Dipodal Tetraamide Derivatives of 1,10-Diaza-18-Crown-6 and Alkylmalonic Acids—Synthesis and Use as Ionophores in Ion Selective Membrane Electrodes
Abstract
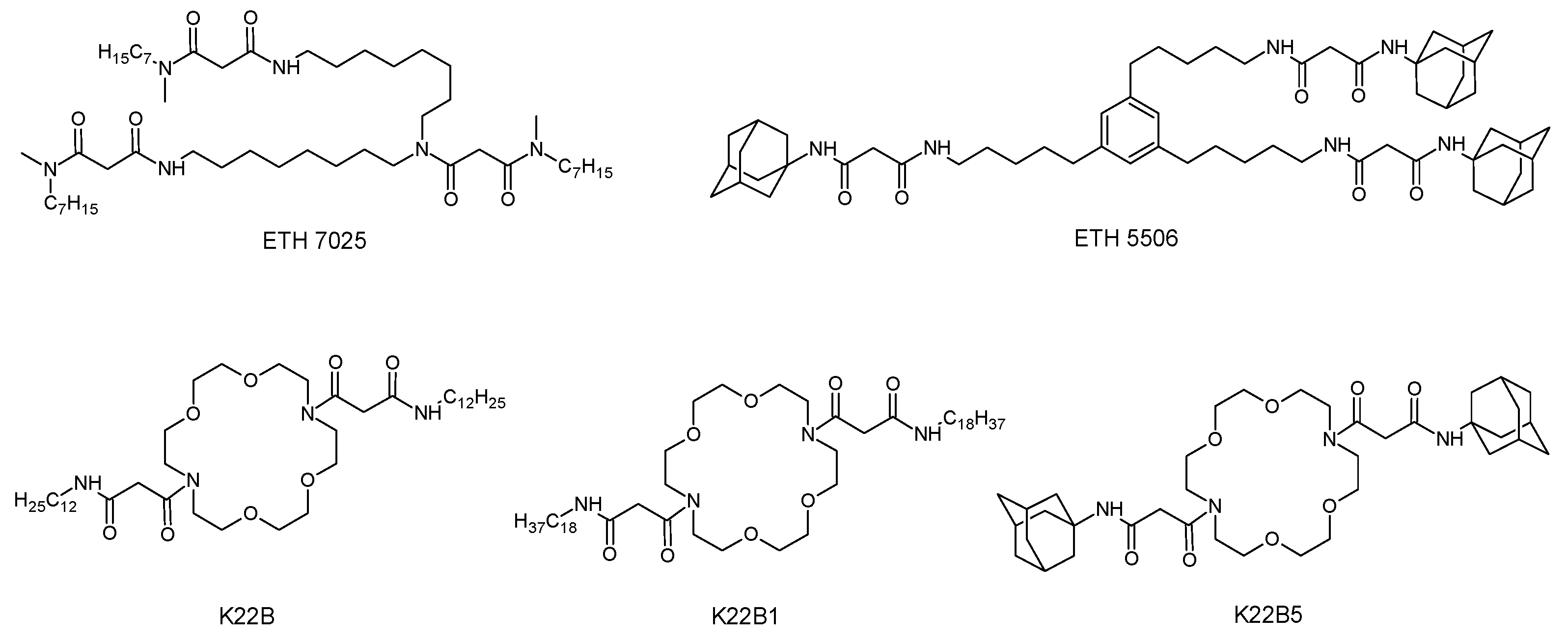
1. Introduction
2. Materials and Methods
2.1. Preparation of Electrodes and Potentiometric Measurements
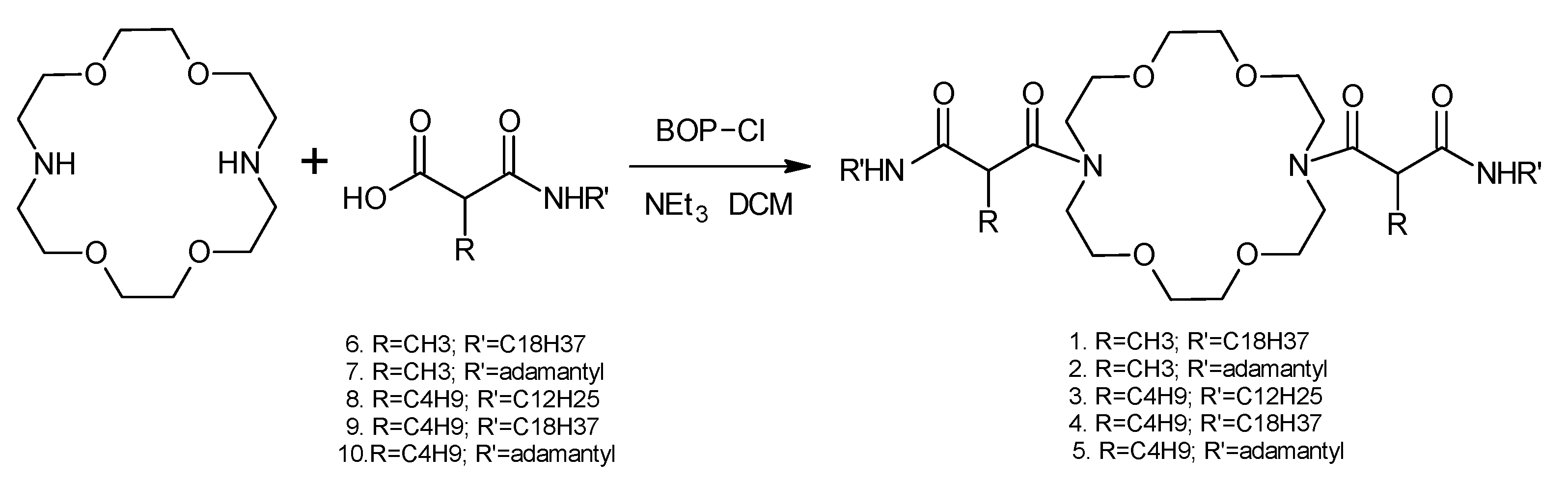
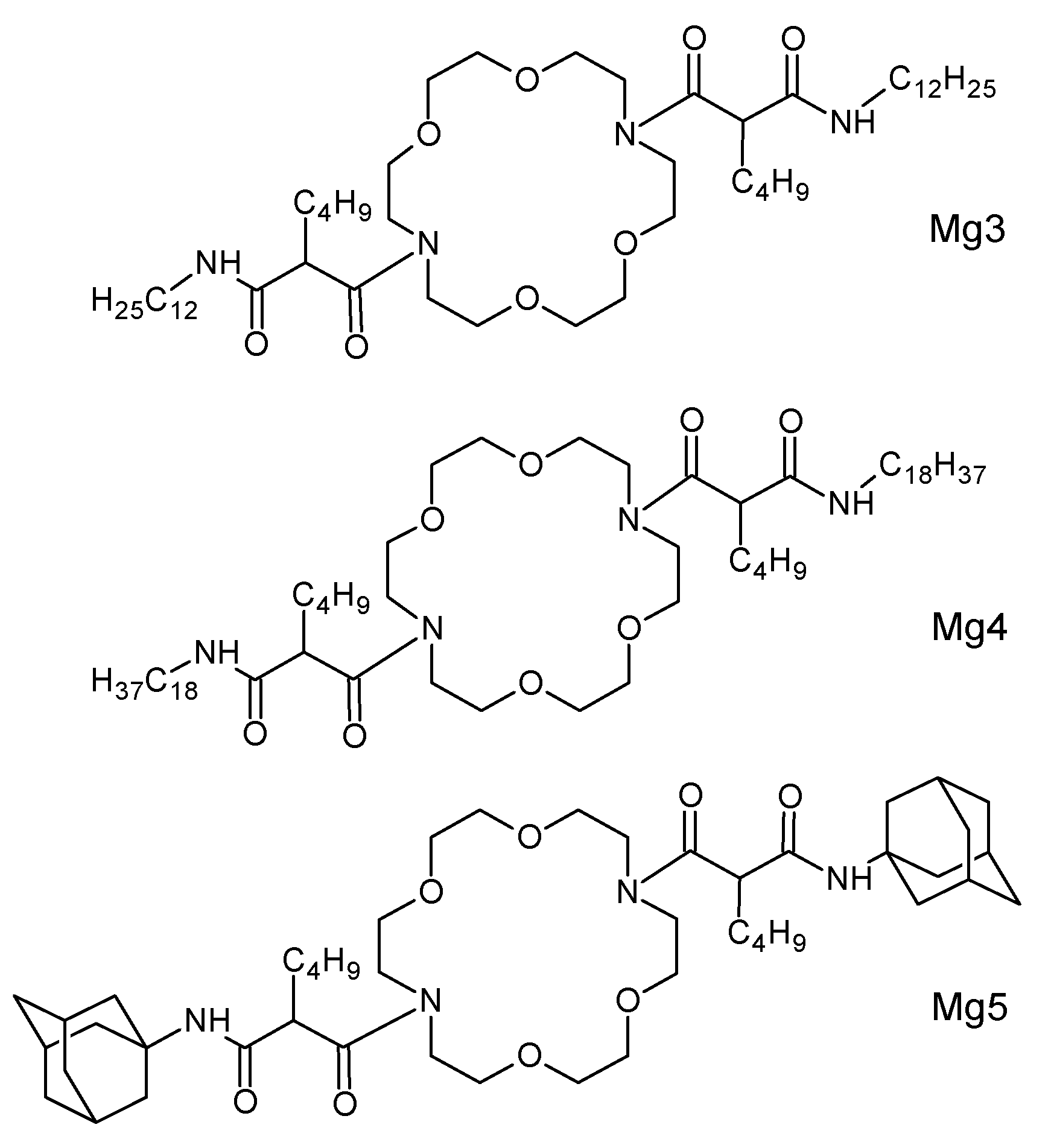
2.2. Synthesis of Novel Ionophores Mg1–Mg5
2.3. Potentiometric Testing of 1,10-Diaza-18-Crown-6 Derivatives Mg1–Mg5—Conventional Electrodes
2.4. Potentiometric Tests Using Glassy Carbon Solid Contact Electrodes
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altura, B.M.; Altura, B.T. Importance of Ionized Magnesium Measurements in Physiology and Medicine and The Need for Ion-selective Electrodes. J. Clin. Case Stud. 2016, 1, 1–4. [Google Scholar]
- Bazydlo, L.A.L.; Needham, M.; Harris, N.S. Calcium, Magnesium, and Phosphate. Lab. Med. 2014, 45, e44. [Google Scholar] [CrossRef]
- Zehtabchi, S.; Sinert, R.; Rinnert, S.; Chang, B.; Heinis, C.; Altura, R.A.; Altura, B.T.; Altura, B.M. Serum Ionized Magnesium Levels and Ionized Calcium-to-Magnesium Ratios in Adult Patients with Sickle Cell Anemia. Am. J. Hematol. 2004, 77, 215–222. [Google Scholar] [CrossRef]
- Farruggia, F.; Iotti, S.; Prodi, L.; Zaccheroni, N.; Montaltu, M.; Savage, P.B.; Andreani, G.; Trapani, G.; Wolf, F.I. A Simple Spectrofluorometric Assay to Measure Total Intracellular Magnesium by a Hydroxyquinoline Derivative. J. Fluoresc. 2009, 19, 11–19. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, N.; Mulrooney, R.C.; Callan, J.F. A Ratiometric Fluorescent Probe for Magnesium Employing Excited State Intramolecular Proton Transfer. Tetrahedron Lett. 2008, 49, 6690–6692. [Google Scholar] [CrossRef]
