors 2021, 21, 3358. [Google Scholar] [CrossRef]
- Zangheri, M.; Cevenini, L.; Anfossi, L.; Baggiani, C.; Simoni, P.; Di Nardo, F.; Roda, A. A simple and compact smartphone accessory for quantitative chemiluminescence-based lateral flow immunoassay for salivary cortisol detection. Biosens. Bioelectron. 2015, 64, 63–68. [Google Scholar] [CrossRef]
- Rong, Z.; Wang, Q.; Sun, N.; Jia, X.; Wang, K.; Xiao, R.; Wang, S. Smartphone-based fluorescent lateral flow immunoassay platform for highly sensitive point-of-care detection of Zika virus nonstructural protein 1. Anal. Chim. Acta 2019, 1055, 140–147. [Google Scholar] [CrossRef]
- Rajendran, V.K.; Bakthavathsalam, P.; Bergquist, P.L.; Sunna, A. Smartphone detection of antibiotic resistance using convective PCR and a lateral flow assay. Sens. Actuators B Chem. 2019, 298, 126849. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, K.; Xiao, K.; Qin, W.; Lu, W.; Tao, W.; Cui, D. Smartphone-based dual-modality imaging system for quantita-tive detection of color or fluorescent lateral flow immunochromatographic strips. Nanoscale Res. Lett. 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Jung, Y.; Heo, Y.; Lee, J.J.; Deering, A.; Bae, E. Smartphone-based lateral flow imaging system for detection of food-borne bacteria E.coli O157:H7. J. Microbiol. Methods 2020, 168, 105800. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Q.; Wang, J.; Liang, Z.; Li, J.; Wu, J.; Shen, X.; Lei, H.; Li, X. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens. Bioelectron. 2020, 158, 112178. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, J.; Fu, Q.; Sheng, L.; Liu, B.; Wang, C.; Li, C.; Li, T. A smartphone-based enzyme-linked immunochromatographic sensor for rapid quantitative detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2021, 329, 129163. [Google Scholar] [CrossRef]
- Zangheri, M.; Di Nardo, F.; Calabria, D.; Marchegiani, E.; Anfossi, L.; Guardigli, M.; Mirasoli, M.; Baggiani, C.; Roda, A. Smartphone biosensor for point-of-need chemiluminescence detection of ochratoxin A in wine and coffee. Anal. Chim. Acta 2021, 1163, 338515. [Google Scholar] [CrossRef]
- Lingervelder, D.; Koffijberg, H.; Kusters, R.; Ijzerman, M.J. Point-of-care testing in primary care: A systematic review on implementation aspects addressed in test evaluations. Int. J. Clin. Pract. 2019, 73, e13392. [Google Scholar] [CrossRef] [Green Version]
- Lattanzio, V.M.T.; von Holst, C.; Visconti, A. Experimental design for in-house validation of a screening immunoassay kit. The case of a multiplex dipstick for Fusarium mycotoxins in cereals. Anal. Bioanal. Chem. 2013, 405, 7773–7782. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.T.; Ciasca, B.; Powers, S.; von Holst, C. Validation of screening methods according to Regulation 519/2014/EU. Determination of deoxynivalenol in wheat by lateral flow immunoassay: A case study. Trends Anal. Chem. 2016, 76, 137–144. [Google Scholar] [CrossRef]
- Lattanzio, V.M.T.; Guarducci, N.; Powers, S.; Ciasca, B.; Pascale, M.; von Holst, C. Validation of a lateral flow immunoassay for the rapid determination of aflatoxins in maize by solvent free extraction. Anal. Methods 2018, 10, 123–130. [Google Scholar] [CrossRef]
- Lattanzio, V.M.T.; von Holst, C.; Lippolis, V.; De Girolamo, A.; Logrieco, A.F.; Mol, H.G.J.; Pascale, M. Evaluation of Mycotoxin Screening Tests in a Verification Study Involving First Time Users. Toxins 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Pecorelli, I.; Guarducci, N.; von Holst, C.; Bibi, R.; Pascale, M.; Ciasca, B.; Logrieco, A.F.; Lattanzio, V.M.T. Critical Comparison of Analytical Performances of Two Immunoassay Methods for Rapid Detection of AflatoxinM1 in Milk. Toxins 2020, 12, 270. [Google Scholar] [CrossRef]
- Offermann, N.; Conrad, K.; Fritzler, M.J.; Fooke Achterrath, M. Development and validation of a lateral flow assay (LFA) for the determination of IgG-antibodies to Pr3 (cANCA) and MPO (pANCA). J. Immunol. Methods 2014, 403, 1–6. [Google Scholar] [PubMed]
- Di Nardo, F.; Anfossi, L.; Ozella, L.; Saccani, A.; Giovannoli, C.; Spano, G.; Baggiani, C. Validation of a qualitative immunochromatographic test for the noninvasive assessment of stress in dogs. J. Chromatogr. B 2016, 1028, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Bheemavarapu, L.P.; Shah, M.I.; Joseph, J.; Sivaprakasam, M. IQVision: An Image-Based Evaluation Tool for Quantitative Lateral Flow Immunoassay Kits. Biosensors 2021, 11, 211. [Google Scholar] [CrossRef]
- Born, P.; Thran, S. The influence of CLIA ‘88 on physician office laboratories. J. Fam. Pract. 1998, 46, 319–327. [Google Scholar] [PubMed]
- Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on In Vitro Diagnostic Medical Devices and Repealing Directive 98/79/EC and Commission Decision 2010/227/EU. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0746 (accessed on 27 July 2021).
