Rapid Estimation of Crop Water Stress Index on Tomato Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Data
2.2. Water Stress Treatment
2.3. Crop Water Stress Index (CWSIW)
2.4. Camera Measurement Functions
2.5. Field Measurements
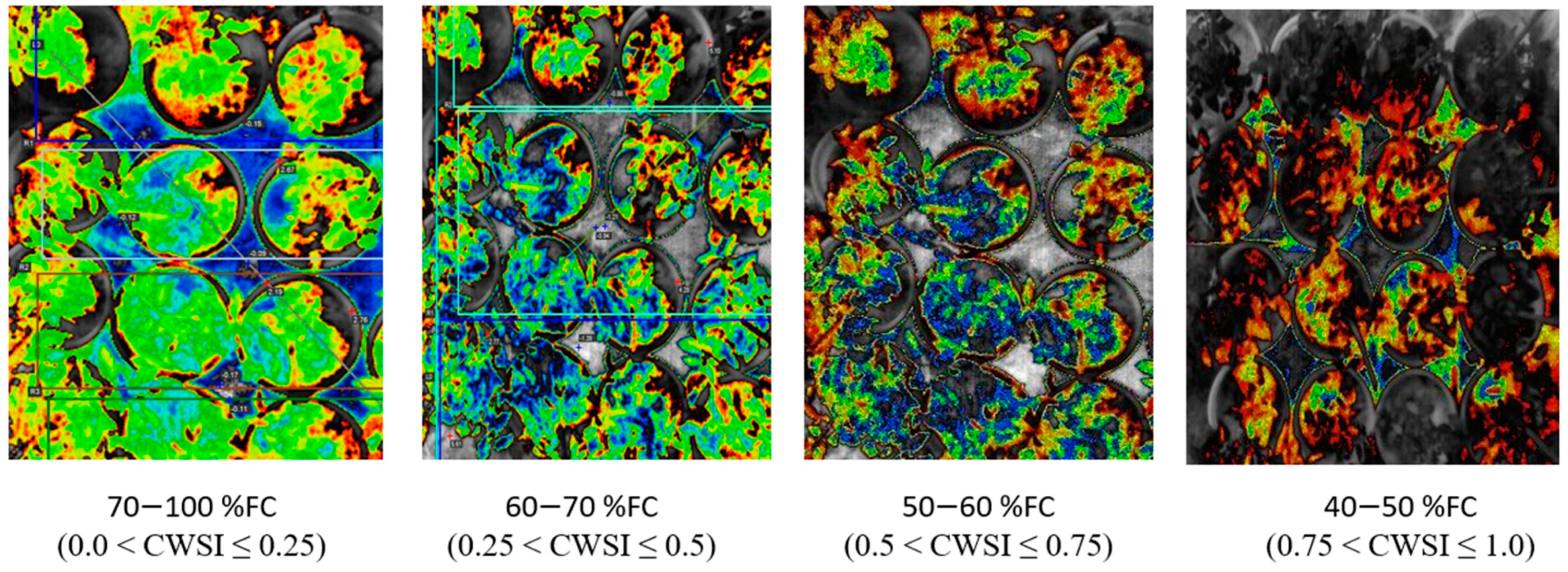
2.5.1. Image Acquisition
2.5.2. Canopy Temperature Measurement
2.5.3. The Conceptualization of CWSI Founded on the Idso Method
2.5.4. Crop Sampling and VWC Calculation
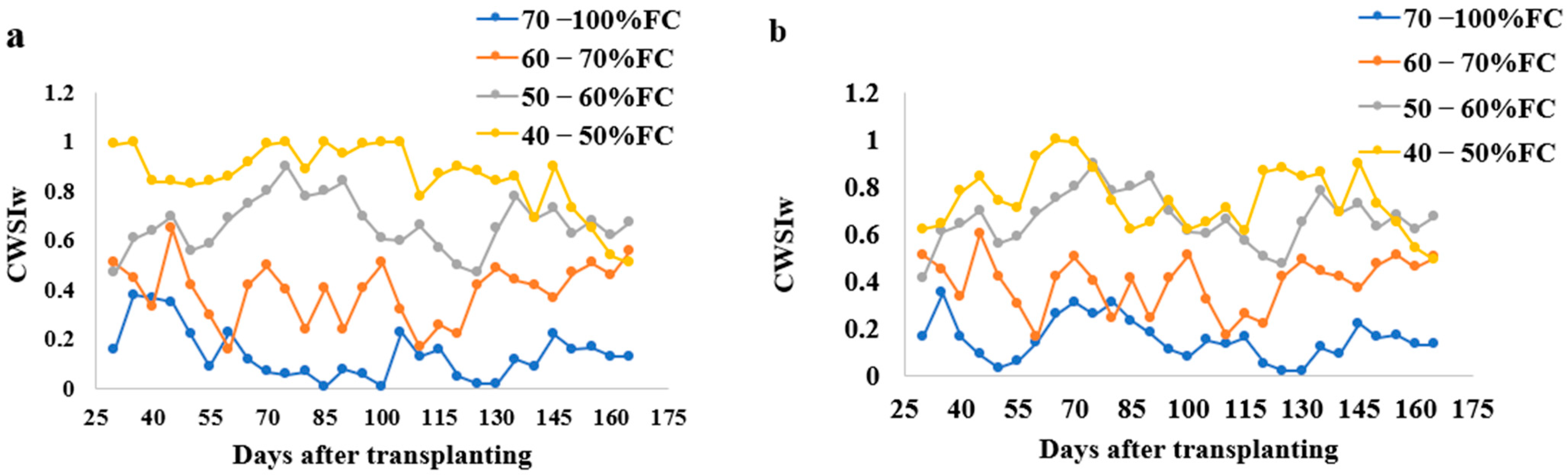
2.5.5. CWSIW Estimation
3. Results
3.1. Soil Water Content Measurement
Soil Moisture Variation
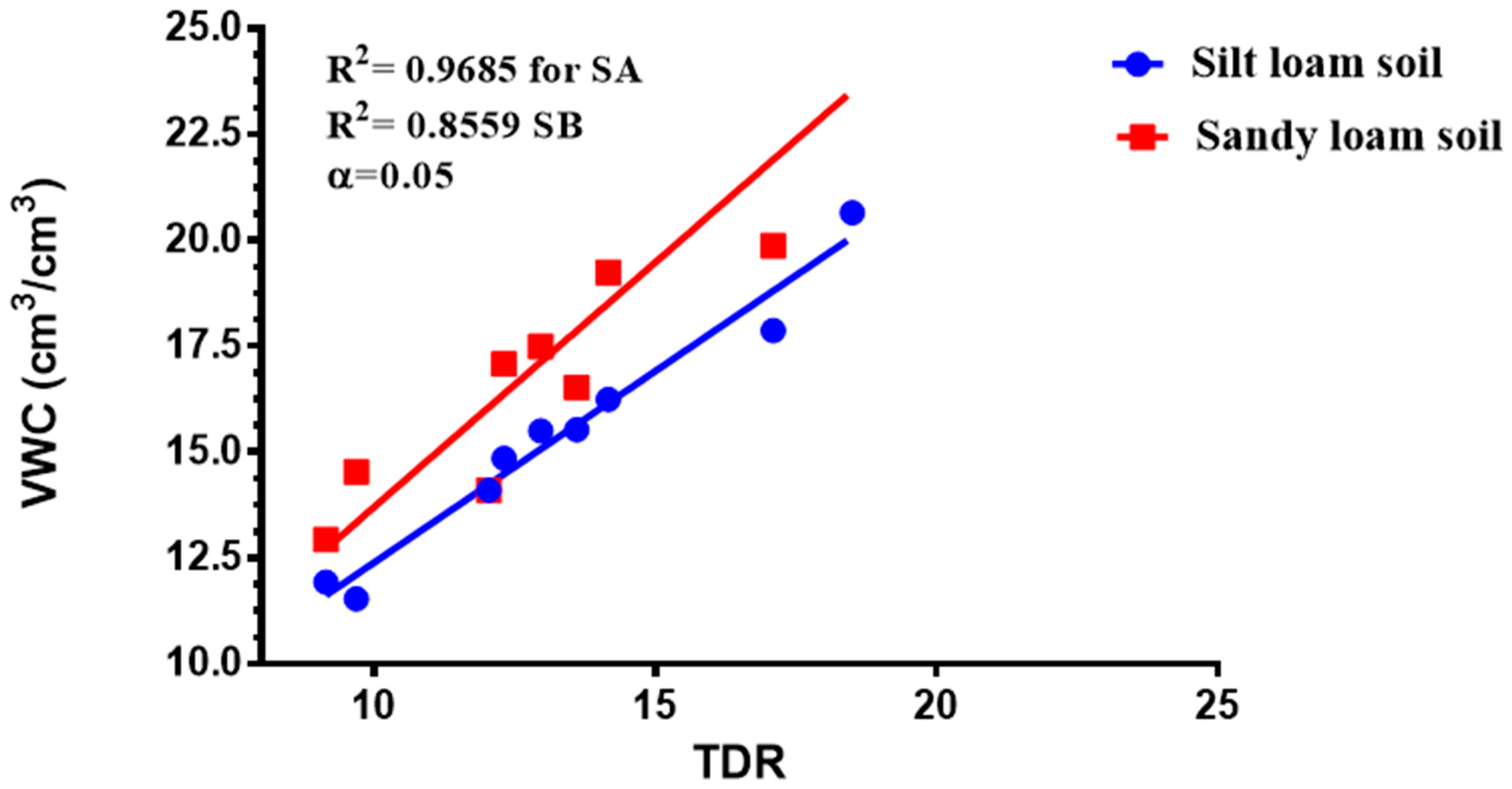
3.2. Crop Water Stress Index (CWSI) and Baseline Equations for Tomatoes
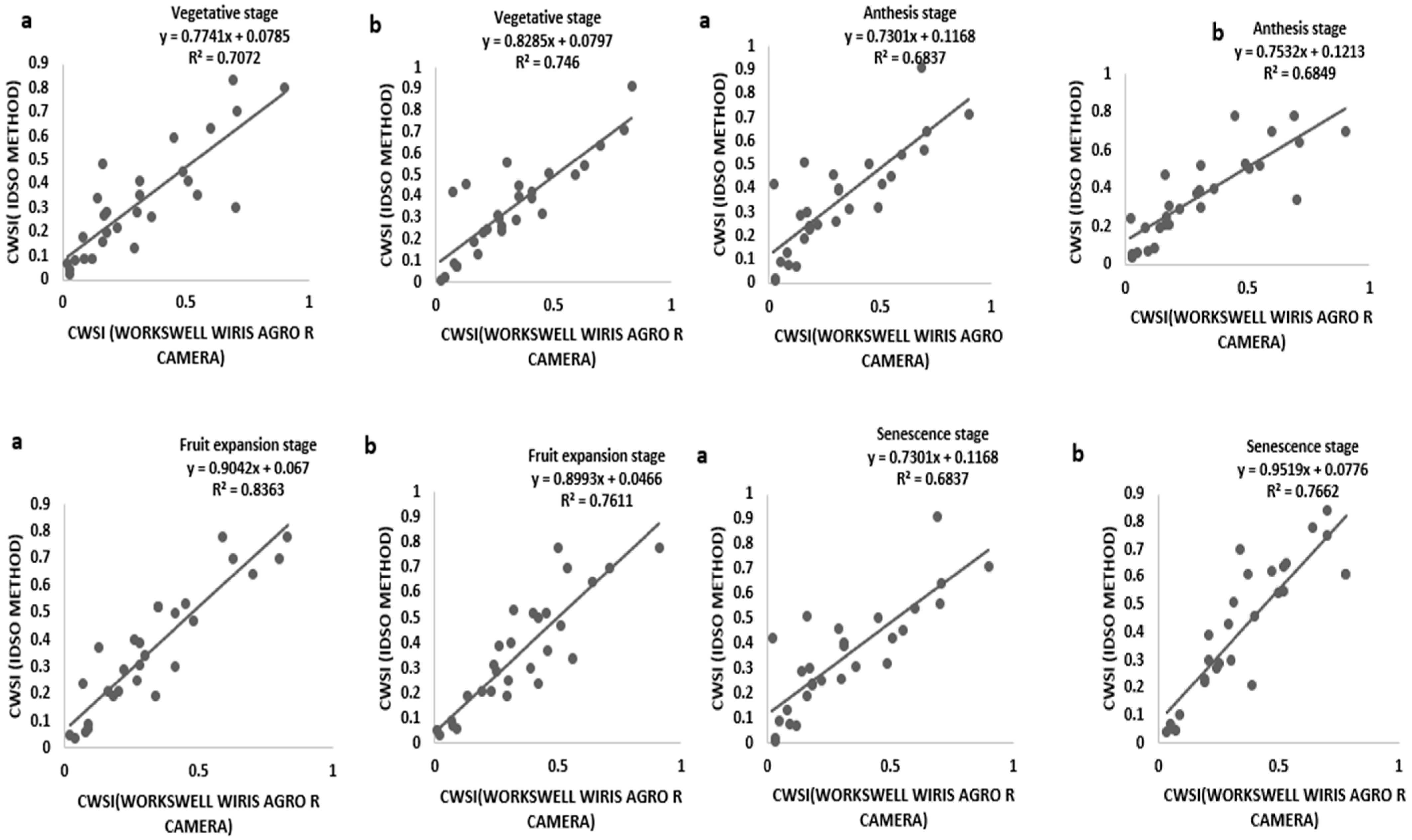
3.3. CWSI Based on the Field Measurement by Idso Method and a WORKSWELL WIRIS AGRO R CAMERA
3.4. Relationship between CWSI Estimated Using WORKSWELL WIRIS AGRO R INFRARED CAMERA and VWC
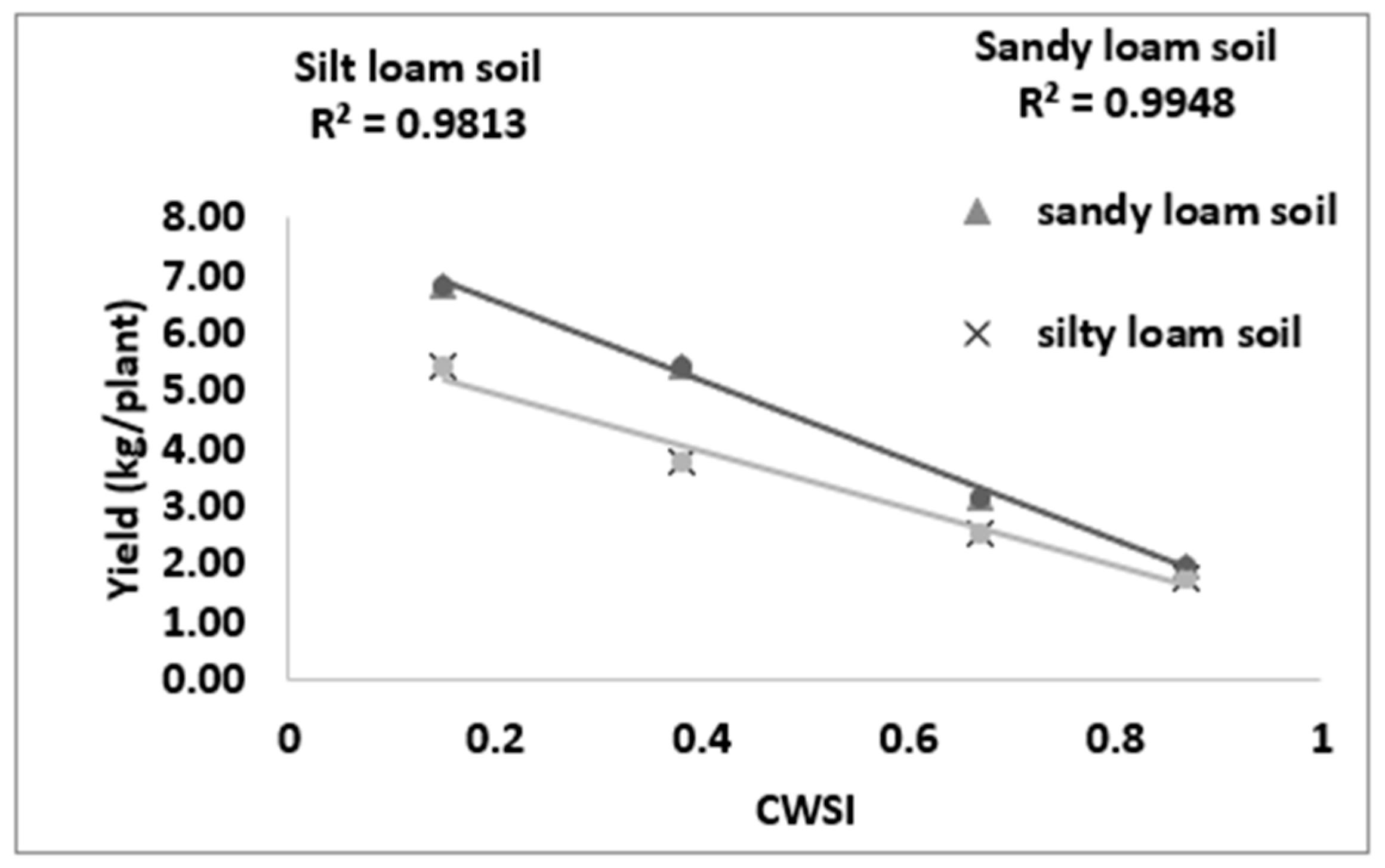
3.5. Correlating CWSIW with Yield
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Xiong, Y.; Huang, G.; Xu, X.; Huang, Q. Effects of water stress on processing tomatoes yield, quality and water use efficiency with plastic mulched drip irrigation in sandy soil of the Hetao irrigation district. Agric. Water Manag. 2017, 179, 205–214. [Google Scholar] [CrossRef]
- Hamouda, Y.A.; Shaghaleh, H.; Sheteiwy, M.; Guoa, X.; Elshaikha, N.; Khand, N.; Oumaroue, A.; Rahima, S. Impact of alternative wetting and soil drying and soil clay content on the morphological and physiological traits of rice roots and their relationships to yield and nutrient use-efficiency. Agric. Water Manag. 2019, 233, 3–9. [Google Scholar]
- Pék, Z.; Szuvandzsiev, P.; Daood, H.; Neményi, A.; Helyes, L. Effect of irrigation on yield parameters and antioxidant profiles of processing cherry tomato. Open Life Sci. 2014, 9, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hu, T.; Feng, P.; Wang, L.; Yang, S. Tomato yield and water use efficiency change with various soil moisture and potassium levels during different growth stages. PLoS ONE 2019, 14, e0213643. [Google Scholar] [CrossRef] [PubMed]