- Chapp, A.D.; Schum, S.; Behnke, J.E.; Hahka, T.; Huber, M.J.; Jiang, E.; Larson, R.A.; Shan, Z.; Chen, Q.-H. Measurement of cations, anions, and acetate in serum, urine, cerebrospinal fluid, and tissue by ion chromatography. Physiol. Rep. 2018, 6, e13666. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Li, X.; Zhu, P.; Xu, X.; Xu, Q.; Kang, Y. Speciation of magnesium in rat plasma using capillary electrophoresis-inductively coupled plasma-atomic emission spectrometry. Electrophoresis 2008, 29, 1534–1539. [Google Scholar] [CrossRef]
- Gupta, V.K.; Chandra, S.; Mangla, R. Magnesium-selective electrodes. Sens. Actuators B 2002, 86, 235–241. [Google Scholar] [CrossRef]
- Gupta, V.K.; Prasad, R.; Kumar, A. Magnesium–tetrazaporphyrin incorporated PVC matrix as a new material for fabrication of Mg2+ selective potentiometric sensor. Talanta 2004, 63, 1027–1033. [Google Scholar] [CrossRef]
- Khalil, S.; Alharthi, S.S. Ion-selective Membrane Sensor for Magnesium Determination in Pharmaceutical Formulations. Int. J. Electrochem. Sci. 2020, 15, 9223–9232. [Google Scholar] [CrossRef]
- Asif, M.H.; Usman Ali, S.M.; Nur, O.; Willander, M.; Englund, U.H.; Elinder, F. Functionalized ZnO nanorod-based selective magnesium ion sensor for intracellular measurements. Biosens. Bioelectron. 2010, 26, 1118–1123. [Google Scholar] [CrossRef]
- Urbanowicz, M.; Pijanowska, D.G.; Jasiński, A.; Ekman, M.; Bocheńska, M.K. A miniaturized solid-contact potentiometric multisensor platform for determination of ionic profiles in human saliva. J. Solid State Electrochem. 2019, 23, 3299. [Google Scholar] [CrossRef]
- Algarra, M.; Jiménez-Herrera, C.M.; da Silva, J.C.G.E. Recent Applications of Magnesium Chemical Sensors in Biological Samples. Crit. Rev. Anal. Chem. 2015, 45, 32–40. [Google Scholar] [CrossRef]
- Oesch, U.; Ammann, D.; Simon, W. Ion-selective membrane electrodes for clinical use. Clin. Chem. 1986, 32, 1448–1459. [Google Scholar] [CrossRef]
- Müller, M.; Rouilly, M.; Rusterholz, B.; Maj-Zurawska, M.; Hu, Z.; Simon, W. Magnesium Selective Electrodes for Blood Serum Studies and Water Hardness Measurement. Microchim. Acta 1988, 96, 283–290. [Google Scholar] [CrossRef]