- Carter, D.J.; Cary, R.B. Lateral flow microarrays: A novel platform for rapid nucleic acid detection based on miniaturized lateral flow chromatography. Nucleic Acids Res. 2007, 35, e74. [Google Scholar] [CrossRef] [Green Version]
- Taranova, N.A.; Byzova, N.A.; Zaiko, V.V.; Starovoitova, T.A.; Vengerov, Y.Y.; Zherdev, A.V.; Dzantiev, B.B. Integration of lateral flow and microarray technologies for multiplex immunoassay: Application to the determination of drugs of abuse. Microchim. Acta 2013, 180, 1165–1172. [Google Scholar] [CrossRef]
- Safenkova, I.V.; Pankratova, G.K.; Zaitsev, I.A.; Varitsev, Y.A.; Vengerov, Y.Y.; Zherdev, A.V.; Dzantiev, B.B. Multiarray on a test strip (MATS): Rapid multiplex immunodetection of priority potato pathogens. Anal. Bioanal. Chem. 2016, 408, 6009–6017. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, L.; Liu, B.; Ge, Q.; Dong, J.; Zhao, X. Rapid and Ultrasensitive Quantification of Multiplex Respiratory Tract Infection Pathogen via Lateral Flow Microarray based on SERS Nanotags. Theranostics 2019, 9, 4849–4859. [Google Scholar] [CrossRef]
- Natarajan, S.; Jayaraj, J.; Prazeres, D.M.F. A Cellulose Paper-Based Fluorescent Lateral Flow Immunoassay for the Quantitative Detection of Cardiac Troponin I. Biosensors 2021, 11, 49. [Google Scholar] [CrossRef]
- Jiang, X.; Lillehoj, P.B. Lateral flow immunochromatographic assay on a single piece of paper. Analyst 2021, 146, 1084–1090. [Google Scholar] [CrossRef]
- Yang, J.M.; Kim, K.R.; Jeon, S.; Cha, H.J.; Kim, C.S. A sensitive paper-based lateral flow immunoassay platform using engineered cellulose-binding protein linker fused with antibody-binding domains. Sens. Actuators B Chem. 2021, 329, 129099. [Google Scholar] [CrossRef]
