Miniaturization of Respiratory Measurement System in Artificial Ventilator for Small Animal Experiments to Reduce Dead Space and Its Application to Lung Elasticity Evaluation
Abstract
:1. Introduction
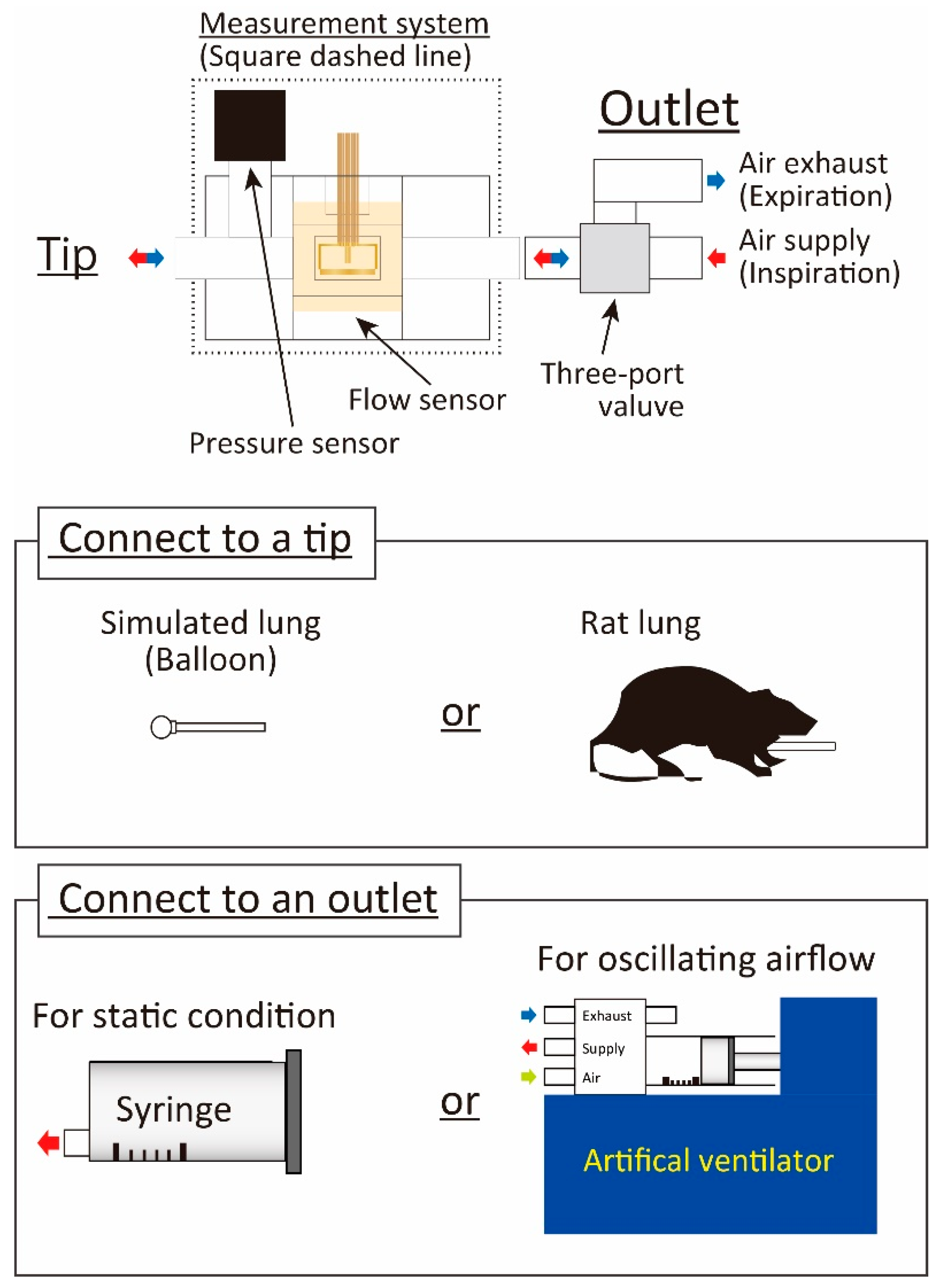
2. Respiratory Measurement System
2.1. Dead Space at Lung System in Animal
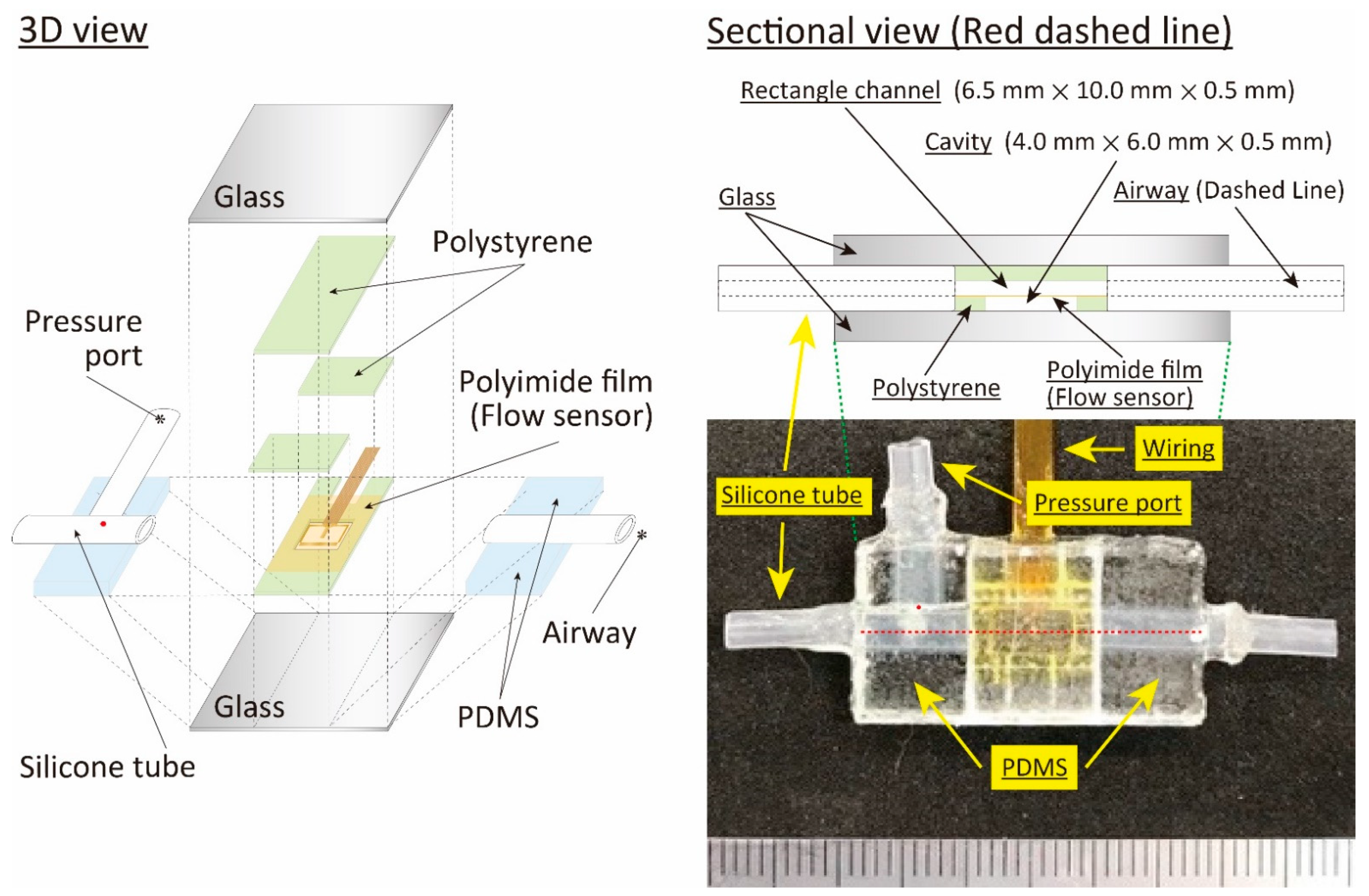
2.2. Structure and Fabrication
- (1)
- Variation of pressure value; the pressure variation insides of the balloon and the respiratory in the rat caused by the airflow supply are assumed up to 10 kPa and 1.0 kPa, respectively.