- Wittamperuma, I.; Hafeez, M.; Pakparvar, M.; Louis, J. Remote-sensing-based biophysical models for estimating LAI of irrigated crops in Murry darling basin. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2012, 34, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Sui, R.; Baggard, J. Development and evaluation of a variable rate irrigation method in Mississippi Delta. Trans. Am. Soc. Agric. Biol. Eng. 2020, 19, 2880. [Google Scholar] [CrossRef] [Green Version]
- Lahoz, I.; Pérez-de-Castro, A.; Valcárcel, M.; Macua, J.I.; Beltránd, J.; Rosellóc, S.; Cebolla-Cornejo, J. Effect of water deficit on the agronomical performance and quality of processing tomato. Sci. Hortic. 2016, 200, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Fernández, J.; González-Zamora, A.; Sánchez, N.; Gumuzzio, A. A soil water based index as a suitable agricultural drought indicator. J. Hydrol. 2015, 522, 265–273. [Google Scholar] [CrossRef]
- Osroosh, Y.; Peters, R.T.; Campbell, C.S.; Zhang, Q. Automatic irrigation scheduling of apple tress using therietical crop water stress index with and innovative dynamic threshold. Comput. Electron. Agric. 2015, 118, 193–203. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Sensitivity of spectral vegetation indices for monitoring water stress in tomato plants. Comput. Electron. Agric. 2019, 163, 104860. [Google Scholar] [CrossRef]
- Ustin, S.; Darling, D.; Kefauver, S.; Greenberg, J.; Cheng, Y.; Whiting, M. Remotely sensed estimates of crop water demand. Int. Soc. Opt. Eng. 2004, 5544, 230–240. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Ustin, S.L. Modeling canopy water content for carbon estimates from MODIS data at land EOS validation sites. Int. Geosci. Remote Sens. 2001, 1, 342–344. [Google Scholar]
- Nemeskéri, E.; Molnár, K.; Vígh, R.; Nagy, J.; Dobos, A. Relationships between stomatal behaviour, spectral traits and water use and productivity of green peas (Pisum sativum L.) in dry seasons. Acta Physiol. Plant 2015, 37, 34. [Google Scholar] [CrossRef]