- Maj-Zurawska, M.; Rouilly, M.; Morf, W.E.; Simon, W. Determination of magnesium and calcium in water with ion-selective electrodes. Anal. Chim. Acta 1989, 218, 47–59. [Google Scholar] [CrossRef]
- Spichiger, U.E.; Eugster, R.; Haase, E.; Rumpf, G.; Gehrig, P.; Schmid, A.; Rusterholz, B.; Simon, W. Critical parameters and optimization of a magnesium-selective liquid membrane electrode for application to human blood serum. Fresenius J. Anal. Chem. 1991, 341, 727–731. [Google Scholar] [CrossRef]
- Zhang, W.; Jenny, L.; Spichiger, U.E. A Comparison of Neutral Mg2+ -Selective Ionophores in Solvent Polymeric Membranes: Complex Stoichiometry and Lipophilicity. Anal. Sci. 2000, 16, 11–18. [Google Scholar] [CrossRef]
- Rouilly, M.; Rusterholz, B.; Spichiger, U.E.; Simon, W. Neutral ionophore-based selective electrode for assaying the activity of magnesium in undiluted blood serum. Clin. Chem. 1990, 36, 466–469. [Google Scholar] [CrossRef]
- Spichiger, U.E.; Eugster, R.; Schimd, A.; Gehrig, P.; Rusterholz, B.; Simon, W. Application of an Ion Selective Magnesium Electrode on Human Blood Serum. In Methodology and Clinical Applications of Blood Gases, pH, Electrolytes and Sensor Technology, Proceedings of an International Symposium of the Working Group on Ion Selective Electrodes, Monterey, CA, USA, 18–20 July 1990; Burnett, W., Gochman, N., Graham, G.A., Maas, A.H.J., Elinwiijk, R.F.M., Van Kesse, A.L., Eds.; IFCC: Utrecht, The Netherlands, 1990; Volume 12. [Google Scholar]
- Eugster, R.; Rusterholz, B.; Schmid, A.; Spichiger, U.E.; Simon, W. Characterization procedure for ion-selective electrode assays of magnesium activity in aqueous solutions of physiological composition. Clin. Chem. 1993, 39, 855–859. [Google Scholar] [CrossRef]
- O’Donnell, J.; Li, H.; Rusterholz, B.; Pedrazza, U.; Simon, W. Development of magnesium-selective ionophores. Anal. Chim. Acta 1993, 281, 129–134. [Google Scholar] [CrossRef]