- (2)
- Resolution; the pressure variation becomes less than 100 Pa under the small airflow volume condition in the case of the rat. Thus, we assumed that a few Pa of resolution was needed in the following animal experiments.
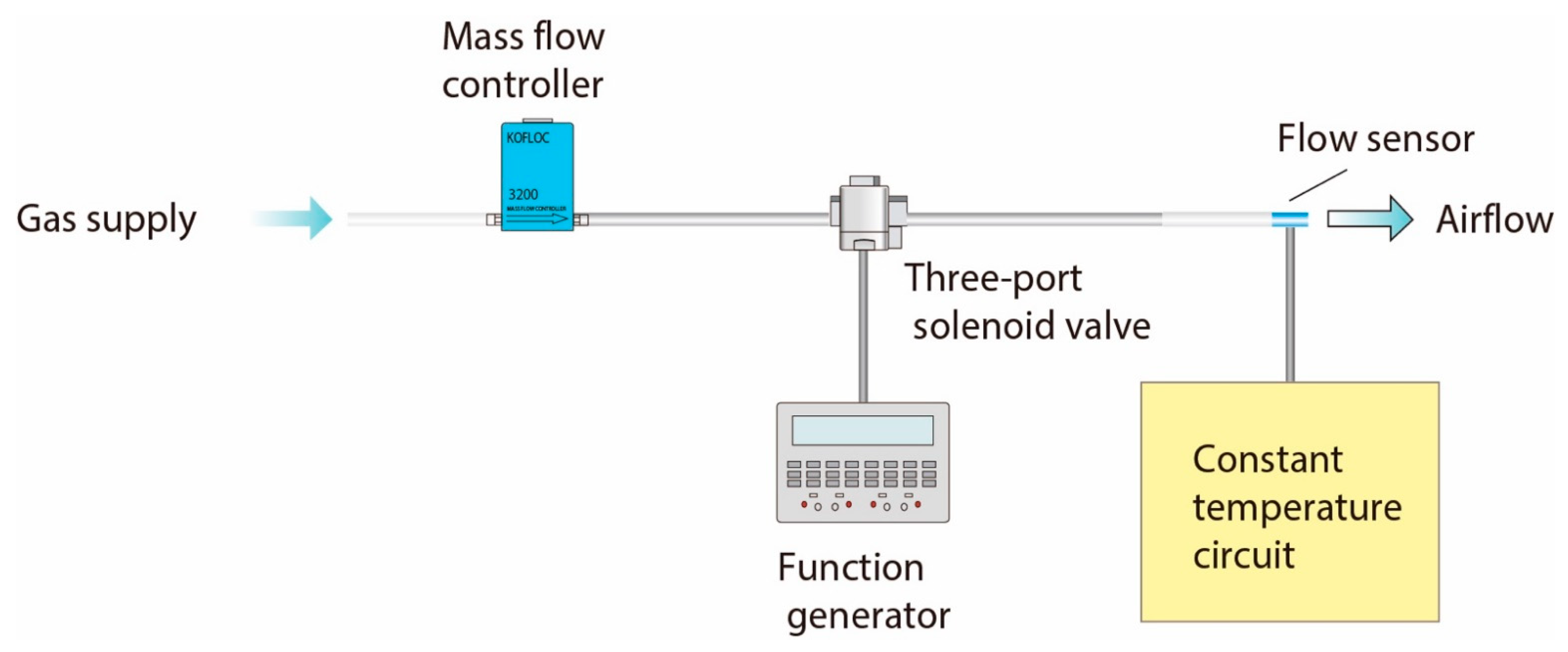
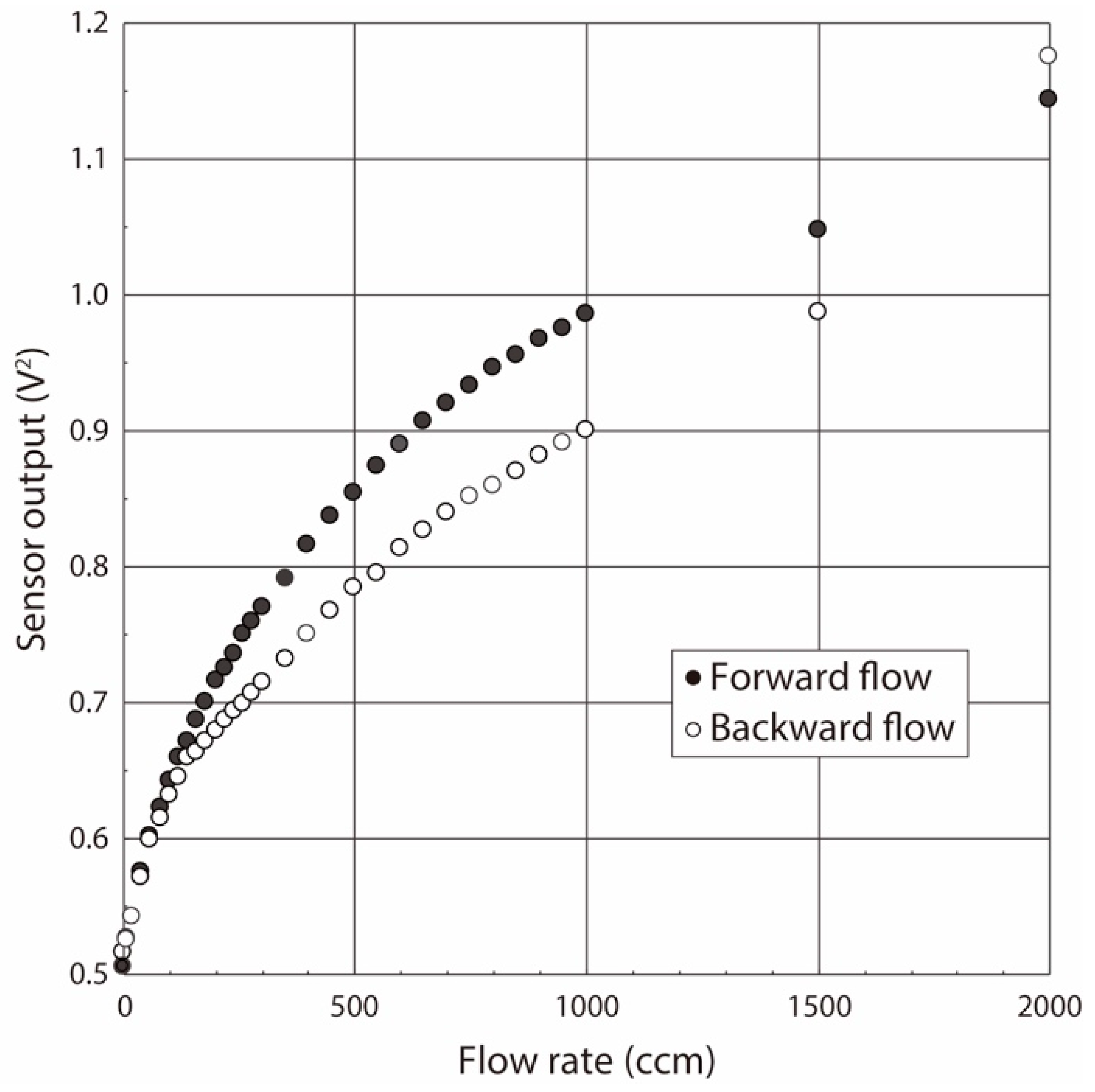
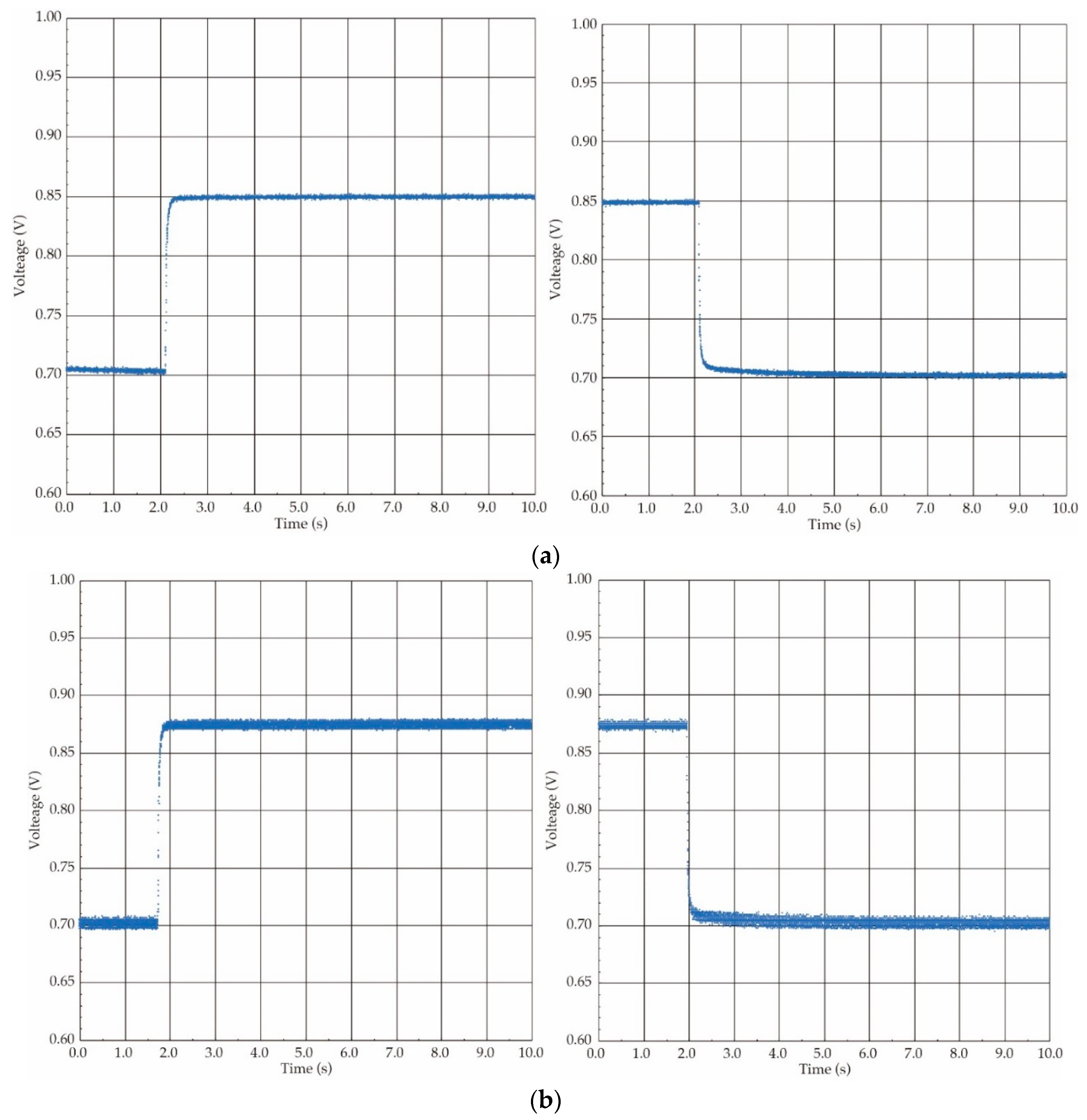
2.3. Calibration Method
3. Experiments
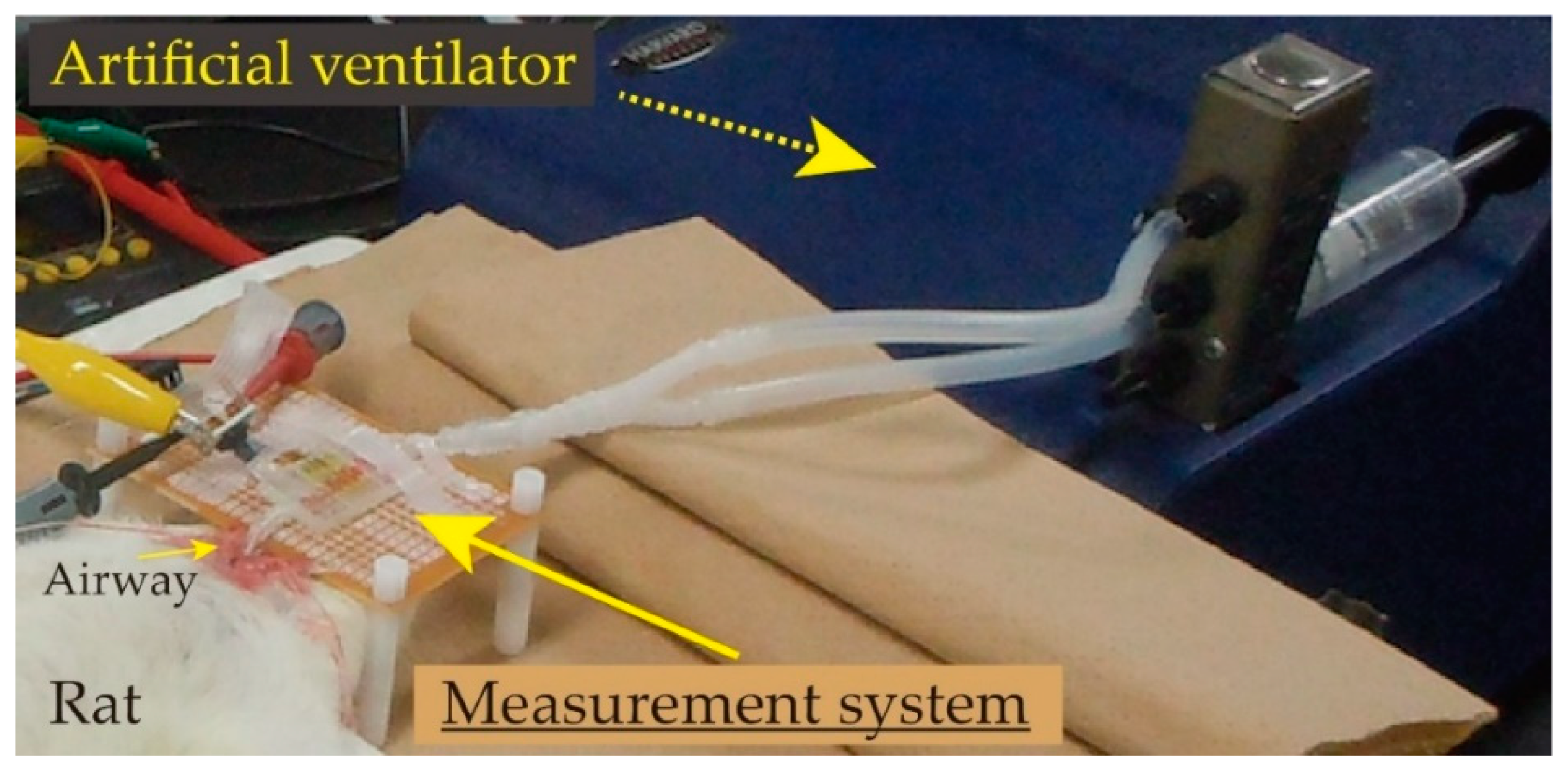
3.1. Outline of Experiments
3.2. Balloon Elasticity Evaluation
