Effectiveness of Robotic Exoskeleton-Assisted Gait Training in Spinocerebellar Ataxia: A Case Report
Abstract
1. Introduction
2. Case Description and Methods
2.1. Case Description
2.2. Methods
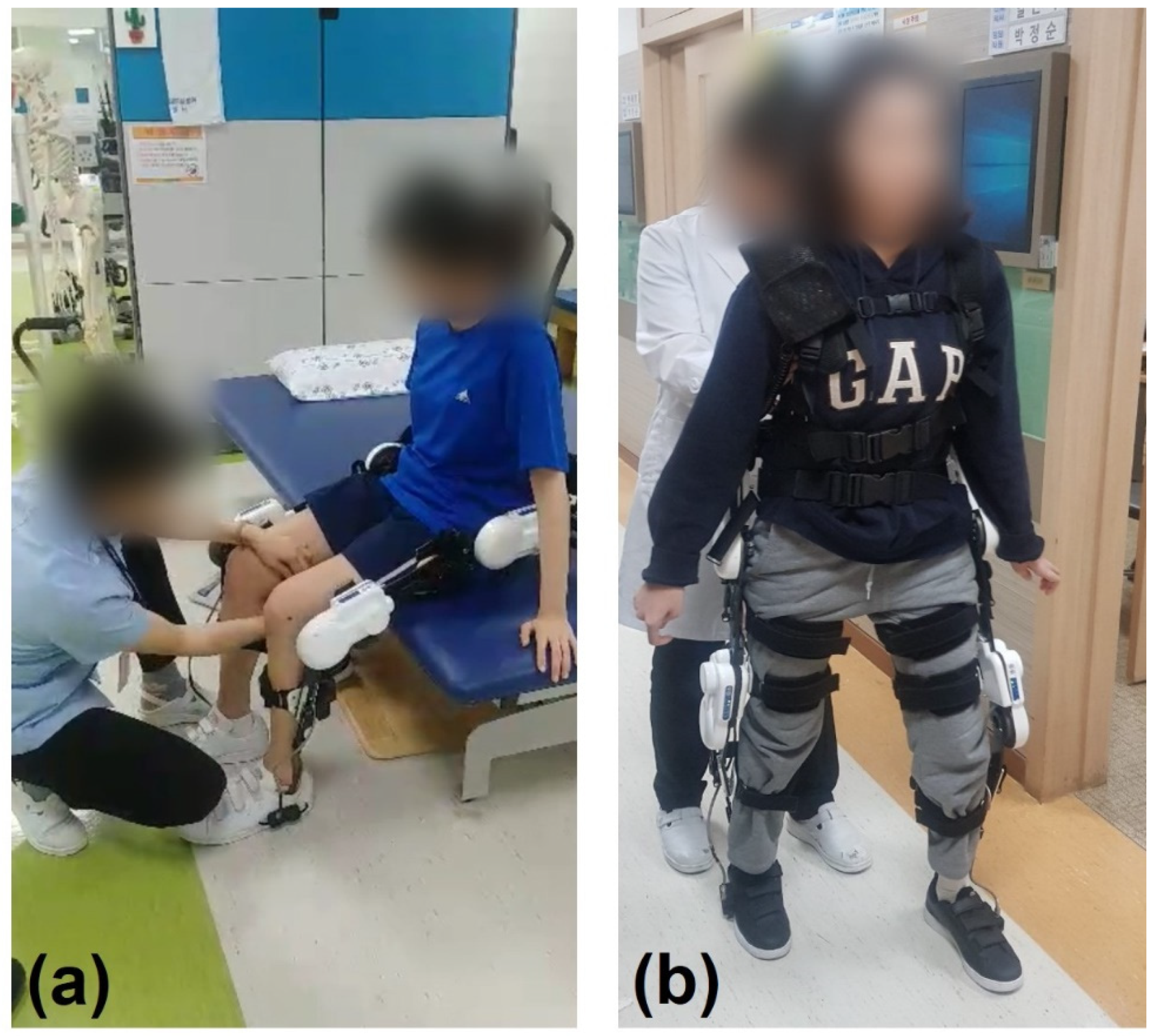
2.2.1. Training Program
2.2.2. Robotic Exoskeletal Device
2.2.3. Functional Assessments
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klockgether, T.; Mariotti, C.; Paulson, H.L. Spinocerebellar ataxia. Nat. Rev. Dis. Primers 2019, 5, 24. [Google Scholar] [CrossRef]
- Sullivan, R.; Yau, W.Y.; O’Connor, E.; Houlden, H. Spinocerebellar ataxia: An update. J. Neurol. 2019, 266, 533–544. [Google Scholar] [CrossRef]
- Salman, M.S. Epidemiology of Cerebellar Diseases and Therapeutic Approaches. Cerebellum 2018, 17, 4–11. [Google Scholar] [CrossRef]
- Ruano, L.; Melo, C.; Silva, M.C.; Coutinho, P. The global epidemiology of hereditary ataxia and spastic paraplegia: A systematic review of prevalence studies. Neuroepidemiology 2014, 42, 174–183. [Google Scholar] [CrossRef]
- Diallo, A.; Jacobi, H.; Cook, A.; Labrum, R.; Durr, A.; Brice, A.; Charles, P.; Marelli, C.; Mariotti, C.; Nanetti, L.; et al. Survival in patients with spinocerebellar ataxia types 1, 2, 3, and 6 (EUROSCA): A longitudinal cohort study. Lancet Neurol. 2018, 17, 327–334. [Google Scholar] [CrossRef]
- Klockgether, T.; Lüdtke, R.; Kramer, B.; Abele, M.; Bürk, K.; Schöls, L.; Riess, O.; Laccone, F.; Boesch, S.; Lopes-Cendes, I.; et al. The natural history of degenerative ataxia: A retrospective study in 466 patients. Brain 1998, 121 Pt 4, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Coarelli, G.; Marcotulli, C.; Leonardi, L.; Piccolo, F.; Spadaro, M.; Frontali, M.; Ferraldeschi, M.; Vulpiani, M.C.; Ponzelli, F.; et al. Riluzole in patients with hereditary cerebellar ataxia: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015, 14, 985–991. [Google Scholar] [CrossRef]
- Lei, L.F.; Yang, G.P.; Wang, J.L.; Chuang, D.M.; Song, W.H.; Tang, B.S.; Jiang, H. Safety and efficacy of valproic acid treatment in SCA3/MJD patients. Parkinsonism Relat. Disord. 2016, 26, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Braga Neto, P.; Pedroso, J.L.; Kuo, S.H.; Marcondes Junior, C.F.; Teive, H.A.; Barsottini, O.G. Current concepts in the treatment of hereditary ataxias. Arq. Neuropsiquiatr. 2016, 74, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ilg, W.; Brötz, D.; Burkard, S.; Giese, M.A.; Schöls, L.; Synofzik, M. Long-term effects of coordinative training in degenerative cerebellar disease. Mov. Disord. 2010, 25, 2239–2246. [Google Scholar] [CrossRef]
- Young, A.J.; Ferris, D.P. State of the Art and Future Directions for Lower Limb Robotic Exoskeletons. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 171–182. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, W.; Zhang, W.; Ding, X. A Review on Lower Limb Rehabilitation Exoskeleton Robots. Chin. J. Mech. Eng. 2019, 32, 74. [Google Scholar] [CrossRef]