- Spichiger, U.E.; Fakler, A. Potentiometric microelectrodes as sensor and detectors. Magnesium-selective electrodes as sensors, and hofmeister electrodes as detectors for histamine in capillary electrophoresis. Electrochim. Acta 1997, 42, 3137–3145. [Google Scholar] [CrossRef]
- Zhang, W.; Fakler, A.; Demuth, C.; Spichiger, U.E. Comparison of different methods for determining the selectivity coeffcient using a magnesium-selective electrode. Anal. Chim. Acta 1998, 375, 211–222. [Google Scholar] [CrossRef]
- Suzuki, K.; Watanabe, K.; Matsumoto, Y.; Kobayashi, M.; Sato, S.; Siswanta, D.; Hisamoto, H. Design and Synthesis of Calcium and Magnesium Ionophores Based on Double-Armed Diazacrown Ether Compounds and Their Application to an Ion-Sensing Component for an Ion-Selective Electrode. Anal. Chem. 1995, 67, 324–334. [Google Scholar] [CrossRef]
- Siswanta, D.; Hisamoto, H.; Sato, S.; Matsumoto, Y.; Koike, Y.; Yamamori, S.; Suzuki, K. Magnesium Ion-Selective Optodes Based on a Neutral Ionophore and a Lipophilic Cationic Dye. Anal. Sci. 1997, 13, 429–435. [Google Scholar] [CrossRef][Green Version]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric Selectivity Coefficients of Ion-Selective Electrodes. Part I. Inorganic Cations (Technical Report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Dinten, O.; Spichiger, U.E.; Chaniotakis, N.; Gehrig, P.; Rusterholz, B.; Morf, W.E.; Simon, W. Lifetime of Neutral-Carrier-Based Liquid Membranes in Aqueous Samples and Blood and the Lipophilicity of Membrane Components. Anal. Chem. 1991, 63, 596–603. [Google Scholar] [CrossRef]
- Guziński, M.; Jarvis, J.M.; Pendley, B.D.; Lindner, E. Equilibration Time of Solid Contact Ion-Selective Electrodes. Anal. Chem. 2015, 87, 6654–6659. [Google Scholar] [CrossRef] [PubMed]
- Pergel, E.; Gyurcsanyi, R.E.; Toth, K.; Lindner, E. Picomolar Detection Limits with Current-Polarized Pb2+ Ion-Selective Membranes. Anal. Chem. 2001, 73, 4249–4253. [Google Scholar] [CrossRef]