- (1)
- The balloon elasticity was almost constant in the region of the small balloon deformation by the airflow inflating.
- (2)
- The balloon elasticity largely changed in the large deformation region when the balloon was inflated over the pressure of 107 kPa.
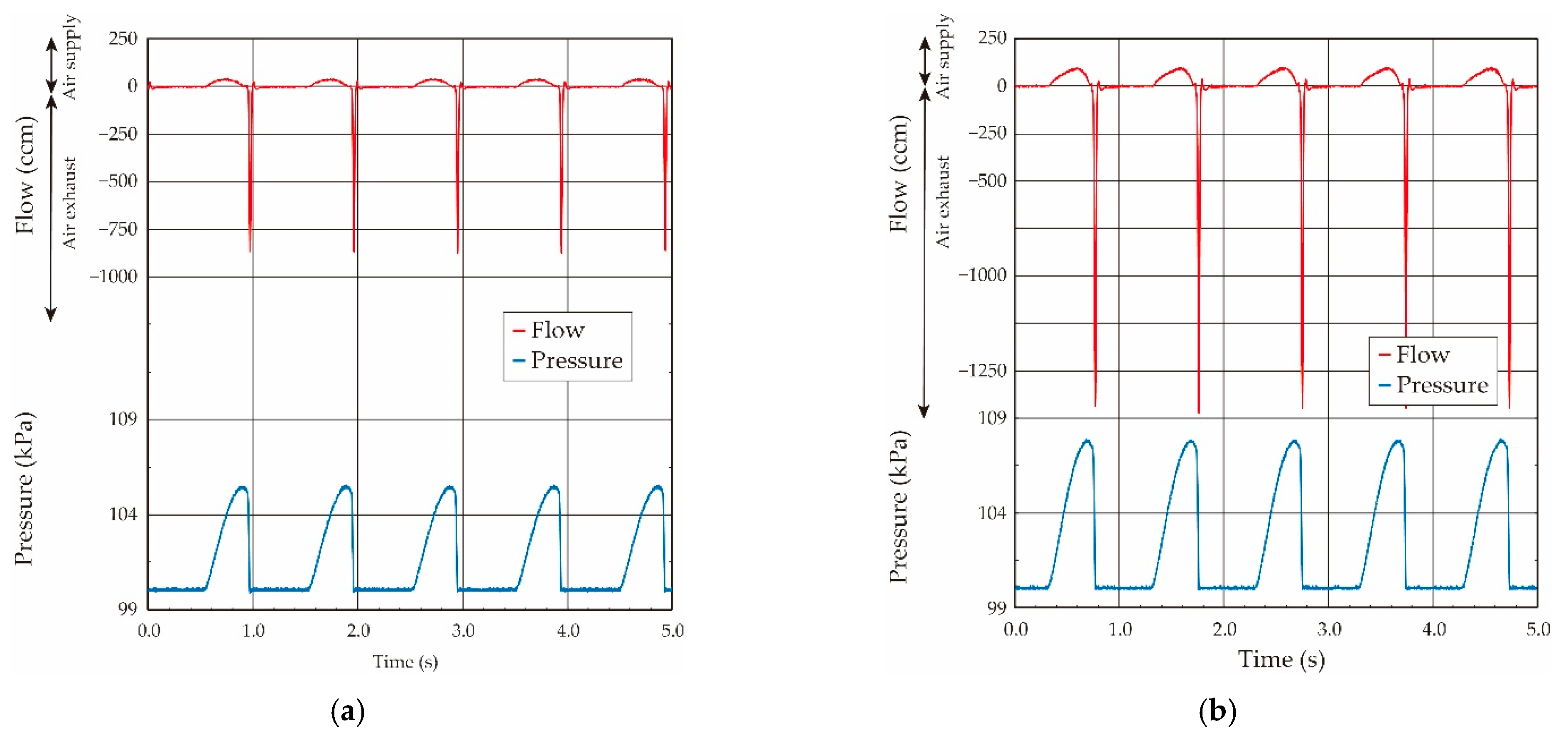
3.3. Elasticity Evaluation of Lung Tissue in Living Rat
- (1)
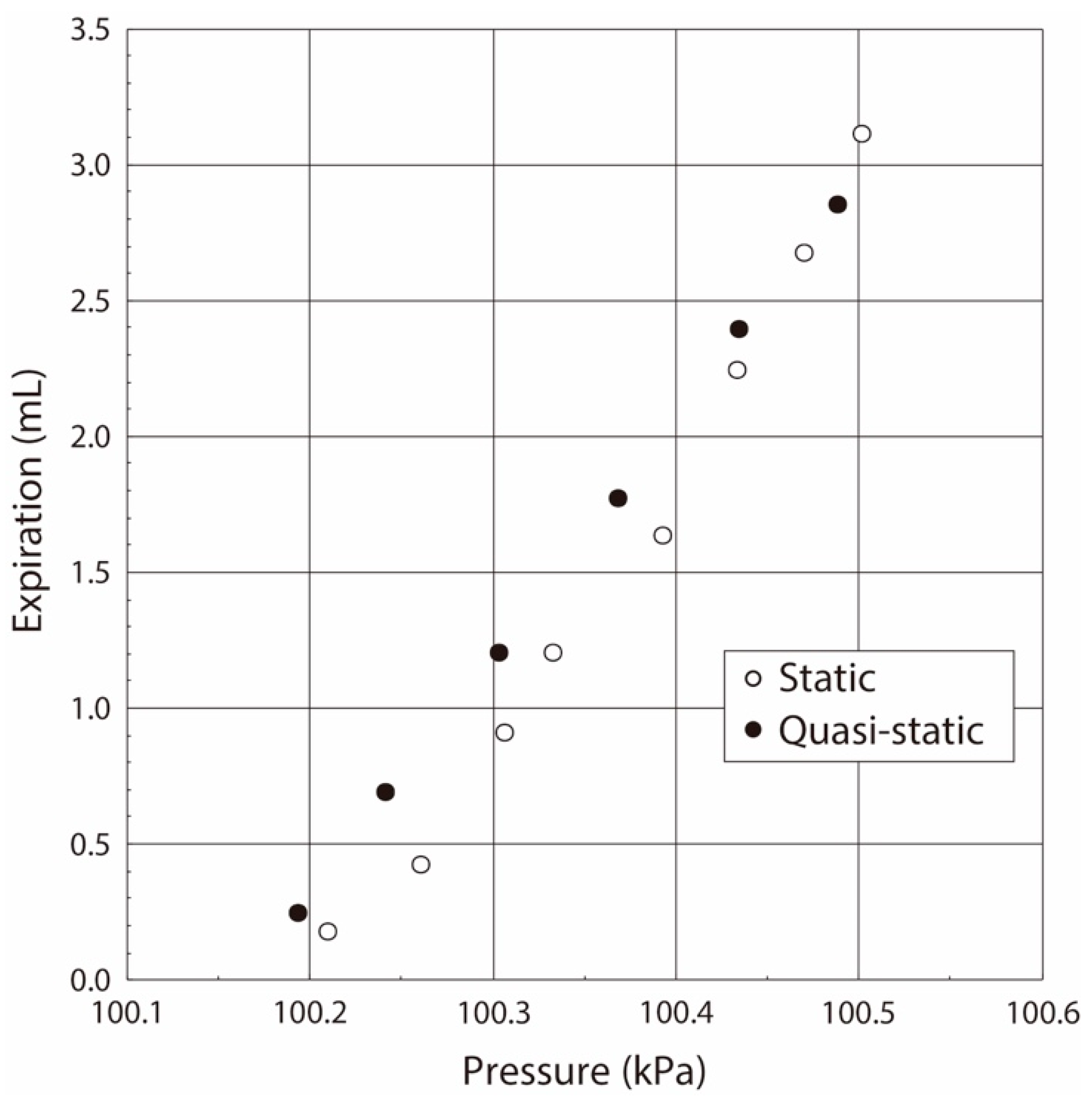
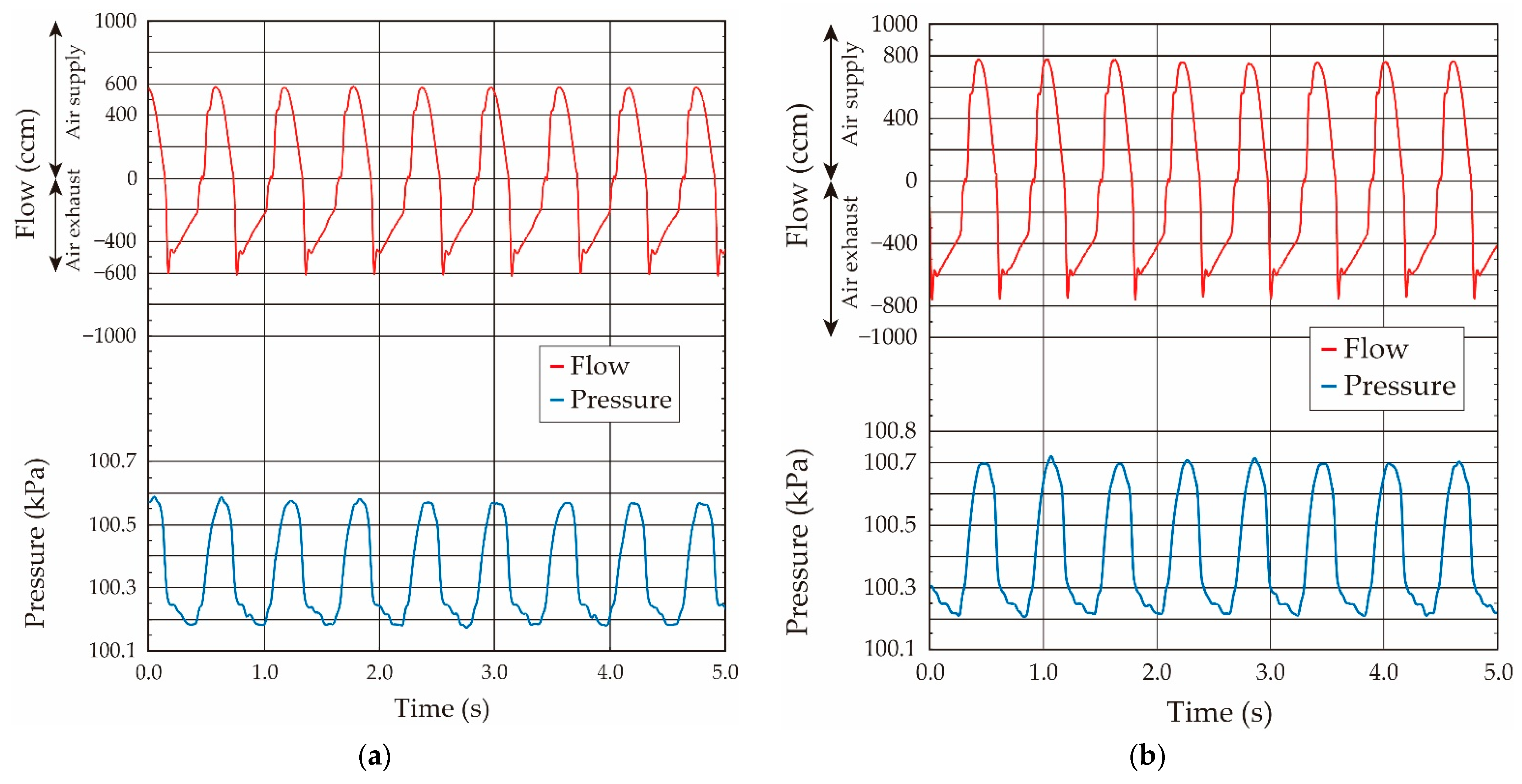
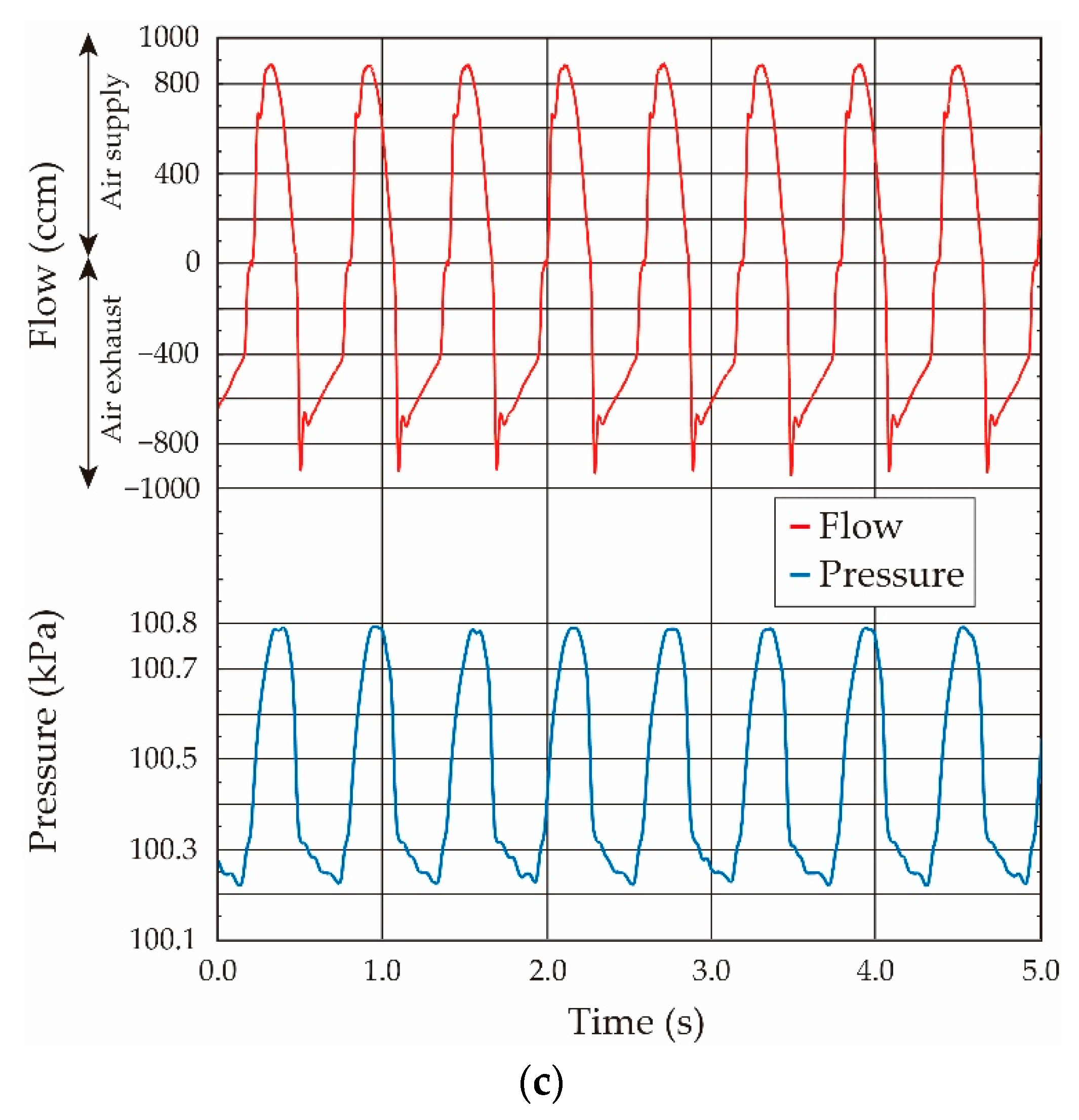
- The variation of the pressure value by the airflow volume depends on the lung tissue elasticity, which demonstrates that we are able to estimate its elasticity by the gradient of the pressure vs. flow volume curve. In the case of our lung system experiment, the curve showed the linear relation in the pressure range from 100.15 kPa to 100.50 kPa (pressure difference: 0.35 kPa), and thus we conclude that the elasticity of the lung tissue is constant in this pressure region.
- (2)
- The experimental results obtained under both the static and quasi-static conditions are generally coincident. This result followed the same trend as the results of the balloon experiment. Therefore, we demonstrated that the developed respiratory measurement system is able to evaluate the elasticity of the lung tissue in a living rat by using the pressure value under the quasi-static condition in the case of ventilation (animal experiments).
4. Conclusions
- (1)
- The elasticity of the lung tissue in a living rat can be evaluated by gradient of the pressure vs. flow volume curve.
- (2)
- It can be also evaluated by using the pressure value obtained under the quasi-static condition in the case of the sinusoidal-shaped oscillating airflow ventilation used in the animal experiments.
- (1)
- The system can evaluate the respiration properties in the case of the spontaneous breathing by simply connecting it to the airway of the experimental animal (under no ventilation condition).