- Toth, L.; Schiffer, A.; Nyitrai, M.; Pentek, A.; Told, R.; Maroti, P. Developing an anti-spastic orthosis for daily home-use of stroke patients using smart memory alloys and 3D printing technologies. Mater. Des. 2020, 195, 109029. [Google Scholar] [CrossRef]
- Gull, M.A.; Bai, S.; Bak, T. A Review on Design of Upper Limb Exoskeletons. Robotics 2020, 9, 16. [Google Scholar] [CrossRef]
- Fonteyn, E.M.; Heeren, A.; Engels, J.J.; Boer, J.J.; van de Warrenburg, B.P.; Weerdesteyn, V. Gait adaptability training improves obstacle avoidance and dynamic stability in patients with cerebellar degeneration. Gait Posture 2014, 40, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Fonteyn, E.M.; Keus, S.H.; Verstappen, C.C.; Schöls, L.; de Groot, I.J.; van de Warrenburg, B.P. The effectiveness of allied health care in patients with ataxia: A systematic review. J. Neurol. 2014, 261, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Milne, S.C.; Corben, L.A.; Georgiou-Karistianis, N.; Delatycki, M.B.; Yiu, E.M. Rehabilitation for Individuals With Genetic Degenerative Ataxia: A Systematic Review. Neurorehabil. Neural Repair 2017, 31, 609–622. [Google Scholar] [CrossRef]
- Zesiewicz, T.A.; Wilmot, G.; Kuo, S.H.; Perlman, S.; Greenstein, P.E.; Ying, S.H.; Ashizawa, T.; Subramony, S.H.; Schmahmann, J.D.; Figueroa, K.P.; et al. Comprehensive systematic review summary: Treatment of cerebellar motor dysfunction and ataxia: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 464–471. [Google Scholar] [CrossRef]
- Marshall, P.; Al-Timman, J.; Riley, R.; Wright, J.; Williams, S.; Hainsworth, R.; Tan, L.B. Randomized controlled trial of home-based exercise training to evaluate cardiac functional gains. Clin. Sci. 2001, 101, 477–483. [Google Scholar] [CrossRef]
- Duncan, P.; Richards, L.; Wallace, D.; Stoker-Yates, J.; Pohl, P.; Luchies, C.; Ogle, A.; Studenski, S. A randomized, controlled pilot study of a home-based exercise program for individuals with mild and moderate stroke. Stroke 1998, 29, 2055–2060. [Google Scholar] [CrossRef]
- Han, D.S.; Chuang, P.W.; Chiu, E.C. Effect of home-based reablement program on improving activities of daily living for patients with stroke: A pilot study. Medicine 2020, 99, e23512. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.L.; Lee, C.M.; Wu, Y.W.; Chen, T.A.; Wu, Y.T. Home-based exercise increases exercise capacity but not quality of life in people with chronic heart failure: A systematic review. Aust. J. Physiother. 2008, 54, 87–93. [Google Scholar] [CrossRef][Green Version]