- Elter, A.; Bock, T.; Spiehl, D.; Russo, G.; Hinz, S.C.; Bitsch, S.; Baum, E.; Langhans, M.; Meckel, T.; Dörsam, E.; et al. Carbohydrate binding module-fused antibodies improve the performance of cellulose-based lateral flow immunoassays. Sci. Rep. 2021, 11, 7880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.Y.; Lee, K.S.; Ong, C.W.; Chan, M.Y.; Ang, L.W.; Leo, Y.S.; Chen, M.I.-C.; Lye, D.C.B.; Young, B.E. Diagnostic performance of COVID-19 serological assays during early infection: A systematic review and meta-analysis of 11 516 samples. Influenza Other Respir Viruses 2021, 15, 529–538. [Google Scholar] [CrossRef]
- Eftekhari, A.; Alipour, M.; Chodari, L.; Maleki Dizaj, S.; Ardalan, M.; Samiei, M.; Sharifi, S.; Zununi Vahed, S.; Huseynova, I.; Khalilov, R.; et al. A Comprehensive Review of Detection Methods for SARS-CoV-2. Microorganisms 2021, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Advice on the Use of Point-Of-Care Immunodiagnostic Tests for COVID-19. WHO Reference Number: WHO/2019-nCoV/Sci_Brief/POC_immunodiagnostics/2020.1. Available online: https://www.who.int/publications/i/item/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19-scientific-brief (accessed on 27 July 2021).
- European Centre for Disease Prevention and Control. Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK. 19 November 2020. ECDC: Stockholm; 2020. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Options-use-of-rapid-antigen-tests-for-COVID-19_0.pdf (accessed on 27 July 2021).
- Scohy, A.; Anantharajah, A.; Bodéus, M.; Kabamba-Mukadi, B.; Verroken, A.; Rodriguez-Villalobos, H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J. Clin. Virol. 2020, 129, 104455. [Google Scholar] [CrossRef]
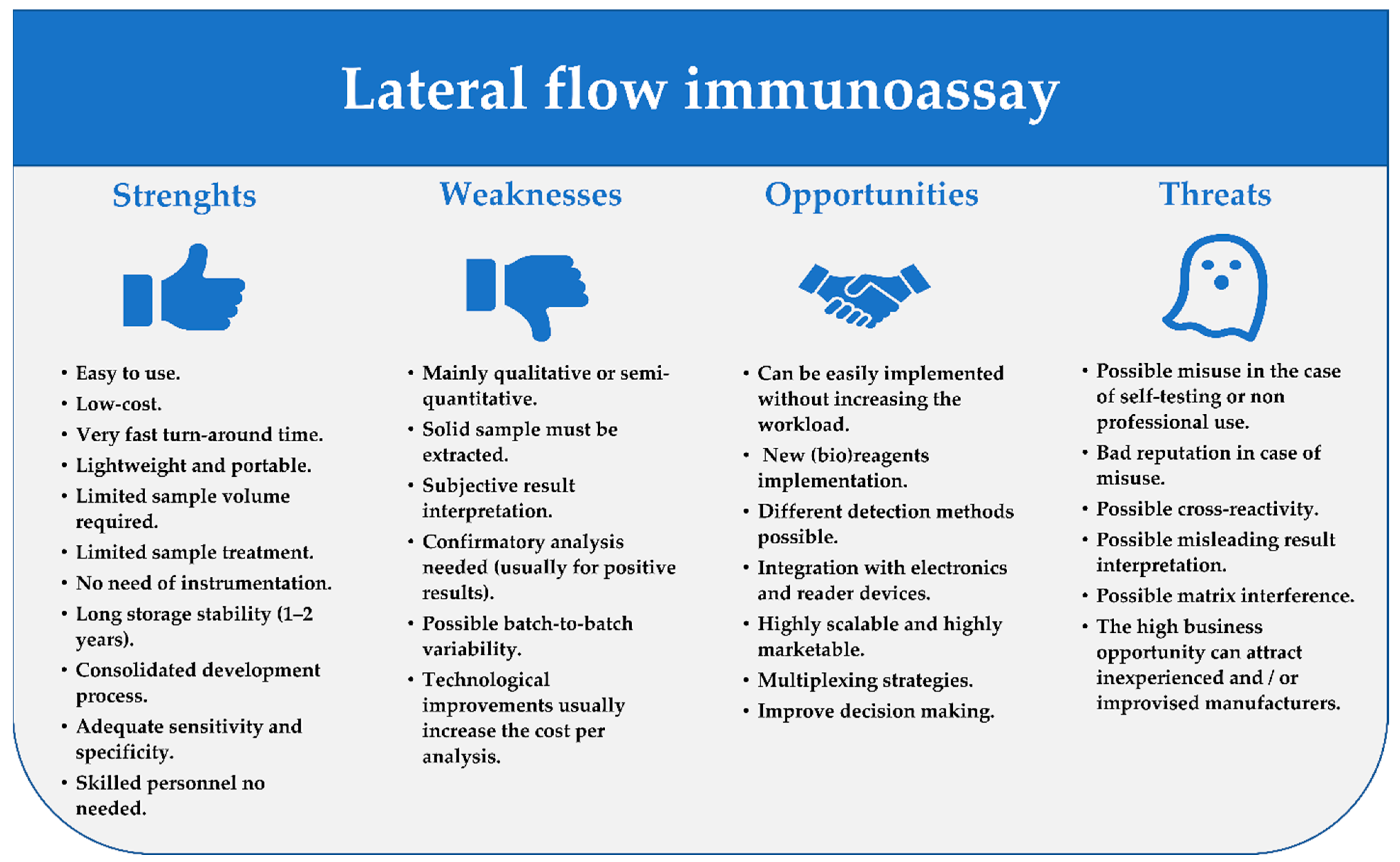
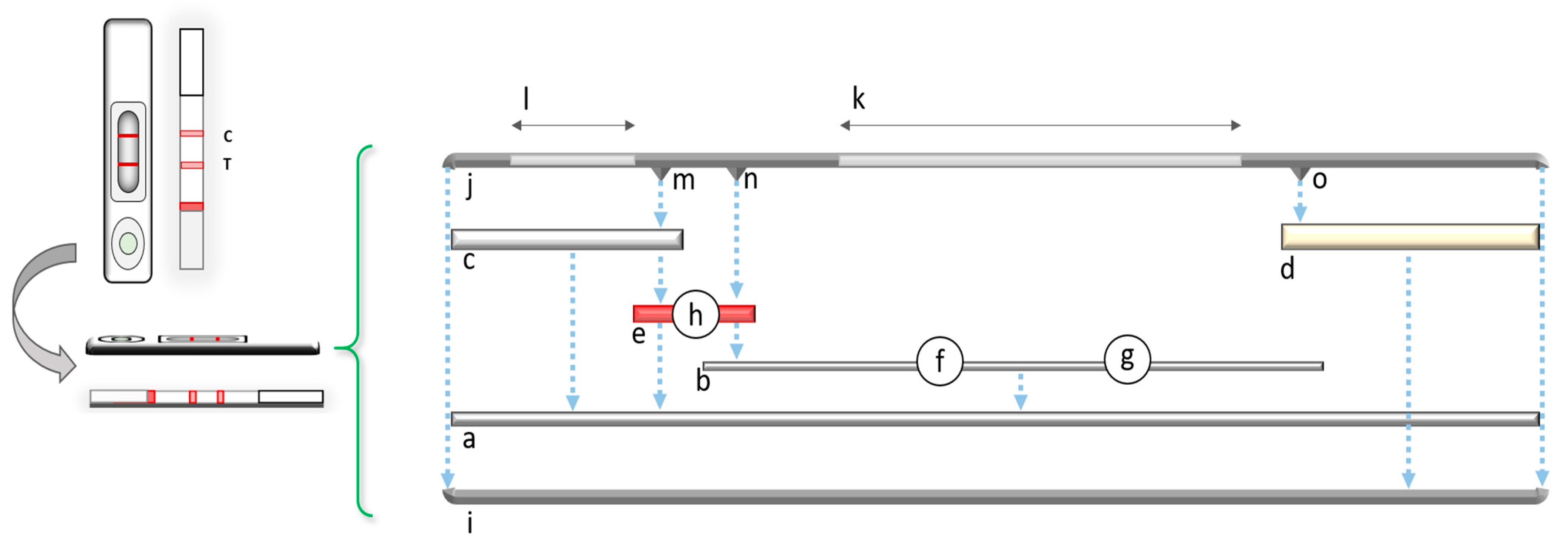
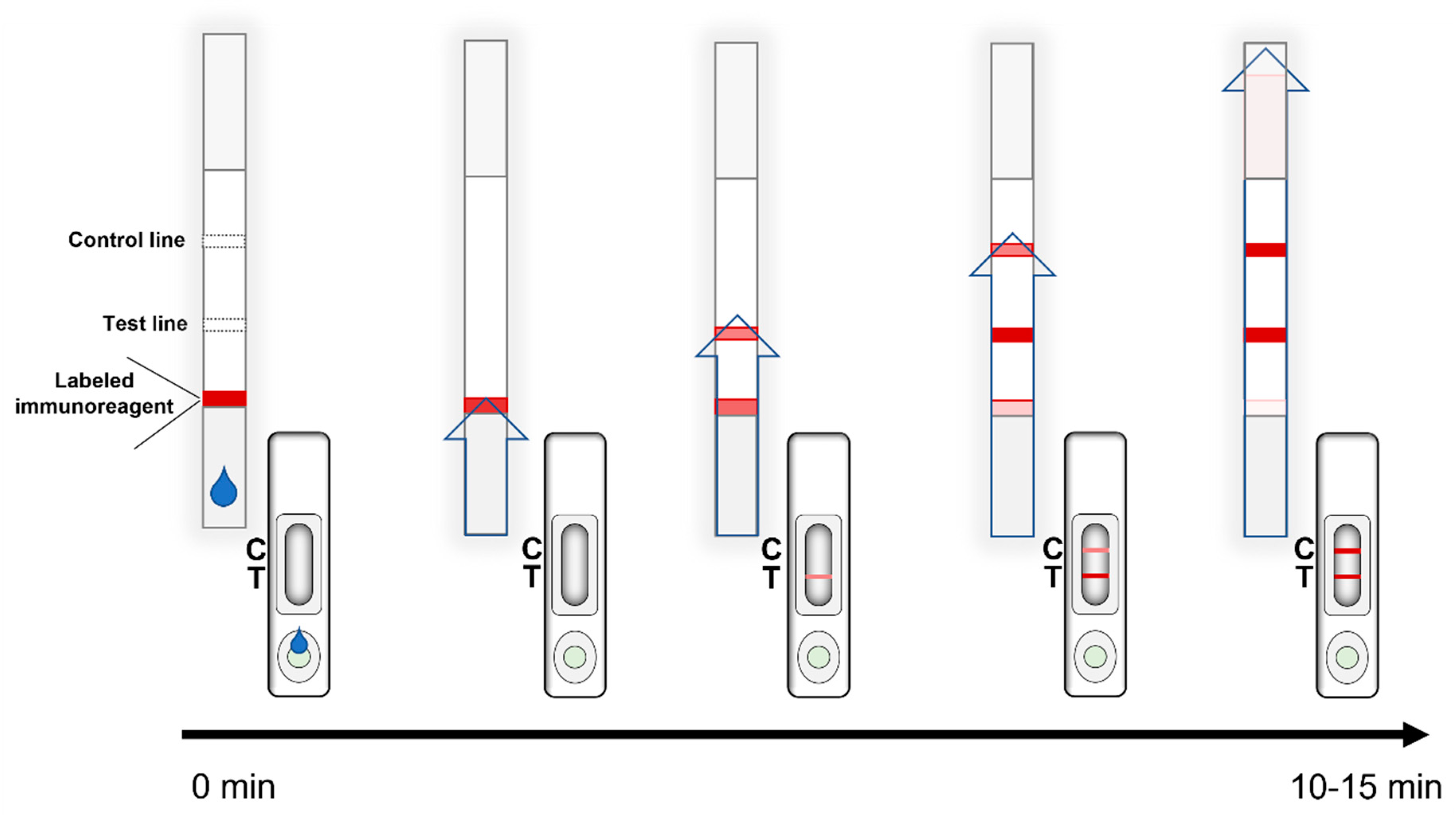
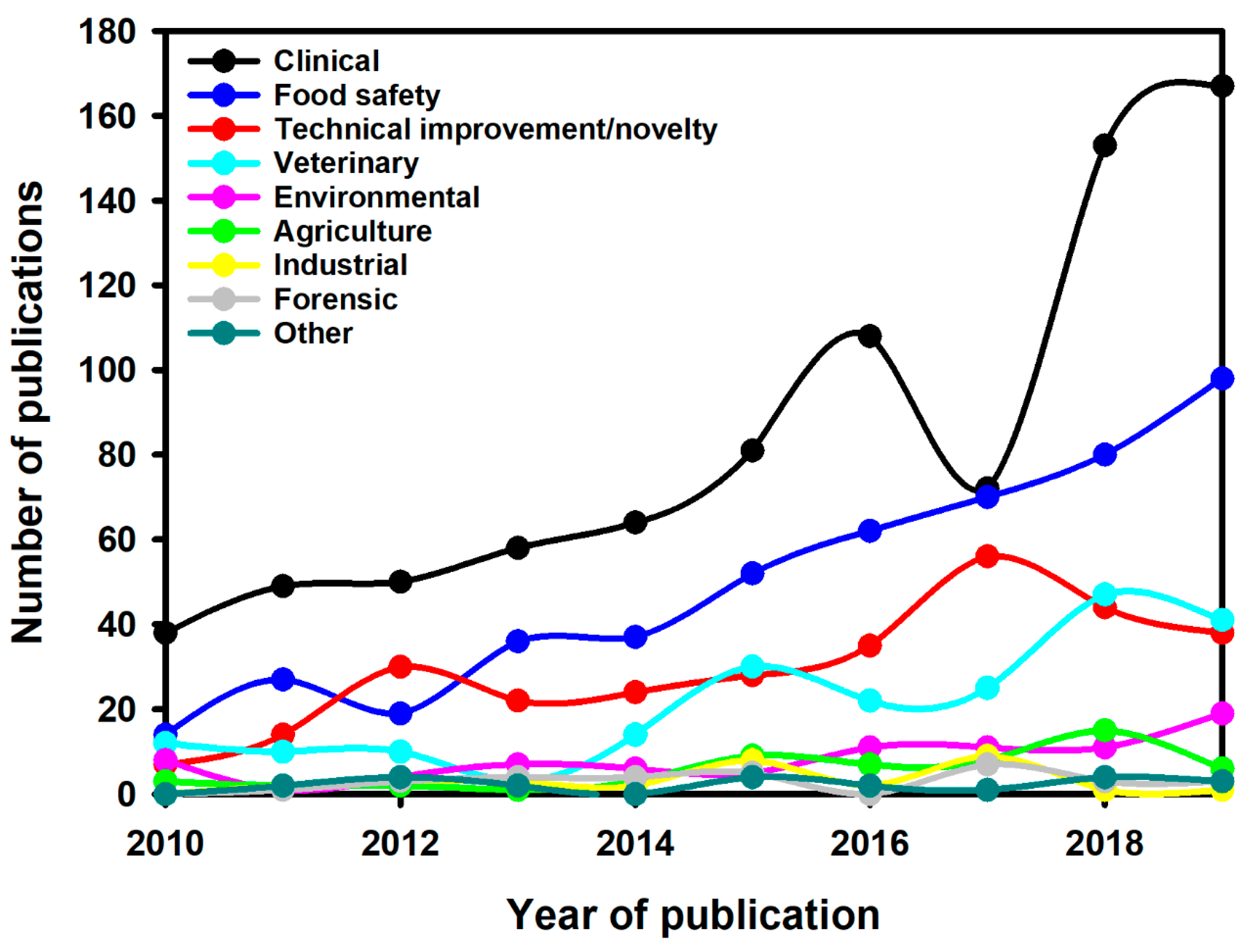
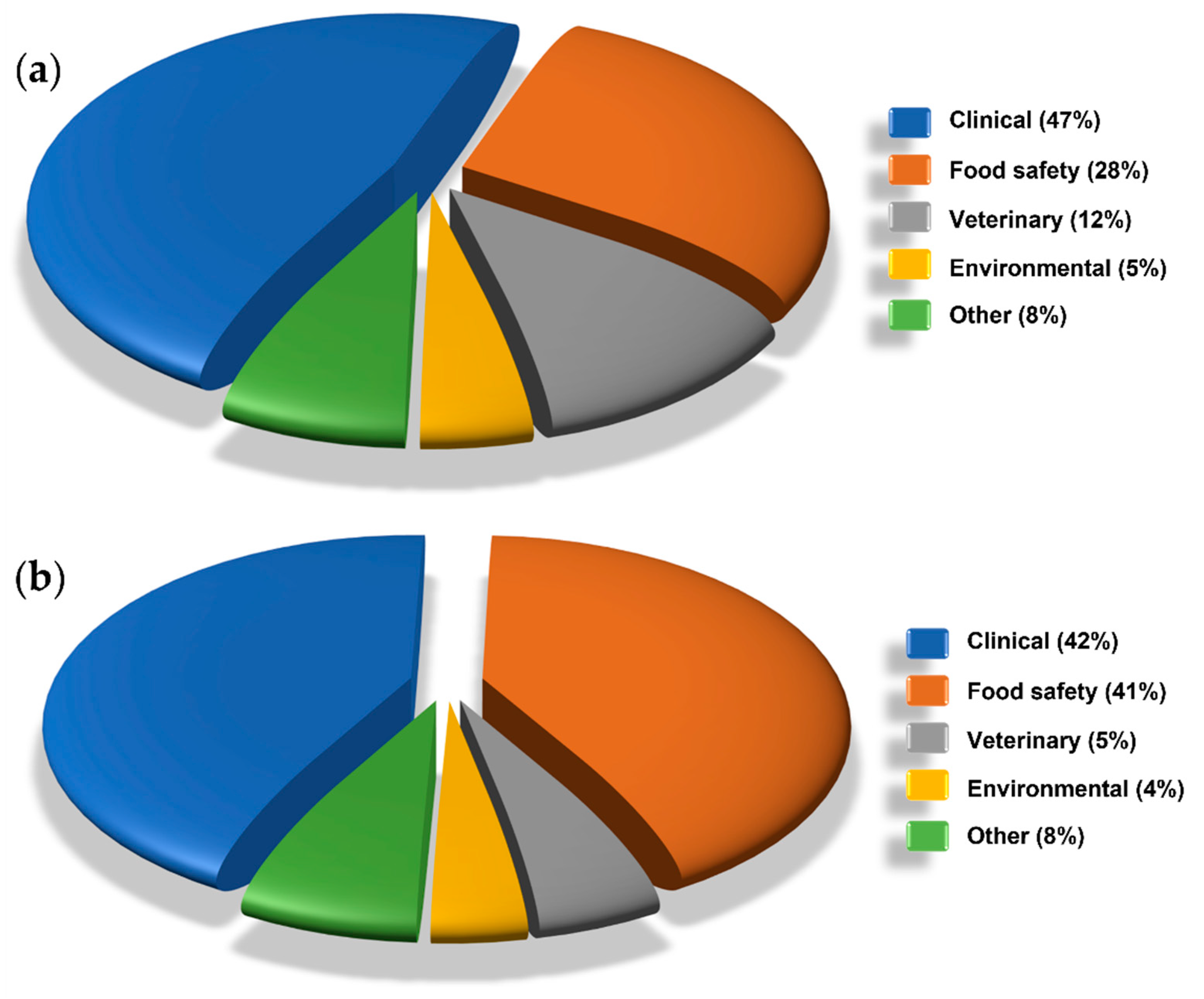
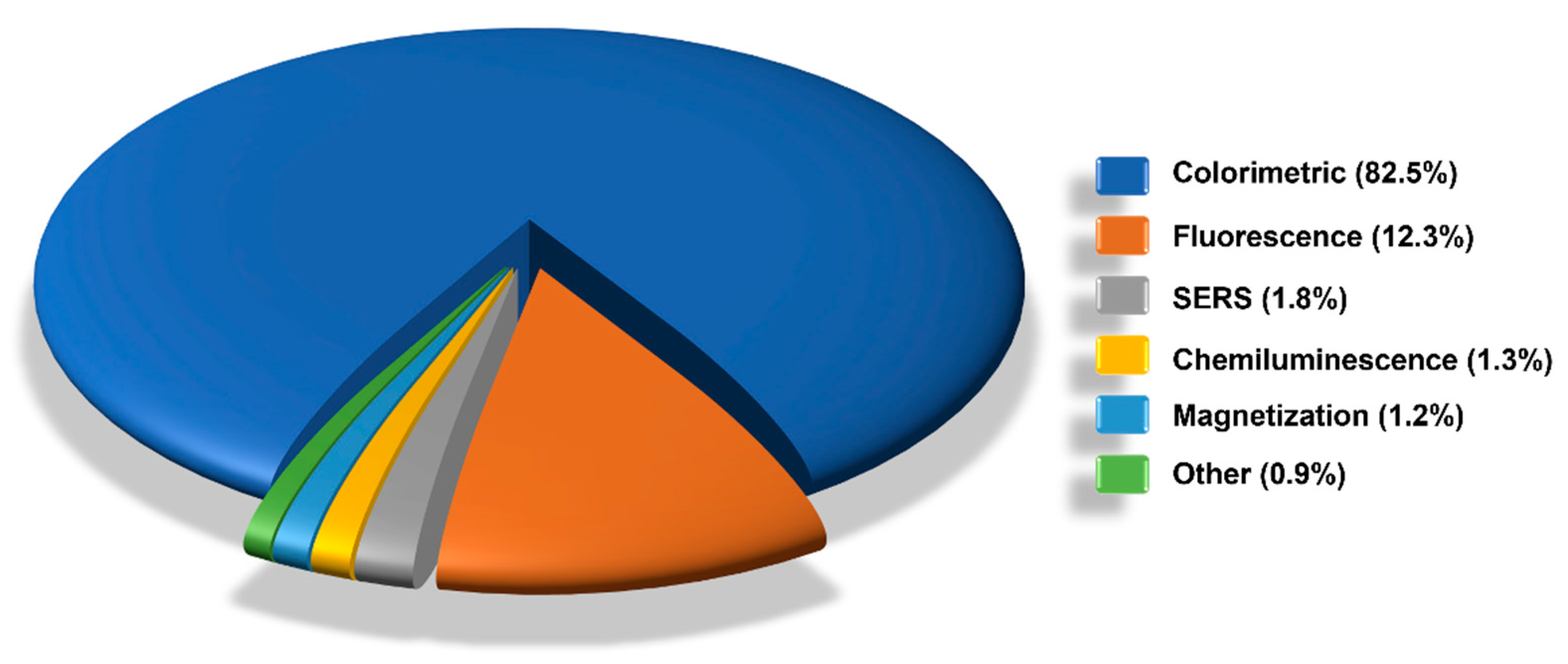
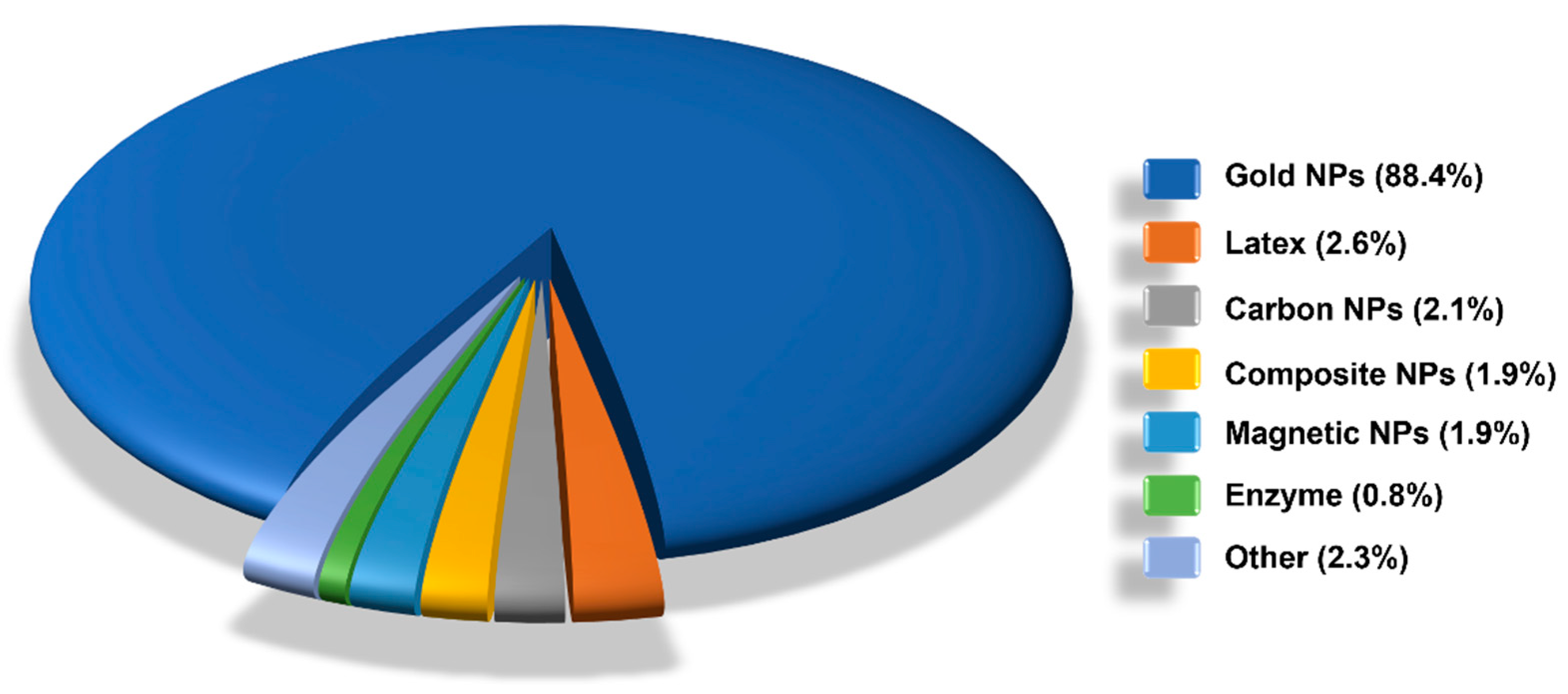
| Application Field | Target | Matrix | Reference | |