- (2)
- By integrating a CO2 sensor working as a capnometer onto the system, it can detect the value of its partial pressure in the breathing, and thus we can estimate the respiration conditions, such as the arrested respiration, and the partial CO2 pressure value in arterial blood.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, B.D.; Corbridge, T.C. Basic Invasive Mechanical Ventilation. South Med. J. 2009, 102, 1238–1245. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.; Brochard, L.J.; Slutsky, A.S. Mechanical Ventilation: State of the Art. Mayo Clin. Proc. 2017, 92, 1382–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, J.M.; Corbridge, T.C.; Singer, B.D. Invasive Mechanical Ventilation. South Med. J. 2018, 111, 746–753. [Google Scholar] [CrossRef]
- Inoue, M. Mechanical ventilation. Kyobu Geka 2018, 71, 733–736. [Google Scholar]
- MacIntyre, N.R. Ventilator-associated pneumonia: The role of ventilator management strategies. Respir. Care 2005, 50, 766–773. [Google Scholar] [PubMed]
- Corona, T.M.; Aumann, M. Ventilator waveform interpretation in mechanically ventilated small animals. J. Vet. Emerg. Crit. Care 2011, 21, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Terragni, P.; Ranieri, V.M.; Brazzi, L. Novel approaches to minimize ventilator-induced lung injury. Curr. Opin. Crit. Care 2015, 21, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Volgyesi, G.A.; Tremblay, L.N.; Webster, P.; Zamel, N.; Slutsky, A.S. A new ventilator for monitoring lung mechanics in small animals. J. Appl. Physiol. 2000, 89, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Gattinoni, L.; Tonetti, T.; Cressoni, M.; Cadringher, P.; Herrmann, P.; Moerer, O.; Protti, A.; Gotti, M.; Chiurazzi, C.; Carlesso, E.; et al. Ventilator-related causes of lung injury: The mechanical power. Intensiv. Care Med. 2016, 42, 1567–1575. [Google Scholar] [CrossRef]
- Tonetti, T.; Vasques, F.; Rapetti, F.; Maiolo, G.; Collino, F.; Romitti, F.; Camporota, L.; Cressoni, M.; Cadringher, P.; Quintel, M.; et al. Driving pressure and mechanical power: New targets for VILI prevention. Ann. Transl. Med. 2017, 5, 286. [Google Scholar] [CrossRef] [Green Version]
- Rahaman, U. Mathematics of ventilator-induced lung injury. Indian J. Crit. Care Med. 2017, 21, 521–524. [Google Scholar] [CrossRef]
- Nieman, G.F.; Satalin, J.; Kollisch-Singule, M.; Andrews, P.; Aiash, H.; Habashi, N.M.; Gatto, L.A. Physiology in Medicine: Understanding dynamic alveolar physiology to minimize ventilator-induced lung injury. J. Appl. Physiol. 2017, 122, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.H.T.; Smith, B.J. Ventilator-induced lung injury and lung mechanics. Ann. Transl. Med. 2018, 6, 378. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Giosa, L.; Bonifazi, M.; Pasticci, I.; Busana, M.; Macri, M.; Romitti, F.; Vassalli, F.; Quintel, M. Targeting transpulmonary pressure to prevent ventilator-induced lung injury. Expert Rev. Respir. Med. 2019, 13, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J.; Rocco, P.R.M.; Gattinoni, L. Static and Dynamic Contributors to Ventilator-induced Lung Injury in Clinical Practice. Pressure, Energy, and Power. Am. J. Respir. Crit. Care Med. 2020, 201, 767–774. [Google Scholar] [CrossRef]
- Silva, P.L.; Ball, L.; Rocco, P.R.M.; Pelosi, P. Power to mechanical power to minimize ventila-tor-induced lung injury? Intensive Care Med. Exp. 2019, 7 (Suppl. 1), 38. [Google Scholar] [CrossRef] [Green Version]
- Marini, J.J. Evolving concepts for safer ventilation. Crit. Care 2019, 23 (Suppl. 1), 114. [Google Scholar] [CrossRef] [Green Version]
- Ngo, H.-D.; Mukhopadhyay, B.; Thanh, V.C.; Mackowiak, P.; Schlichting, V.; Obermeier, E.; Lang, K.-D.; Giuliani, A.; Drera, L.; Arancio, D. Liquid-free, piezoresistive, SOI-based pressure sensor for high temperature measurements up to 400 °C. In Proceedings of the SENSORS, 2012 IEEE, Taipei, Taiwan, 28–31 October 2012; pp. 958–961. [Google Scholar]
- Hu, C.-F.; Lin, C.-M.; Fang, W. Integration of PDMS-infiltrated CNTs and Si bulk-micromachining for monolithic physical sensors application. In Proceedings of the 17th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS & EUROSENSORS XXVII), Barcelona, Spain, 16–20 June 2013; pp. 1565–1568. [Google Scholar] [CrossRef]
- Ebefors, T.; Kälvesten, E.; Stemme, G. Three dimensional silicon triple-hot-wire anemometer based on polyimide joints. In Proceedings of the Eleventh Annual International Workshop on Micro Electro Mechanical Systems. An Investigation of Micro Structures, Sensors, Actuators, Machines and Systems, Heidelberg, Germany, 25–29 January 1998; pp. 93–98. [Google Scholar] [CrossRef]
- Xue, D.; Zhou, W.; Ni, Z.; Wang, J.; Li, X. A dront-side micro-fabricated tiny-size thermoresistive gas flow sensor with low cost, high sensitivity, and quick response. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1945–1948. [Google Scholar]
- Lin, Q.; Xu, Y.; Jiang, F.; Tai, Y.C.; Ho, C.M. A parameterized three-dimensional model for MEMS thermal shear-stress sensors. J. Microelectromech. Syst. 2005, 14, 625–633. [Google Scholar]
- Liu, C.; Huang, J.-B.; Zhu, Z.; Jiang, F.; Tung, S.; Tai, Y.-C.; Ho, C.-M. A micromachined flow shear-stress sensor based on thermal transfer principles. J. Microelectromech. Syst. 1999, 8, 90–99. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Funabashi, H. A Z-axis differential capacitive SOI accelerometer with vertical comb electrodes. Sens. Actuators A Phys. 2004, 116, 378–383. [Google Scholar] [CrossRef]
- Li, C.; Wen, Y.; Fan, S.; Kan, B.; Wang, C. Design of a new differential silicon resonant accelerometer with dual proofmasses using two-stage microlever. In Proceedings of the 2015 IEEE SENSORS, Busan, Korea, 1–4 November 2015; pp. 116–119. [Google Scholar]