- Jackson, R. Canopy Temperature and Crop Water Stress; Academic Press: New York, NY, USA, 1982; Volume 1. [Google Scholar]
- Idso, S. Non-water stressed baselines: A key to measuring and interpreting plant water stress. Agric. Meteorol. 1982, 27, 59–70. [Google Scholar] [CrossRef]
- Idso, S.B.; Jackson, R.D.; Pinter, P.J.; Hatfield, J.H. Normalizing the stress degree-day parameter for environmental variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Veysi, S.; Ali Naseri, A.B.D.; Hamzeh, S.; Bartholomeus, H. A satellite based crop water stress index for irrigation scheduling in sugarcane fields. Agric. Water Manag. 2017, 189, 70–86. [Google Scholar] [CrossRef]
- Prashar, A.; Jones, H.G. Assessing drought responses using thermal infrared imaging. Methods Mol. Biol. 2016, 1398, 209–219. [Google Scholar] [PubMed] [Green Version]
- Jones, H. Remote sensing of plant stresses and its use in irrigation management. VII Int. Symp. Irrig. Hortic. Crops 2012, 1038, 239–247. [Google Scholar] [CrossRef]
- Matese, A.; Baraldi, R.; Berton, A.; Cesaraccio, C.; Di-Gennaro, S.; Duce, P.; Zaldei, A. Estimation of Water Stress in Grapevines Using Proximal and Remote Sensing Methods. Remote Sens. 2018, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- Jackson, T.; Chen, D.; Cosh, M.; Li, F.; Anderson, M.; Walthall, C.; Doriaswamy, P.; Hunt, E. Vegetation water content mapping using Landsat data derived normalized difference water index for corn and soybeans. Remote Sens. Environ. 2004, 92, 475–482. [Google Scholar] [CrossRef]
- Chen, D.; Huang, J.; Jackson, T.J. Vegetation water content estimation for corn and soybeans using spectral indices derived from MODIS near- and short-wave infrared bands. Remote Sens Environ. 2005, 98, 225–236. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, C.; Guo, N.; Li, Y.; Jian, S.; Yu, K. Determining the canopy water stress for spring wheat using Canopy Hyperspectral Reflectance Data in Loess Plateau Semiarid Regions. Lett. Spectrosc. 2015, 48, 492–498. [Google Scholar] [CrossRef]