- Tsukahara, A.; Yoshida, K.; Matsushima, A.; Ajima, K.; Kuroda, C.; Mizukami, N.; Hashimoto, M. Effects of gait support in patients with spinocerebellar degeneration by a wearable robot based on synchronization control. J. Neuroeng. Rehabil. 2018, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, A.; Yoshida, K.; Matsushima, A.; Ajima, K.; Kuroda, C.; Mizukami, N.; Hashimoto, M. Evaluation of walking smoothness using wearable robotic system curara® for spinocerebellar degeneration patients. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 1494–1499. [Google Scholar] [CrossRef]
- Gopura, R.A.R.C.; Bandara, D.S.V.; Kiguchi, K.; Mann, G.K.I. Developments in hardware systems of active upper-limb exoskeleton robots: A review. Robot. Auton. Syst. 2016, 75, 203–220. [Google Scholar] [CrossRef]

| T0 | T1 | T2 | |
|---|---|---|---|
| General Assessment | |||
| MMT (right leg/left leg) | (4/4/4)/(4/4/4) | (4/4/4)/(4/4/4) | (4/4/4)/(4/4/4) |
| MAS (right ankle/left ankle) | Gr 0/Gr 0 | Gr 0/Gr 0 | Gr 0/Gr 0 |
| K-MBI | 69 | 73 | 73 |
| BBS | 19 | 23 | 23 |
| SARA | 25 | 23 | 23 |
| EQ-5D | 8 | 8 | 8 |
| Posturography—SD of COP AP/COP ML (mm) | |||
| open eyes | 389/404 | 20/14 | 17/17 |
| closed eyes | 446/495 | 30/20 | 20/29 |
| 10-m walking test (s) | |||
| comfortable pace | 38.12 | 24.30 | 18.81 |
| fast pace | 34.50 | 21.88 | 16.20 |
| Timed up-and-go test (s) | 31.94 | 25.97 | 20.91 |
| Gait analysis | |||
| cadence (steps/min) | 78.8 | 89.8 | 92.2 |
| speed (cm/s) | 40.8 | 47.3 | 57.0 |
| stride length (cm) | 62.6 | 64.2 | 74.9 |
| right/left stride length (cm) | 63.2/61.9 | 64.5/63.9 | 73.7/76.1 |
| right/left step length (cm) | 27.6/35.0 | 24.5/39.2 | 32.3/42.6 |
| step width (cm) | 16.2 | 17.5 | 16.7 |
| Oxygen consumption during comfortable walking | |||
| VO2 (mL/min) | 940.463 | 1274.272 | 1257.204 |
| MET | 5.229 | 7.13 | 7.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Han, J.-Y.; Song, M.-K.; Choi, I.-S.; Park, H.-K. Effectiveness of Robotic Exoskeleton-Assisted Gait Training in Spinocerebellar Ataxia: A Case Report. Sensors 2021, 21, 4874. https://doi.org/10.3390/s21144874
Kim S-H, Han J-Y, Song M-K, Choi I-S, Park H-K. Effectiveness of Robotic Exoskeleton-Assisted Gait Training in Spinocerebellar Ataxia: A Case Report. Sensors. 2021; 21(14):4874. https://doi.org/10.3390/s21144874
Chicago/Turabian StyleKim, San-Ha, Jae-Young Han, Min-Keun Song, In-Sung Choi, and Hyeng-Kyu Park. 2021. "Effectiveness of Robotic Exoskeleton-Assisted Gait Training in Spinocerebellar Ataxia: A Case Report" Sensors 21, no. 14: 4874. https://doi.org/10.3390/s21144874
APA StyleKim, S.-H., Han, J.-Y., Song, M.-K., Choi, I.-S., & Park, H.-K. (2021). Effectiveness of Robotic Exoskeleton-Assisted Gait Training in Spinocerebellar Ataxia: A Case Report. Sensors, 21(14), 4874. https://doi.org/10.3390/s21144874