|---|---|---|---|---|
| Clinical | Viruses and related infections | Ebola virus | Whole blood, plasma | [85,86,87] |
| HIV-1 and -2 | Blood, serum | [84,88] | ||
| Noroviruses | Stool | [89] | ||
| Influenza A/B | Nasopharyngeal (nasal) swab | [90] | ||
| Chikungunya virus | Serum | [91] | ||
| Dengue virus | Blood | [92] | ||
| Herpes simplex virus type 2 | Plasma, serum | [93] | ||
| SARS-CoV-2 | Serum, blood, saliva | [94,95,96,97,98,99,100] | ||
| Bacteria and related infections | Brucellosis | Serum, plasma, whole blood | [101] | |
| Helicobacter pylori | Stool | [102] | ||
| Pneumococcal pneumonia | Pleural fluid | [103] | ||
| Plasmodium falciparum infections | Whole blood | [104] | ||
| Scrub typhus | Serum | [105] | ||
| Other infectious diseases | Burkholderia pseudomallei infections | Blood, urine, other bodily fluids | [106] | |
| Pneumocystis pneumonia | Serum | [107] | ||
| Strongyloidiasis | Serum | [108,109] | ||
| Candidiasis | Pharyngeal swabs | [110] | ||
| Progressive disseminated histoplasmosis | Serum | [111] | ||
| Toxoplasmosis | Serum | [112] | ||
| Allergic bronchopulmonary aspergillosis | Serum | [113,114] | ||
| Fascioliasis | Serum | [115] | ||
| Chagas disease | Serum | [116] | ||
| Cerebral angiostrongyliasis | Serum | [117] | ||
| Other diseases | Systemic lupus erythematosus | Serum | [118] | |
| Sepsis | Serum | [119] | ||
| Acute hyperglycemia and diabetes mellitus | Serum | [120,121] | ||
| Diabetic retinopathy | Urine | [122] | ||
| Alpha thalassaemia | Whole blood | [123] | ||
| Kidney injury | Urine | [124] | ||
| Cancers | Gastric | Plasma | [125] | |
| Cervical | Urine | [126] | ||
| Ovarian | Serum | [127] | ||
| Bladder | Urine | [128] | ||
| Prostate | Urine | [129] | ||
| Health status (bio)markers | Folate | Serum | [130] | |
| Hormones | Saliva, urine, serum | [131,132,133,134,135,136] | ||