- Zhang, W. Investigation of Mg2+ -Selective Electrodes for Measurements in Biological Fluids. Ph.D. Thesis, Swiss Federal Institute of Technology Zurich, Zurich, Switzerland, 2000. [Google Scholar]









| Membrane Number | KTpClPB/Ionophore (Molar Ratio) | % Ionophore by Weight | % Lipophilic Salt by Weight | % o-NPOE (104 mg) by Weight | % PVC (50 mg) by Weight |
|---|---|---|---|---|---|
| 1. | 0.4:1 | 2.09 | 0.43 | 65.83 | 31.65 |
| 2. | 0.6:1 | 2.08 | 0.64 | 65.69 | 31.58 |
| 3. | 0.8:1 | 1.02 | 0.42 | 66.56 | 32.00 |
| 4. | 0.8:1 | 2.08 | 0.86 | 65.55 | 31.51 |
| 5. | 1:1 | 2.08 | 1.04 | 65.43 | 31.46 |
| 6. | 1:1 | 1.27 | 0.64 | 66.24 | 31.85 |
| 7. | 1:1 | 3.97 | 1.98 | 63.51 | 30.53 |
| 8. | 1.2:1 | 1.02 | 0.63 | 66.42 | 31.93 |
| 9. | 1.2:1 | 2.07 | 1.28 | 65.27 | 31.38 |
| 10. | 1.5:1 | 2.06 | 1.60 | 65.06 | 31.28 |
| Membrane Number | KTpClPB/Ionophore (Molar Ratio) | % Ionophore by Weight | % Lipophilic Salt by Weight | % o-NPOE (104 mg) by Weight | % PVC (50 mg) by Weight |
|---|---|---|---|---|---|
| 11. | 0.6:1 | 1.02 | 0.42 | 66.56 | 32.00 |
| 12. | 0.8:1 | 2.07 | 1.13 | 65.37 | 31.43 |
| 13. | 0.8:1 | 1.02 | 0.56 | 66.47 | 31.95 |
| 14. | 1:1 | 2.07 | 1.38 | 65.20 | 31.35 |
| 15. | 1:1 | 0.96 | 0.64 | 66.45 | 31.95 |
| 16. | 1.2:1 | 1.02 | 0.83 | 66.28 | 31.87 |
| Ionophore | Log PTLC | Ionophore | Log PTLC [25] |
|---|---|---|---|
| Mg1 | >20 | K22B1 | 15.0 ± 0.3 |
| Mg2 | 3.3 | K22B5 | 3.0 ± 0.4 |
| Mg3 | 12.7 | K22B | 7.1 ±0.4 |
| Mg4 | >20 | K22B1 | 15.0 ± 0.3 |
| Mg5 | 5.3 | K22B5 | 3.0 ± 0.4 |
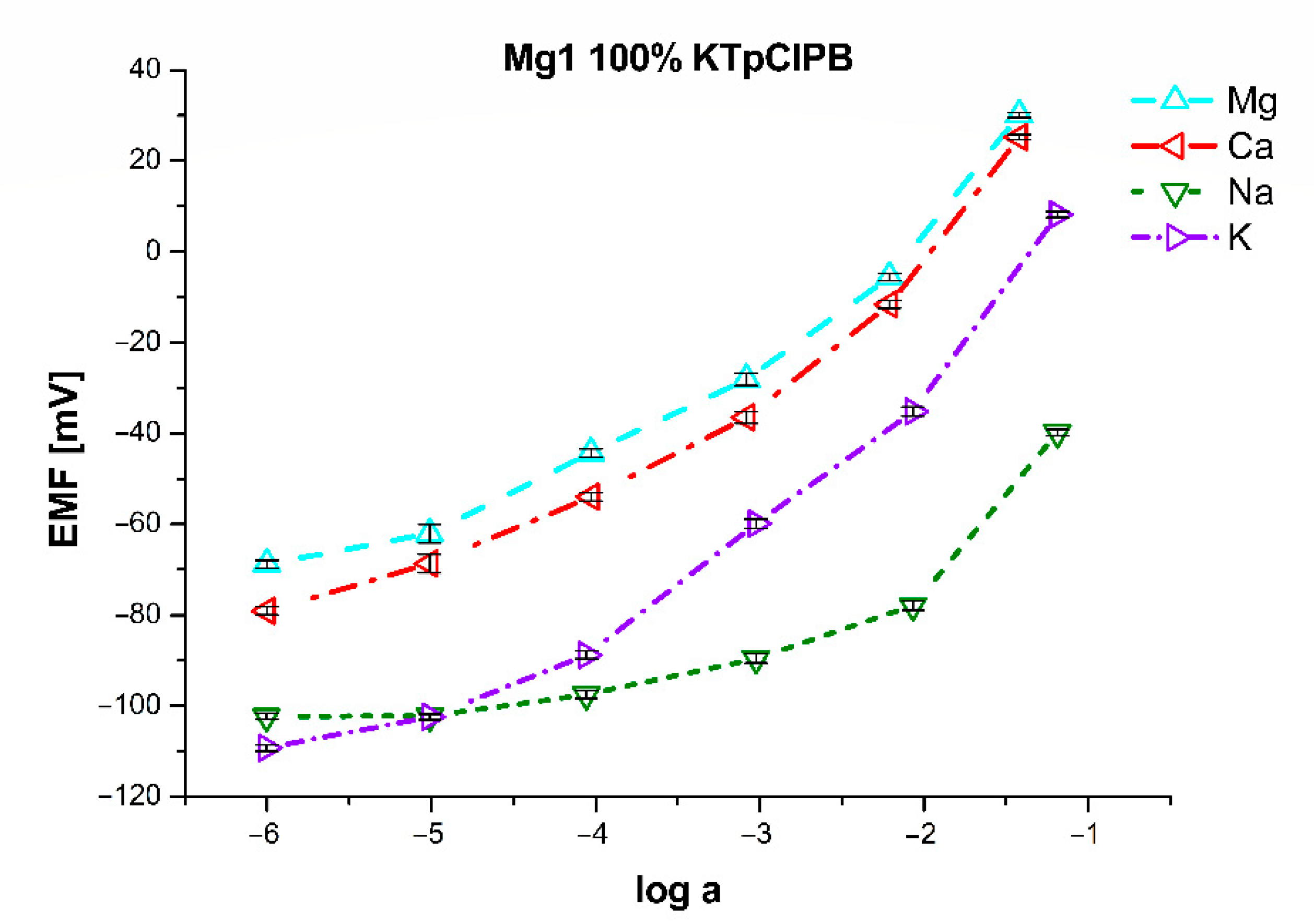
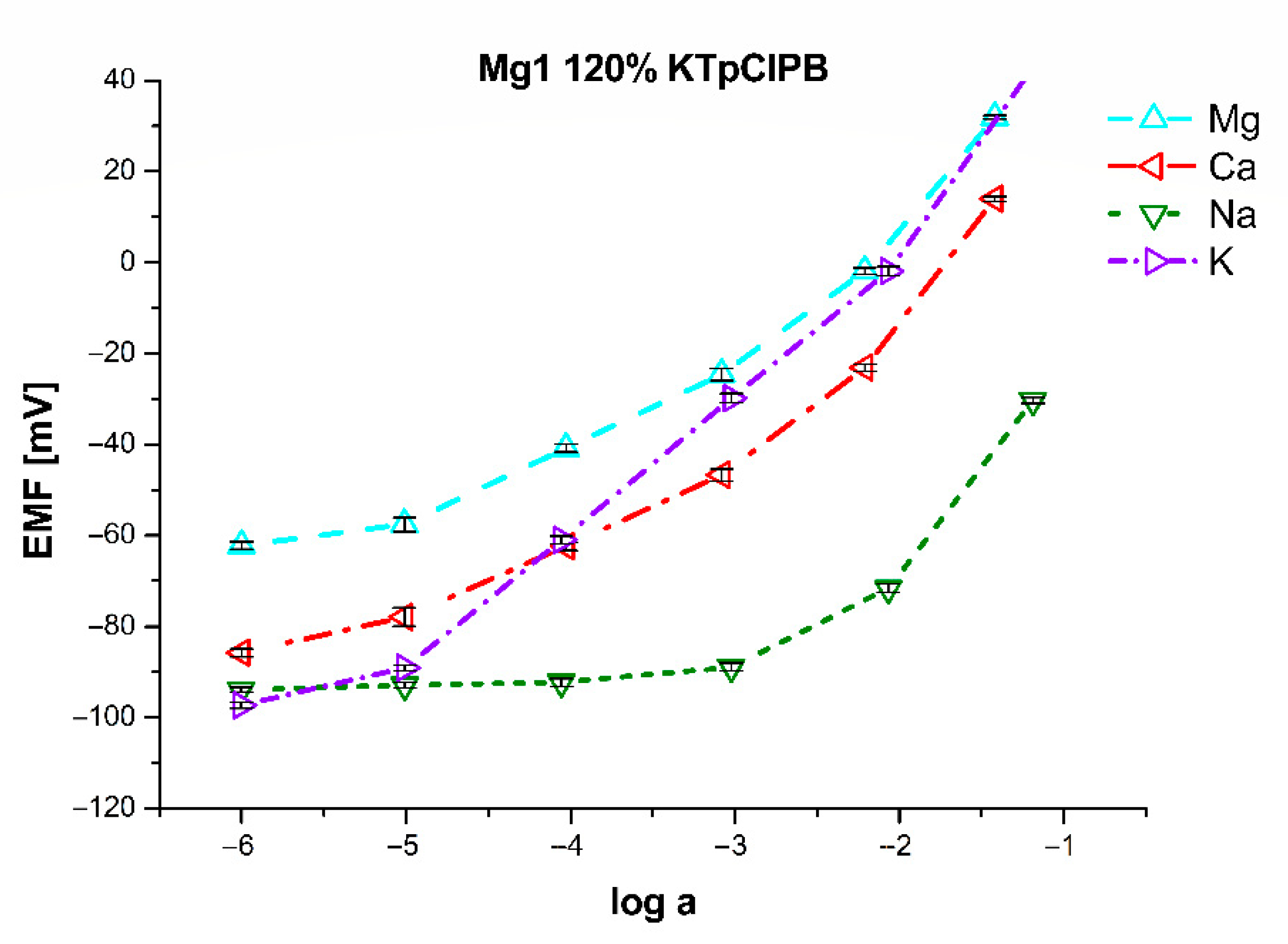
| Mg1 | 80% of KTpClPB | 100% of KTpClPB | 120% of KTpClPB | 140% of KTpClPB | ||||
|---|---|---|---|---|---|---|---|---|
| LR (log(a)) | −5 to −1 | −5 to −1 | −5 to −1 | −5 to −1 | ||||
| LDL (log(a)) | −5.21 | −5.20 | −5.18 | −5.19 | ||||