- Courteaud, J.; Crespy, N.; Combette, P.; Sorli, B.; Giani, A. Studies and optimization of the frequency response of a micromachined thermal accelerometer. Sens. Actuators A Phys. 2008, 147, 75–82. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Kageyama, Y.; Funabashi, H.; Sakata, J. Polysilicon vibrating gyroscope vacuum-encapsulated in an on-chip micro chamber. Sens. Actuators A Phys. 2001, 90, 49–55. [Google Scholar] [CrossRef]
- Liu, S.Q.; Zhu, R.; Ding, H.G. A temperature compensation method for micromachined thermal gas gyroscope. In Proceedings of the 2015 IEEE SENSORS, Busan, Korea, 1–4 November 2015; pp. 638–641. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Shimizu, T.; Miyaji, T.; Shikida, M.; Sasaki, H.; Sato, K. Itoigawa, A micromachined active tactile sensor for hardness detection. Sens. Actuators A 2004, 114, 141–146. [Google Scholar] [CrossRef]
- Chun, K.J.; Wise, K.D. A capacitive silicon tactile image array. In Proceedings of the 1985 International Conference on Solid-state Sensors and Actuators, Philadelphia, PA, USA, 11–14 June 1985; pp. 22–25. [Google Scholar]
- Kane, B.J.; Kovacs, G.T.A. A CMOS Compatible Traction Stress Sensing Element for Use in High Resolution Tactile Imaging. In Proceedings of the International Solid-State Sensors and Actuators Conference, Stockholm, Sweden, 25–29 June 1995; pp. 648–651. [Google Scholar] [CrossRef]
- Shikida, M.; Yoshikawa, K.; Iwai, S.; Sato, K. Flexible flow sensor for large-scale air-conditioning network systems. Sens. Actuators A Phys. 2012, 188, 2–8. [Google Scholar] [CrossRef]
- Shikida, M.; Yamazaki, Y.; Yoshikawa, K.; Sato, K. A MEMS flow sensor applied in a variable-air-volume unit in a building air-conditioning system. Sens. Actuators A Phys. 2013, 189, 212–217. [Google Scholar] [CrossRef]
- Yoshida, H.; Hasegawa, Y.; Taniguchi, K.; Matsushima, M.; Kawabe, T.; Shikida, M. Development of breathing monitoring system for artificial ventilator in animal experiment. In Proceedings of the 32nd International Microprocesses and Nanotechnology Conference (MNC2019), Hiroshima, Japan, 28–31 October 2019; p. 30D-4-3. [Google Scholar]
- Yoshida, H.; Hasegawa, Y.; Matsushima, M.; Sugiyama, T.; Kawabe, T.; Shikida, M. Micro-machined respiratory monitoring system development for artificial ventilator in animal experiment. Microsyst. Technol. 2020, 26, 3715–3724. [Google Scholar] [CrossRef]
- Robertson, H.T. Dead space: The physiology of wasted ventilation. Eur. Respir. J. 2014, 45, 1704–1716. [Google Scholar] [CrossRef] [Green Version]
- Anatomy, Anatomic Dead Space, Statpearls. Available online: https://www.statpearls.com/articlelibrary/viewarticle/17518/ (accessed on 1 July 2021).
- Physiology, Lung Dead Space, Statpearls. Available online: https://www.statpearls.com/articlelibrary/viewarticle/24497/ (accessed on 1 July 2021).
- Ristic, L. Sensor Technology and Devices; Artch House Inc.: Norwood, MA, USA, 1994. [Google Scholar]
- Senturia, S.D. Microsystem Design; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK, 2001. [Google Scholar]
- Muller, R.S.; Howe, R.T.; Senturia, S.D.; Smith, R.L.; White, R.M. Microsensors; IEEE Press: New York, NY, USA, 1990. [Google Scholar]
- Fatikow, S.; Rembold, U. Microsystem Technology and Microrobotics; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Meijer, G.C.M.; Herwaarden, A.W. Thermal Sensor; IOP Publishing: Philadelphia, PA, USA, 1994. [Google Scholar]
- Gianchandani, Y.B.; Tabata, O.; Zappe, H. Comprehensive Microsystems; Elsevier B.V.: Amsterdam, The Netherlands, 2008; Volume 2–3. [Google Scholar]
- Trautweiler, S.F. Silicon Hot Film Flow Sensor; DISS. ETH No. 12185; Swiss Federal Institute of Technology: Zurich, Switzerland, 1997. [Google Scholar]
- Hasegawa, Y.; Kawaoka, H.; Yamada, T.; Matsushima, M.; Kawabe, T.; Shikida, M. Respiration and heartbeat signal detection from airflow at airway in rat by catheter flow sensor with temperature compensation function. J. Micromech. Microeng. 2017, 27, 125016. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kawaoka, H.; Mitsunari, Y.; Matsushima, M.; Kawabe, T.; Shikida, M. Catheter type thermal flow sensor with small footprint for measuring breathing function. Microsyst. Technol. 2018, 24, 3455–3465. [Google Scholar] [CrossRef]
- King, L.V. XII. On the convection of heat from small cylinders in a stream of fluid: Determination of the convection constants of small platinum wires with applications to hot-wire anemometry. Philos. Trans. R. Soc. Lond. 1914, 214, 373–432. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, H.; Hasegawa, Y.; Matsushima, M.; Sugiyama, T.; Kawabe, T.; Shikida, M. Miniaturization of Respiratory Measurement System in Artificial Ventilator for Small Animal Experiments to Reduce Dead Space and Its Application to Lung Elasticity Evaluation. Sensors 2021, 21, 5123. https://doi.org/10.3390/s21155123
Yoshida H, Hasegawa Y, Matsushima M, Sugiyama T, Kawabe T, Shikida M. Miniaturization of Respiratory Measurement System in Artificial Ventilator for Small Animal Experiments to Reduce Dead Space and Its Application to Lung Elasticity Evaluation. Sensors. 2021; 21(15):5123. https://doi.org/10.3390/s21155123
Chicago/Turabian StyleYoshida, Homare, Yoshihiro Hasegawa, Miyoko Matsushima, Tomoshi Sugiyama, Tsutomu Kawabe, and Mitsuhiro Shikida. 2021. "Miniaturization of Respiratory Measurement System in Artificial Ventilator for Small Animal Experiments to Reduce Dead Space and Its Application to Lung Elasticity Evaluation" Sensors 21, no. 15: 5123. https://doi.org/10.3390/s21155123
APA StyleYoshida, H., Hasegawa, Y., Matsushima, M., Sugiyama, T., Kawabe, T., & Shikida, M. (2021). Miniaturization of Respiratory Measurement System in Artificial Ventilator for Small Animal Experiments to Reduce Dead Space and Its Application to Lung Elasticity Evaluation. Sensors, 21(15), 5123. https://doi.org/10.3390/s21155123