- Mzid, N.; Cantore, V.; De-Mastro, G.; Albrizio, R.; Sellami, M.; Todorovic, M. The Application of Ground Based and Satellite Remote Sensing for Estimation of Bio-Physiological Parameters of Wheat Grown Under Different Water Regimes. Water 2020, 12, 2095. [Google Scholar] [CrossRef]
- El Jaafari, S. Durum wheat breeding for abiotic stresses resistance: Defining physiological traits and criteria. Options Mediterr. 2000, 40, 251–256. [Google Scholar]
- Zhang, L.; Zhang, H.; Niu, Y.; Han, W. Mapping Maize Water Stress Based on UAV Multispectral Remote Sensing. Remote Sens. 2019, 11, 605. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhou, G. Deriving a light use efciency estimation algorithm using in situ hyperspectral and eddy covariance measurements for a maize canopy in Northeast China. Ecol. Evol. 2017, 7, 4735–4744. [Google Scholar] [CrossRef]
- Taghvaeian, S.; Chavez, J.L.; Hansen, N.C. Infrared Thermometry to Estimate Crop Water Stress Index and Water Use of Irrigated Maize in Northeastern Colorado. Remote Sens. 2012, 4, 3619–3637. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Wang, Q.; Zheng, C. Best hyperspectral indices for tracing leaf water status as determined from leaf dehydration experiments. Ecol. Indic. 2015, 54, 96–100. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Katsoulas, N.; Elvanidi, A.; Ferentinos, K.P.; Bartzanas, T.; Kittas, C. Crop reflectance monitoring as a tool for water stress detection in greenhouses: A review. Biosyst. Eng. 2016, 151, 374–398. [Google Scholar] [CrossRef]
- Çolaka, Y.B.; Yazarb, A.; Çolakc, I.; Akçaa, H.; Duraktekina, G. Evaluation of Crop Water Stress Index (CWSI) for Eggplant under Varying Irrigation Regimes Using Surface and Subsurface Drip Systems. Agric. Agric. Sci. Procedia 2015, 4, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Magney, T.S.; Vierling, L.A.; Eitel, J.U.; Huggins, D.R.; Garrity, S.R. Response of high frequency Photochemical Reflectance Index (PRI) measurements to environmental conditions in wheat. Remote Sens. Environ. 2016, 173, 84–97. [Google Scholar] [CrossRef]