| Cardiac biomarker | Serum, finger-prick blood | [137,138,139] | ||
| Ferritin | Serum | [140] | ||
| Myoglobin | ||||
| Therapeutic drug monitoring | Tenofovir | Urine | [141,142] | |
| Application Field | Target | Matrix | Reference | |
|---|---|---|---|---|
| Food safety | Toxins | Botulinum neurotoxin type A and Staphylococcal enterotoxin B | Milk, grape juice | [143] |
| Staphylococcal enterotoxin B | Milk, honey | [144] | ||
| Tetrodotoxin | Crucian, clam | [145] | ||
| Amatoxins | Mushrooms | [146] | ||
| Mycotoxins | Aflatoxin B1 and fumonisins | Maize flour | [82] | |
| Zearalenone | Maize, cereals | [147,148] | ||
| T-2 toxin | Tap water | [149] | ||
| Aflatoxin B1, zearalenone and deoxynivalenol | Feedstuff | [150] | ||
| Fumonisin B1 and deoxynivalenol | Grain | [151] | ||
| Antimicrobials | Tylosin and tilmicosin | Milk, pork | [152] | |
| Sulfamethazine | Egg, honey, pork | [153] | ||
| Bacitracin | Milk | [154] | ||
| Diclazuril | Chicken | [155] | ||
| Lincomycin | Milk, eggs, honey | [156] | ||
| Carbadox and Cyadox | Chicken breast | [157] | ||
| Bacitracin zinc | Milk | [158] | ||
| Lincomycin and tylosin | Milk, eggs, honey | [159] | ||
| β-lactams | Milk | [160] | ||
| Imidocarb | Milk, beef | [161] | ||
| Colistin and bacitracin | Milk | [162] | ||
| Bacteria | Staphylococcus aureus | Orange juice, lettuce salad, fish | [163] | |
| Escherichia coli O157:H7 | Milk, beef, pork, chicken, bread, jelly | [164,165] | ||
| Salmonella spp. | Chicken, eggs | [166,167] | ||
| Campylobacter jejuni | Milk, chicken | [168] | ||
| Vibrio parahaemolyticus | Clam, white clam, flower clam, razor lam, yellow croaker, fresh shrimp | [169] | ||
| Allergens | Milk casein, egg chicken albumin, hazelnut protein | Bakery products | [170] | |
| Tropomyosin | Various food products | [171] | ||
| Casein and β-lactoglobulin | Several food matrices | [172] | ||
| Major peanut allergen | Peanut oils | [173] | ||