| S (mV/dec) | 24.0 ± 1.2 | 24.6 ± 1.1 | 24.0 ± 0.8 | 23.7 ± 1.2 | ||||
| log(a) | 0 | −1 | 0 | −1 | 0 | −1 | 0 | −1 |
| logKMg/Ca | 0.43 ± 0.08 | 0.29 ± 0.08 | −0.19 ± 0.06 | −0.22 ± 0.06 | −0.79 ± 0.05 | −0.81 ± 0.05 | −0.74 ± 0.08 | −0.79 ± 0.08 |
| logKMg/Na | −2.48 ± 0.12 | −1.66 ± 0.10 | −2.94 ± 0.13 | −1.92 ± 0.14 | −2.26 ± 0.15 | −1.58 ± 0.09 | −2.59 ± 0.12 | −1.70 ± 0.12 |
| logKMg/K | −1.54 ± 0.09 | −0.76 ± 0.08 | −0.97 ± 0.07 | −0.12 ± 0.05 | 0.75 ± 0.05 | 1.35 ± 0.08 | 0.99 ± 0.09 | 1.56 ± 0.15 |
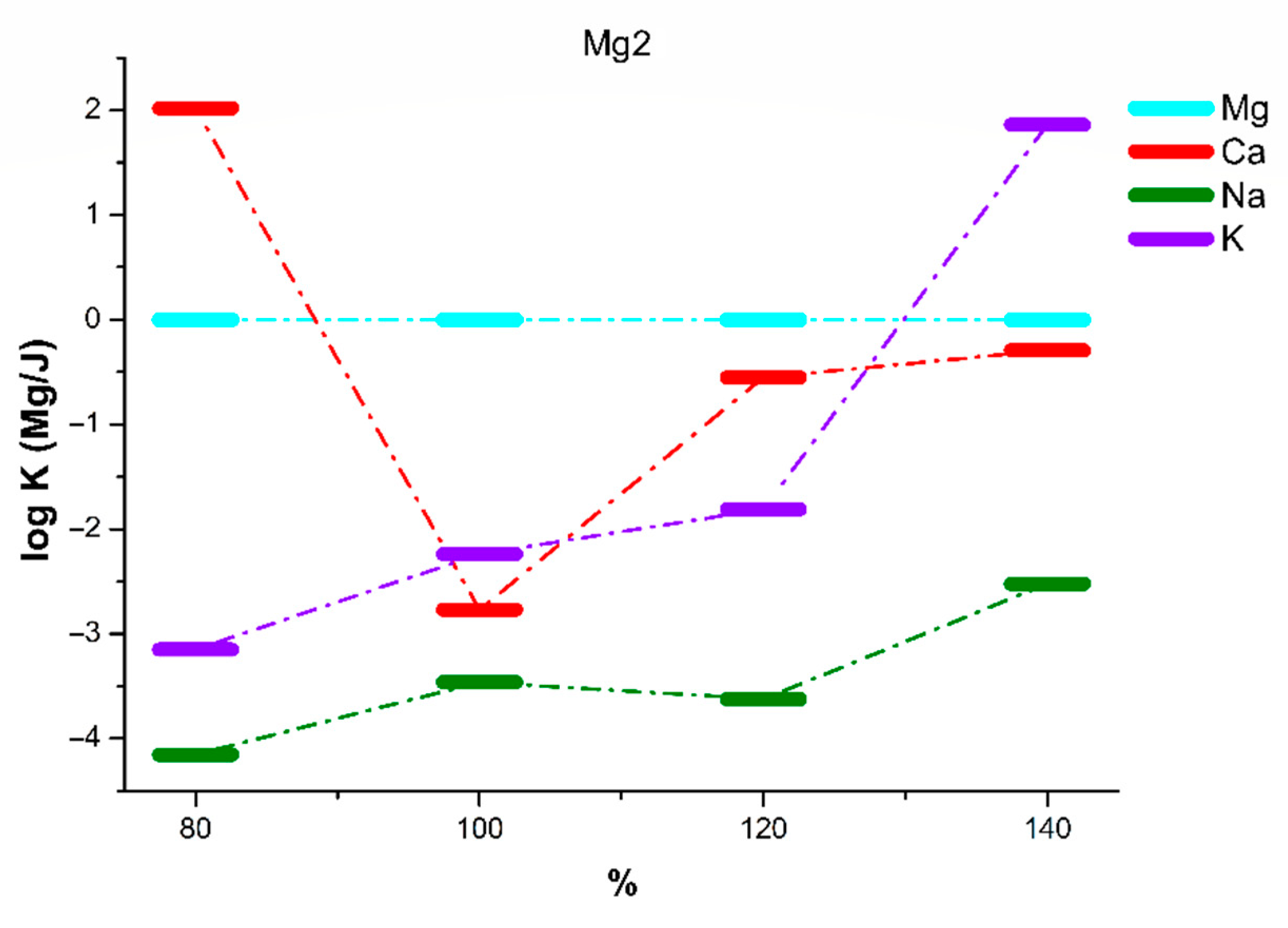
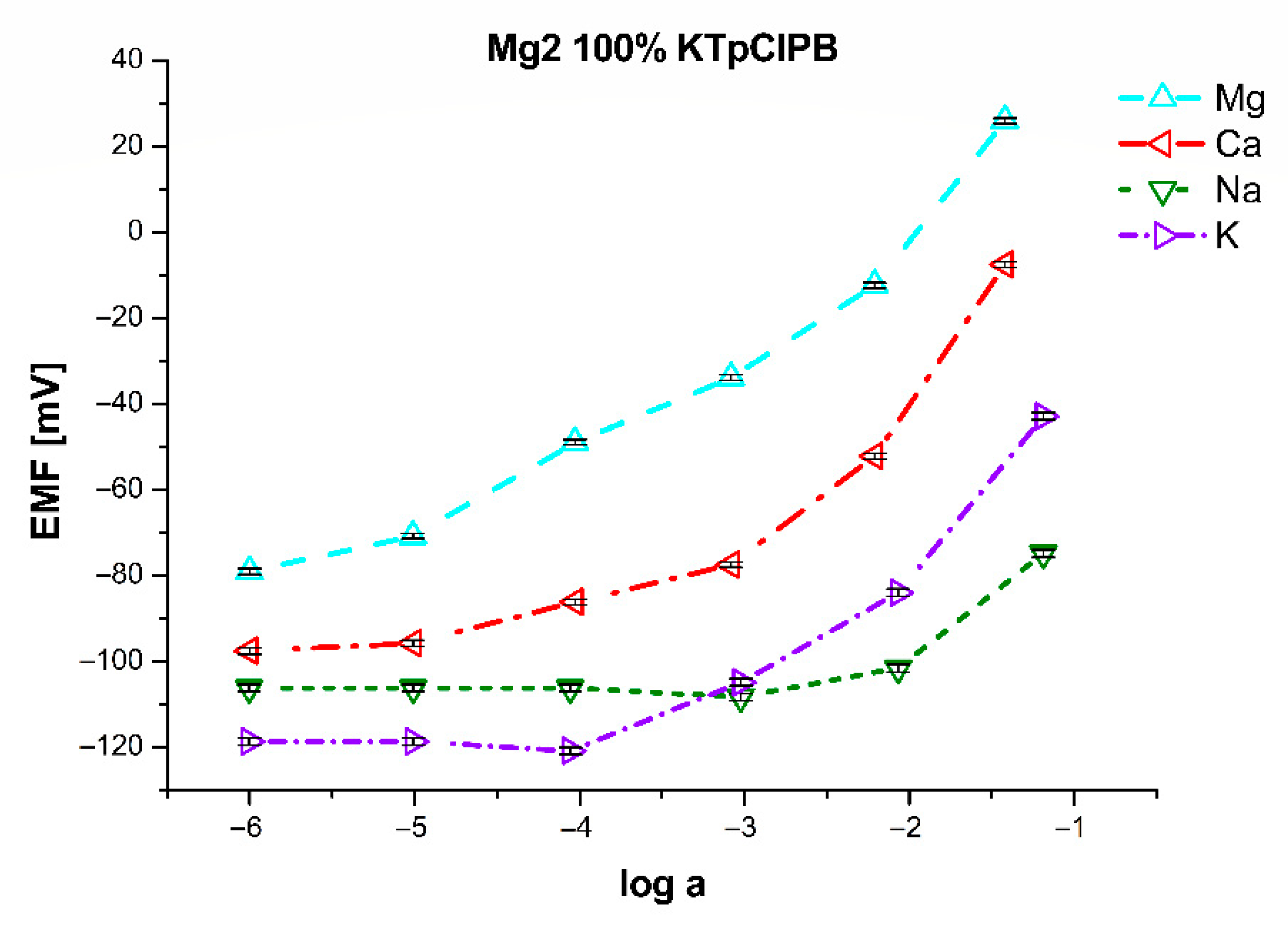
| Mg2 | 80% of KTpClPB | 100% of KTpClPB | 120% of KTpClPB | 140% of KTpClPB | ||||
| LR (log(a)) | −5 to −1 | −5 to −1 | −5 to −1 | −5 to −1 | ||||
| LDL (log(a)) | −5.27 | −5.37 | −5.34 | −5.10 | ||||
| S (mV/dec) | 24.9 ± 1.3 | 20.3 ± 1.4 | 24.9 ± 1.2 | 26.7 ± 1.3 | ||||
| log(a) | 0 | −1 | 0 | −1 | 0 | −1 | 0 | −1 |
| logKMg/Ca | 2.02 ± 0.05 | 1.18 ± 0.08 | −2.77 ± 0.08 | −2.49 ± 0.07 | −0.55 ± 0.07 | −0.73 ± 0.06 | −0.29 ± 0.07 | −0.29 ± 0.06 |
| logKMg/Na | −4.15 ± 0.09 | −3.79 ± 0.10 | −3.46 ± 0.11 | −2.95 ± 0.13 | −3.62 ± 0.11 | −2.48 ± 0.12 | −2.52 ± 0.11 | −1.39 ± 0.12 |
| logKMg/K | −3.15 ± 0.11 | −2.76 ± 0.09 | −2.24 ± 0.07 | −1.72 ± 0.09 | −1.81 ± 0.10 | −0.79 ± 0.09 | 1.86 ± 0.08 | 2.37 ± 0.10 |
| Mg3 | 80% of KTpClPB | 100% of KTpClPB | 120% of KTpClPB | 140% of KTpClPB | ||||
| LR (log(a)) | −5 to −1 | −5 to −1 | −5 to −1 | −5 to −1 | ||||
| LD (log(a)) | −5.34 | −5.26 | −5.34 | −5.11 | ||||
| S (mV/dec) | 19.6 ± 1.1 | 22.0 ± 1.0 | 20.5 ± 1.5 | 22.1 ± 1.0 | ||||
| log(a) | 0 | −1 | 0 | −1 | 0 | −1 | 0 | −1 |
| logKMg/Ca | 3.71 ± 0.12 | 3.00 ± 0.12 | 2.24 ± 0.08 | 1.65 ± 0.07 | 0.83 ± 0.06 | 0.59 ± 0.06 | 0.65 ± 0.05 | 0.59 ± 0.05 |
| logKMg/Na | −2.90 ± 0.10 | −1.63 ± 0.11 | −2.70 ± 0.08 | −1.99 ± 0.09 | −1.61 ± 0.12 | −1.02 ± 0.08 | −0.91 ± 0.06 | −0.03 ± 0.06 |
| logKMg/K | −2.99 ± 0.15 | −2.08 ± 0.11 | −3.46 ± 0.12 | −2.05 ± 0.07 | 1.89 ± 0.11 | 2.40 ± 0.12 | 2,78 ± 0,13 | 3.34 ± 0.15 |