- Sakamoto, T.; Gitelson, A.; Arkebauer, T. MODIS-based corn grain yield estimation model incorporating crop phenology information. Remote Sens. Environ. 2013, 131, 215–231. [Google Scholar] [CrossRef]
- Ceccato, P.; Gobron, N.; Flasse, S.; Pinty, B.; Tarantola, S. Designing a spectral index to estimate vegetation water content from remote sensing data: Part 1. Theoretical approach. Remote Sens. Environ. 2002, 82, 198–207. [Google Scholar] [CrossRef]
- Tanriverdi, C.; Atilgan, A.; Degirmenci, H.; Akyuz1, A. Comparasion of Crop Water Stress Index (CWSI) and Water Deficit Index (WDI) by using Remote Sensing (RS). Infrastruct. Ecol. Rural AREAS 2017, 879–894. [Google Scholar] [CrossRef]
- Sepaskhah, A.R.; Kashefipour, S.M. Relationships between leaf water potential, CWSI, yield and fruit quality of sweet lime under drip irrigation. Agric. Water Manag. 1994, 25, 13–21. [Google Scholar] [CrossRef]
- Espinoza, C.Z.; Khot, L.R.; Sankaran, S.; Jacoby, P.W. High resolution multispectral and thermal remote sensing-based water stress assessment in subsurface irrigated grapevines. Remote Sens. 2017, 9, 961. [Google Scholar] [CrossRef] [Green Version]
- Trenberth, K.E.; Zhang, Y.; Fasullo, J.T. Relationships among top-of-atmosphere radiation and atmospheric state variables in observations and CESM. Adv. Earth Space Sci. 2015, 120, 10074–10090. [Google Scholar] [CrossRef] [Green Version]
- Gerhards, M.; Schlerf, M.; Mallick, K.; Udelhoven, T. Challenges and Future Perspectives of Multi- Hyperspectral Thermal Infrared Remote Sensing for Crop Water-Stress Detection: A Review. Remote Sens. 2019, 11, 1240. [Google Scholar] [CrossRef] [Green Version]
- Poblete-Echeverría, C.; Espinace, D.; Sepúlveda-Reyes, D.; Zúñiga, M.; Sanchez, M. Analysis of crop water stress index (CWSI) for estimating stem water potential in grapevines: Comparison between natural reference and baseline approaches. Acta Hortic. 2017, 1150, 189–194. [Google Scholar] [CrossRef]