| Parvalbumin | Fish | [174] | ||
| Gluten | Grain flours, food dough, burger patty, ice cream, soup | [175,176] | ||
| β-conglycinin | Skimmed milk | [177] | ||
| Hormones | Dexamethasone | Milk, pork meat | [178] | |
| 17β-estradiol | Chicken, fish, prawn, pork | [179] | ||
| Diethylstilbestrol and estradiol | Milk, shrimp tissue | [105] | ||
| Pesticides | Triazophos | Cucumber | [180] | |
| Spirotetramat and spirotetramat-enol | Wine, grape juice, and grapes. | [181] | ||
| Adulterants/food identification/illegal additives | Melamine | Milk, animal feed; | [182,183] | |
| Specific buffalo’s milk protein | Cow’s milk | [184] | ||
| Saffron genomic DNA | Dried herbal materials | [185] | ||
| Duck meat | Beef meat | [186] | ||
| Horse and donkey meat | Several foods | [187] | ||
| Horse, pork beef, sheep meat | Fresh meat | [188] | ||
| Pork meat | Several meats | [189] | ||
| Goose meat | Raw, cooked food products | [190] | ||
| Chicken meat | Meat products | [191] | ||
| Horse meat | Raw, processed meat products | [192] | ||
| Sibutramine | Diet food | [193] | ||
| Chlorpheniramine | Herbal teas | [194] | ||
| Application Field | Target | Matrix | Reference | |
|---|---|---|---|---|
| Veterinary | Viruses and related infections | African swine fever | Blood, spleen, tissue | [195,196] |
| Rabies | Brain tissue | [197,198] | ||
| Porcine epidemic diarrhea | Colostrum, stool | [199,200] | ||
| Bovine rotavirus | Stool | [201] | ||
| Avian leukosis virus | Chicken meconium | [202] | ||
| Avian infectious bronchitis virus | Chicken throat and cloacal swab | [203] | ||
| Newcastle disease | Chicken serum | [204] | ||
| Foot-and-mouth disease | Serum, several tissue samples | [205,206] | ||
| Canine adenovirus | Canine serum, rectal swabs | [207] | ||
| Brucellosis | Serum | [208] | ||
| Gumboro disease | Poultry | [209] | ||
