Gas Monitoring in Human Frontal Sinuses–Stability Considerations and Gas Exchange Studies
Abstract
1. Introduction
1.1. Background
1.2. Gas in Scattering Media Absorption Spectroscopy
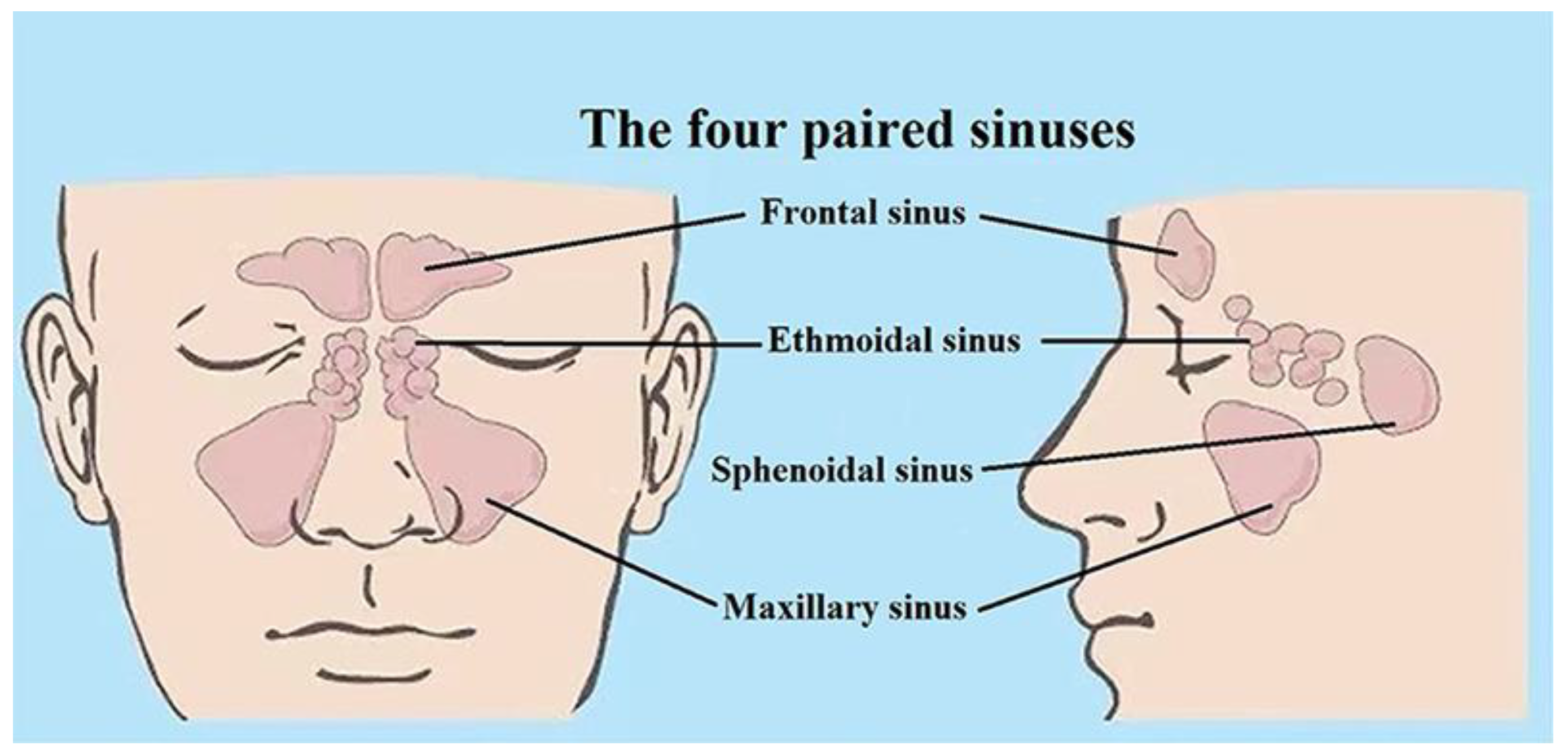