- Aghda, S.F.; Ganjalipour, K.; Nabiollahi, K. Comparison of performance of inclinometer casing and TDR technique. J. Appl. Geophys. 2018, 150, 182–194. [Google Scholar] [CrossRef]
- Cataldo, A.; De-Benedetto, E.; Cannazza, G.; Monti, G.; Piuzzi, E. TDR-based monitoring of rising damp through the embedding of wire-like sensing elements in building structures. Measurement 2017, 98, 355–360. [Google Scholar] [CrossRef]
- Tanriverdi, C. Using TDR in the agricultural water management. KSUJ Sci. Eng. 2005, 2, 108–115. [Google Scholar]
- Chung, C.C.; Lin, C.P.; Wang, K.; Lin, C.; Sheng-Ngui, Y.J. Improved TDR Method for Quality Control of Soil-Nailing Works. J. Geotech. Geoenviron. Eng. 2016, 142, 06015011. [Google Scholar] [CrossRef]
- Menziani, M. Soil volumetric water content measurement using TDR. Ann. Geofis. 1996, 91–96. [Google Scholar]
- Abdullah, N.H.; Kuan, N.; Ibrahim, A.; Ismail, B.; Majid, M.R.; Ramli, R.; Mansor, N. Determination of Soil Water Content Using Time Domain Reflectometer (TDR) for Clayey Soil. In Proceedings of the Advances in Civil Engineering and Science Technology, Penang, Malaysia, 5–6 September 2018; pp. 1–5. [Google Scholar]
- Degirmenci, H.; Gonen, E.; Boyaci, S.; Drip, S.; Components, Y.; Resources, W.; Location, T. A comparison of the gravimetric and TDR methods in terms of determining the soil water content of the corn plant. Ser. A Agron. 2016, 59, 152–158. [Google Scholar]
- Harmanto, V.M.; Salokhe, V.M.; Babel, M.S.; Tantau, H.J. Water requirement of drip irrigated tomatoes grown in greenhouse in tropical environment. Agric. Water Manag. 2005, 71, 225–242. [Google Scholar] [CrossRef]
- Yang, L.; Wei, W.; Chen, L.; Chen, W.; Wang, J. Response of temporal variation of soil moisture to vegetation restoration in semi-arid Loess Plateau, China. CATENA 2014, 115, 123–133. [Google Scholar] [CrossRef]