| Bacteria and related infections | Bovine mastitis | Milk | [210,211,212] | |
| Mycobacterium bovis infection | Bovine serum, whole blood; wild boar serum | [213,214] | ||
| Brucellosis | Dromedary camels serum | [215] | ||
| Other infectious diseases | Bovine babesiosis | Blood | [216,217] | |
| Trypanosomosis | Equine serum | [218] | ||
| Fasciolosis | Sheep serum | [219] | ||
| Canine visceral leishmaniasis | Serum | [220,221] | ||
| Toxoplasmosis | Cat serum | [222] | ||
| Health status (bio)markers | Amyloid A | Horses’ serum | [223] | |
| Progesterone | Cattle plasma | [224] | ||
| Application Field | Target | Matrix | Reference | |
|---|---|---|---|---|
| Environmental | Pesticides | Carbofuran and 3-hydroxy-carbofuran | Water | [225] |
| Paraquat | Water | [226] | ||
| Atrazine and acetochlor | Water | [227] | ||
| Acetochlor and fenpropathrin | Tap water | [228] | ||
| Pathogens | E. coli O157:H7 | River water | [229] | |
| Human adenovirus | Wastewater | [230] | ||
| Yersinia pestis | Suspicious white powders, aerosol samples | [231] | ||
| Heavy metals | Lead (II) | Drinking water | [232] | |
| Other pollutants | Free chlorine | Aqueous soluions | [233] | |
| Karenia mikimotoi | Marine water | [234] | ||
| Karlodinium veneficum | Seawater | [235] | ||
| Microcystin-LR toxin | Water and fish | [236] | ||
| Aflatoxin B1 | Potable water | [237] | ||
| Bisphenol A | Snow | [238] | ||
| Norfloxacin | Tap and river water | [239] | ||
| 3-phenoxybenzoic acid | Lake water | [240] | ||
| Application Field | Target | Matrix | Reference | |
|---|---|---|---|---|
| Other | Agriculture | Banana bract mosaic virus | Banana leaf tissues | [241] |
| Citrus tristeza virus | Citrus leaves | [242] | ||
| Metalaxyl | Tobacco leaves | [243] | ||
| Erwinia amylovora | Different plant parts | [244] | ||
| Potato spindle tuber viroid | Plant leanves | [245] | ||
| Dickeya solani | Potato tubers | [246] | ||