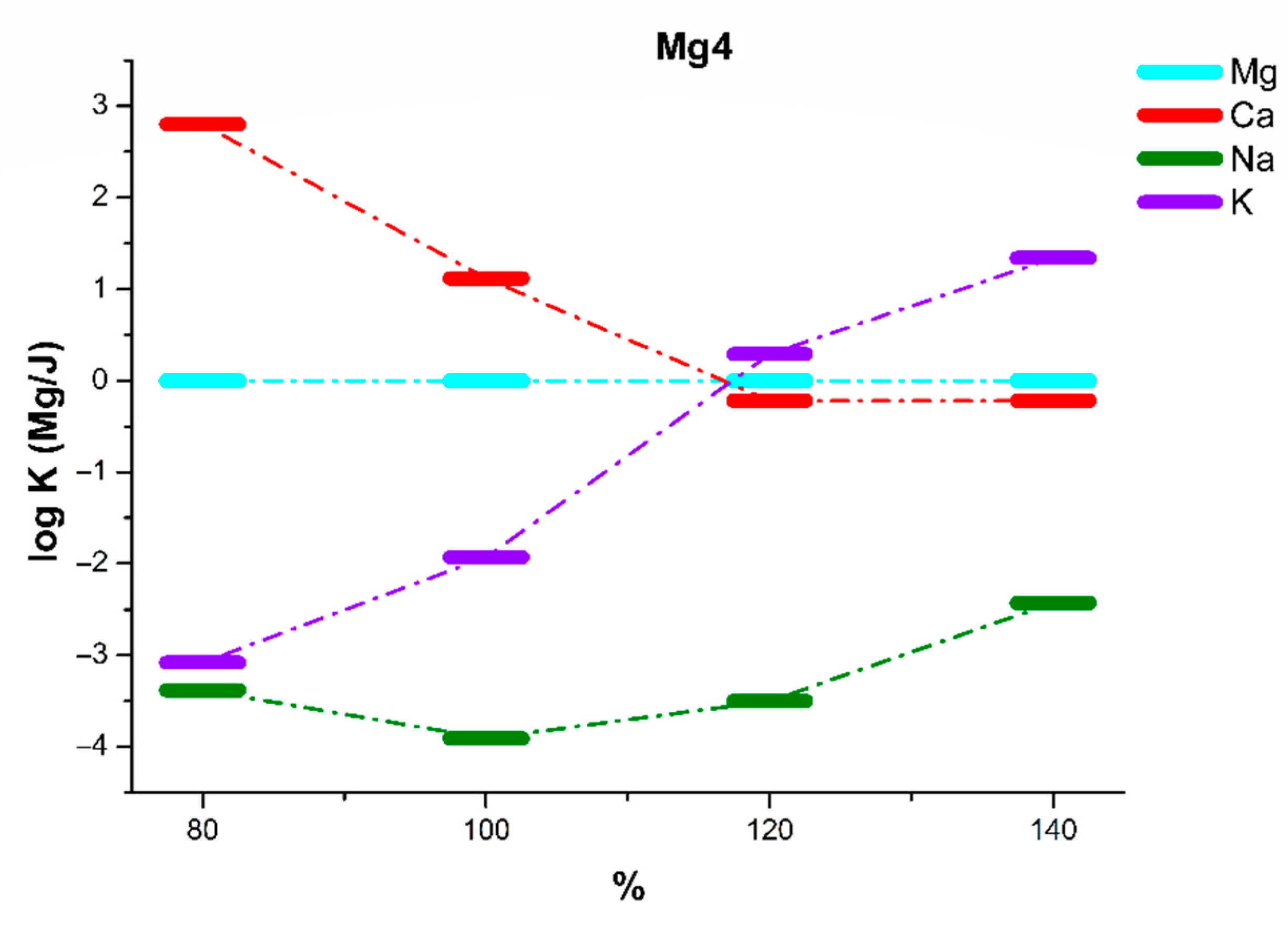
| Mg4 | 80% of KTpClPB | 100% of KTpClPB | 120% of KTpClPB | 140% of KTpClPB | ||||
| LR (log(a)) | −5 to −1 | −5 to −1 | −5 to −1 | −5 to −1 | ||||
| LDL (log(a)) | −5.23 | −5.31 | −5.22 | −5.32 | ||||
| S (mV/dec) | 23.8 ± 1.4 | 23.6 ± 1.2 | 22.9 ± 1.2 | 25.2 ± 1.4 | ||||
| log(a) | 0 | −1 | 0 | −1 | 0 | −1 | 0 | −1 |
| logKMg/Ca | 2.80 ± 0.11 | 2.44 ± 0.11 | 1.12 ± 0.12 | 0.87 ± 0.10 | −0.22 ± 0.06 | −0.32 ± 0.05 | −0.21 ± 0.06 | −0.13 ± 0.05 |
| logKMg/Na | −3.38 ± 0.15 | −1.92 ± 0.12 | −3.91 ± 0.12 | −2.53 ± 0.13 | −3.50 ± 0.11 | −2.07 ± 0.12 | −2.43 ± 0.13 | −1.18 ± 0.09 |
| logKMg/K | −3.08 ± 0.15 | −1.93 ± 0.14 | −1.93 ± 0.10 | −1.18 ± 0.12 | 0.29 ± 0.06 | 0.97 ± 0.08 | 1.34 ± 0.12 | 1.92 ± 0.08 |
| Mg5 | 80% of KTpClPB | 100% of KTpClPB | 120% of KTpClPB | 140% of KTpClPB | ||||
| LR (log(a)) | −5 to −1 | −5 to −1 | −5 to −1 | −5 to −1 | ||||
| LDL (log(a)) | −5.31 | −5.30 | −5.34 | −5.29 | ||||
| S (mV/dec) | 23.2 ± 1.1 | 21.6 ± 1.1 | 22.9 ± 1.3 | 21.4 ± 1.2 | ||||
| log(a) | 0 | −1 | 0 | −1 | 0 | −1 | 0 | −1 |
| logKMg/Ca | 0.79 ± 0.06 | 0.84 ± 0.05 | 0.99 ± 0.05 | 0.96 ± 0.05 | 1.41 ± 0.05 | 1.37 ± 0.05 | 1.39 ± 0.12 | 1.29 ± 0.11 |
| logKMg/Na | −4.57 ± 0.10 | −2.77 ± 0.12 | −3.99 ± 0.15 | −2.23 ± 0.11 | −4.46 ± 0.12 | −2.67 ± 0.10 | −2.79 ± 0.05 | −1.25 ± 0.06 |
| logKMg/K | −1.92 ± 0.10 | −1.04 ± 0.08 | −1.85 ± 0.12 | −0.95 ± 0.10 | −3.10 ± 0.15 | −1.86 ± 0.10 | 2.25 ± 0.11 | 2.29 ± 0.10 |
| M# | 1gc | 2gc | 3gc | 4gc | 5gc | 6gc | 7gc | 8gc | 9gc | 10gc |
|---|---|---|---|---|---|---|---|---|---|---|
| salt/ionophore molar ratio | 0.4:1 | 0.6:1 | 0.8:1 | 0.8:1 | 1:1 | 1:1 | 1:1 | 1.2:1 | 1,2:1 | 1,5:1 |
| % ionophore by weight | 2.09 | 2.08 | 1.02 | 2.08 | 2.08 | 1.27 | 3.97 | 1.02 | 2.07 | 2.06 |
| DL (log(a)) | −5.8 | −4.2 | −3.8 | −4.6 | −5.6 | −4.8 | −4.7 | −4.2 | −4.8 | −4.7 |