- Silber, A.; Israeli, Y.; Elingold, I.; Levi, M.; Levkovitch, I.; Russo, D.; Assouline, S. Irrigation with desalinated water: A step toward increasing water saving and crop yields. Water Resour. Res. 2015, 51, 450–464. [Google Scholar] [CrossRef]
- Mohamed, F.; EL-Aziz, A.B.D. The Effect of Silicon on Minimizing the Implications of Water Stress on Tomato Plants. Environ. Biodivers. Soil Secur. 2020, 4, 137–148. [Google Scholar]
- Gebregziabher, G.; Namara, E.R.; Holden, S. Poverty reduction with irrigation investment: An empirical case study from Tigray, Ethiopia. Agric. Water Dev. 2009, 96, 1837–1843. [Google Scholar] [CrossRef]
- Hussain, I.; Hanjra, M.A. Irrigation and poverty alleviation: Review of the empirical evidence. Irrig. Drain. 2004, 53, 1–15. [Google Scholar] [CrossRef]
- Wang, X.; Fuller, D.; Setemberg, L.; Miralles-Wilhelm, F. Foliar nutrient and water content in subtropical tree islands: A new chemohydrodynamic link between satellite vegetation indices and foliar d15N values. Remote Sens. Environ. 2011, 3, 923–930. [Google Scholar] [CrossRef]
- Lisar, S.Y.; Motafakkerazad, R.; Hossain, M.; Ismail, M.M. Water Stress in Plants: Causes, Effects and Responses. Water Stress 2012, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kirnak, H.; Kaya, C.; Tas, I.; Higgs, D. The influence of water deficit on vegetative growth, physiology, fruit yield and quality in eggplants. Bulg. J. Plant Physiol. 2001, 27, 34–46. [Google Scholar]
- Nemeskéri, E.; Neményi, A.; Bocs, A.; Pék, Z.; Helyes, L. Physiological factors and their relationship with the productivity of processing tomato under different water supplies. Water 2019, 11, 586. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Han, M.; Chavez, J.L.; Lan, Y. Improvement in estimation of soil water deficit by integrating airborne imagery data into a soil water balance model. Int. J. Agric. Biol. Eng. 2017, 10, 37–46. [Google Scholar]






| Soil Texture | Sand (%) | Silt (%) | Clay (%) | Bulk Density (g/cm3) | Saturation Point (%) | Field Capacity (%) | Permanent Wilting Point |