| Forensic | Fentanyl | Human urine and serum | [247,248,249] | |
| Morphine, fentanyl and methamphetamine | Human urine | [250] | ||
| Tetrahydrocannabinol | Human oral fluids | [251,252,253] | ||
| Methamphetamine | Surface | [254] | ||
| Prostate specific antigen and salivary amylase | Vaginal swab | [255] | ||
| Human hemoglobin | Bloodstain | [256] | ||
| Higenamine | Plant samples | [257] | ||
| Hallucinogenic phenethylamines | Human Urine | [258] | ||
| Industrial | Pantothenic acid | Pharmaceutical, food products | [259] | |
| Chlorogenic acid and luteoloside | Flos Lonicerae Japonicae | [260] | ||
| Dexamethasone | Commercial facial masks | [261] | ||
| Dihydroartemisinin and piperaquine | Commercial artemisinin-based combination therapy drugs | [262] | ||
| Artesunate | Pharmaceutical formulation | [263] | ||
| Artemisinin derivatives | Antimalarial drugs | [264] | ||
| Folic acid | Orange, apple, banana, grape juice | [265] | ||
| Other | Cotinine | Human urine | [266] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nardo, F.; Chiarello, M.; Cavalera, S.; Baggiani, C.; Anfossi, L. Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives. Sensors 2021, 21, 5185. https://doi.org/10.3390/s21155185
Di Nardo F, Chiarello M, Cavalera S, Baggiani C, Anfossi L. Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives. Sensors. 2021; 21(15):5185. https://doi.org/10.3390/s21155185
Chicago/Turabian StyleDi Nardo, Fabio, Matteo Chiarello, Simone Cavalera, Claudio Baggiani, and Laura Anfossi. 2021. "Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives" Sensors 21, no. 15: 5185. https://doi.org/10.3390/s21155185
APA StyleDi Nardo, F., Chiarello, M., Cavalera, S., Baggiani, C., & Anfossi, L. (2021). Ten Years of Lateral Flow Immunoassay Technique Applications: Trends, Challenges and Future Perspectives. Sensors, 21(15), 5185. https://doi.org/10.3390/s21155185