| S [mV/dec] | 43.5 | 34.1 | 33.0 | 34.5 | 42.4 | 35.8 | 37.5 | 41.5 | 39.7 | 41.3 |
| LogKMg/Ca | −1.65 | −1.27 | −0.94 | −1.38 | −1.26 | −0.43 | −0.24 | −0.77 | −0.69 | −0.84 |
| LogKMg/K | −1.17 | −1.02 | −1.03 | −1.51 | −0.52 | −0.39 | −0.16 | −0.03 | −0.16 | −0.30 |
| LogKMg/Na | −1.61 | −1.72 | −1.57 | −2.13 | −1.32 | −0.83 | −0.70 | −1.15 | −0.98 | −1.24 |
| M# | 11gc | 12gc | 13gc | 14gc | 15gc | 16gc |
|---|---|---|---|---|---|---|
| salt/ionophore molar ratio | 0.6:1 | 0.8:1 | 0.8:1 | 1:1 | 1:1 | 1.2:1 |
| % ionophore by weight | 1.02 | 2.07 | 1.02 | 2.07 | 0.96 | 1.02 |
| DL (log(a)) | −4.1 | −4.7 | −4.8 | −5.2 | −4.4 | −4.7 |
| S [mV/dec] | 44.5 | 45.7 | 37.9 | 43.1 | 41.2 | 41.3 |
| LogKMg/Ca | 0.10 | 0.03 | −0.08 | −2.62 | −1.96 | 0.49 |
| LogKMg/K | −2.69 | −2.55 | −0.70 | −3.54 | −1.55 | −2.43 |
| LogKMg/Na | −2.22 | −2.07 | −1.25 | −2.65 | −1.48 | −1.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomećko, R.; Luboch, E.; Jeszke, M. Dipodal Tetraamide Derivatives of 1,10-Diaza-18-Crown-6 and Alkylmalonic Acids—Synthesis and Use as Ionophores in Ion Selective Membrane Electrodes. Sensors 2021, 21, 4984. https://doi.org/10.3390/s21154984
Pomećko R, Luboch E, Jeszke M. Dipodal Tetraamide Derivatives of 1,10-Diaza-18-Crown-6 and Alkylmalonic Acids—Synthesis and Use as Ionophores in Ion Selective Membrane Electrodes. Sensors. 2021; 21(15):4984. https://doi.org/10.3390/s21154984
Chicago/Turabian StylePomećko, Radosław, Elżbieta Luboch, and Maciej Jeszke. 2021. "Dipodal Tetraamide Derivatives of 1,10-Diaza-18-Crown-6 and Alkylmalonic Acids—Synthesis and Use as Ionophores in Ion Selective Membrane Electrodes" Sensors 21, no. 15: 4984. https://doi.org/10.3390/s21154984
APA StylePomećko, R., Luboch, E., & Jeszke, M. (2021). Dipodal Tetraamide Derivatives of 1,10-Diaza-18-Crown-6 and Alkylmalonic Acids—Synthesis and Use as Ionophores in Ion Selective Membrane Electrodes. Sensors, 21(15), 4984. https://doi.org/10.3390/s21154984