|---|---|---|---|---|---|---|---|
| Sandy loam | 75.4 | 20 | 4.6 | 1.34 | 48 | 21 | 9 |
| Silt loam | 43.53 | 39.93 | 16.63 | 1.32 | 45.73 | 31 | 19 |
| Soil Texture | pH | O.M (g/kg) | Total N (g/kg) | Total P (g/kg) | Total K (G/KG) | Alkalized N (mg/kg) | Avail. P (mg/kg) | Avail. K (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| Sandy loam | 5.64 | 15.91 | 1.23 | 0.88 | 9.30 | 450.28 | 195.72 | 428.43 |
| Silt loam | 5.30 | 22.97 | 1.518 | 0.865 | 19.59 | 72.71 | 28.25 | 85.50 |
| Growth Stages | Vegetative Stage | Anthesis Stage | Fruit Expansion Stage | Senescence Stage |
|---|---|---|---|---|
| Air Temperature (°C) | 30.6 | 25.1 | 23.9 | 27.4 |
| Relative humidity (%) | 74.6 | 80.45 | 70.2 | 83.2 |
| Vapor Pressure Deficit | 4.13 | 5.17 | 5.02 | 3.11 |
| Growth Stages | Minimum (%) | Maximum (%) | Mean (%) | |||
|---|---|---|---|---|---|---|
| Soil Type | SA | SB | SA | SB | SA | SB |
| Vegetative Stage | 75 | 69 | 83 | 76 | 79 | 72.5 |
| Anthesis Stage | 80 | 73 | 84 | 80 | 82 | 76.5 |
| Friut Expansion Stage | 79 | 82 | 85 | 86 | 82 | 84 |
| Senescence Stage | 73 | 65 | 80 | 74 | 76.5 | 69.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alordzinu, K.E.; Li, J.; Lan, Y.; Appiah, S.A.; AL Aasmi, A.; Wang, H. Rapid Estimation of Crop Water Stress Index on Tomato Growth. Sensors 2021, 21, 5142. https://doi.org/10.3390/s21155142
Alordzinu KE, Li J, Lan Y, Appiah SA, AL Aasmi A, Wang H. Rapid Estimation of Crop Water Stress Index on Tomato Growth. Sensors. 2021; 21(15):5142. https://doi.org/10.3390/s21155142
Chicago/Turabian StyleAlordzinu, Kelvin Edom, Jiuhao Li, Yubin Lan, Sadick Amoakohene Appiah, Alaa AL Aasmi, and Hao Wang. 2021. "Rapid Estimation of Crop Water Stress Index on Tomato Growth" Sensors 21, no. 15: 5142. https://doi.org/10.3390/s21155142
APA StyleAlordzinu, K. E., Li, J., Lan, Y., Appiah, S. A., AL Aasmi, A., & Wang, H. (2021). Rapid Estimation of Crop Water Stress Index on Tomato Growth. Sensors, 21(15), 5142. https://doi.org/10.3390/s21155142