1.3. Previous Work on Frontal Sinus Cavities
2. Study Design
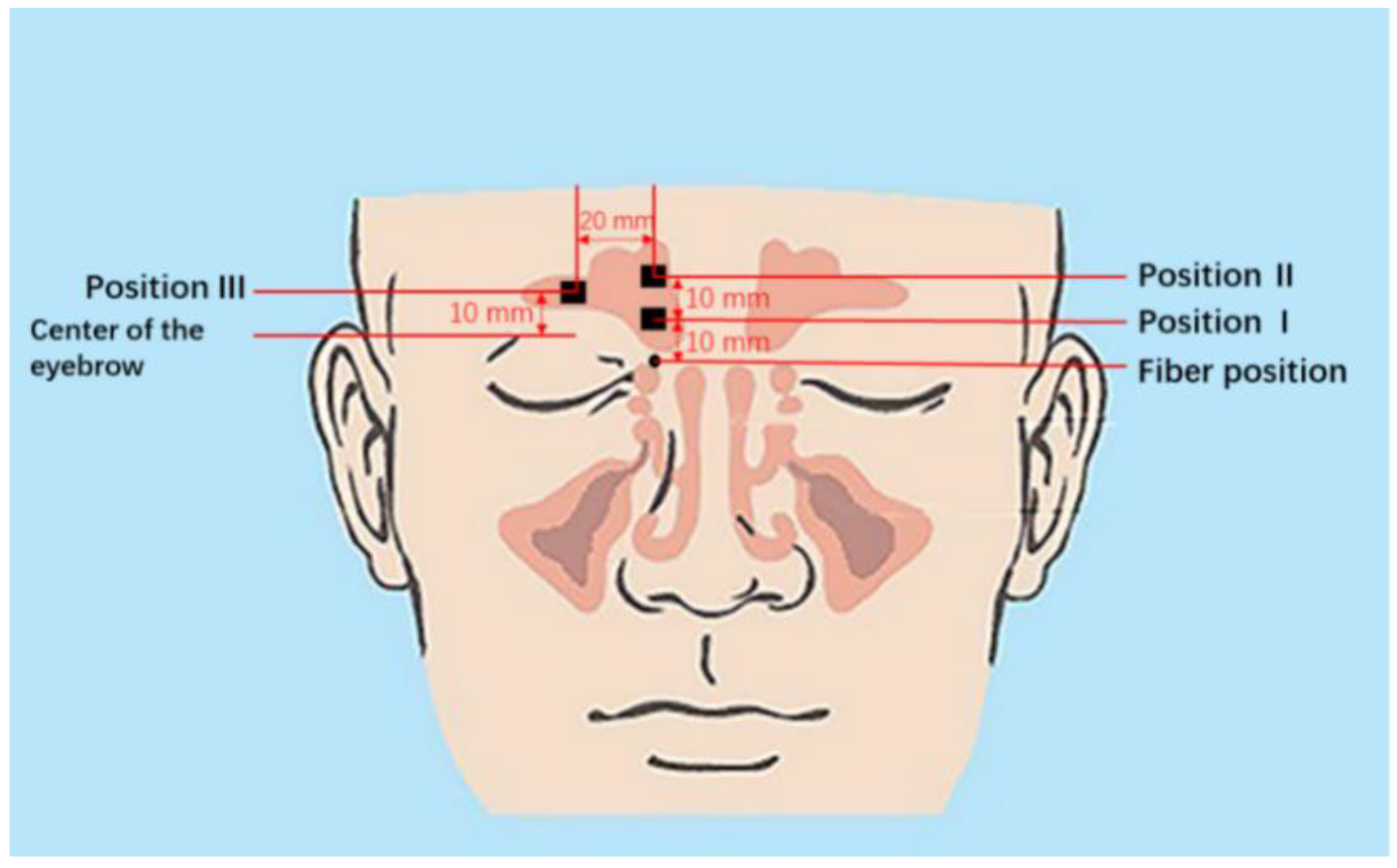
2.1. Volunteers
2.2. Gas Monitoring Systems
3. Measurements and Results
3.1. Instrument Calibration
3.2. Signal Stability and Reproducibility
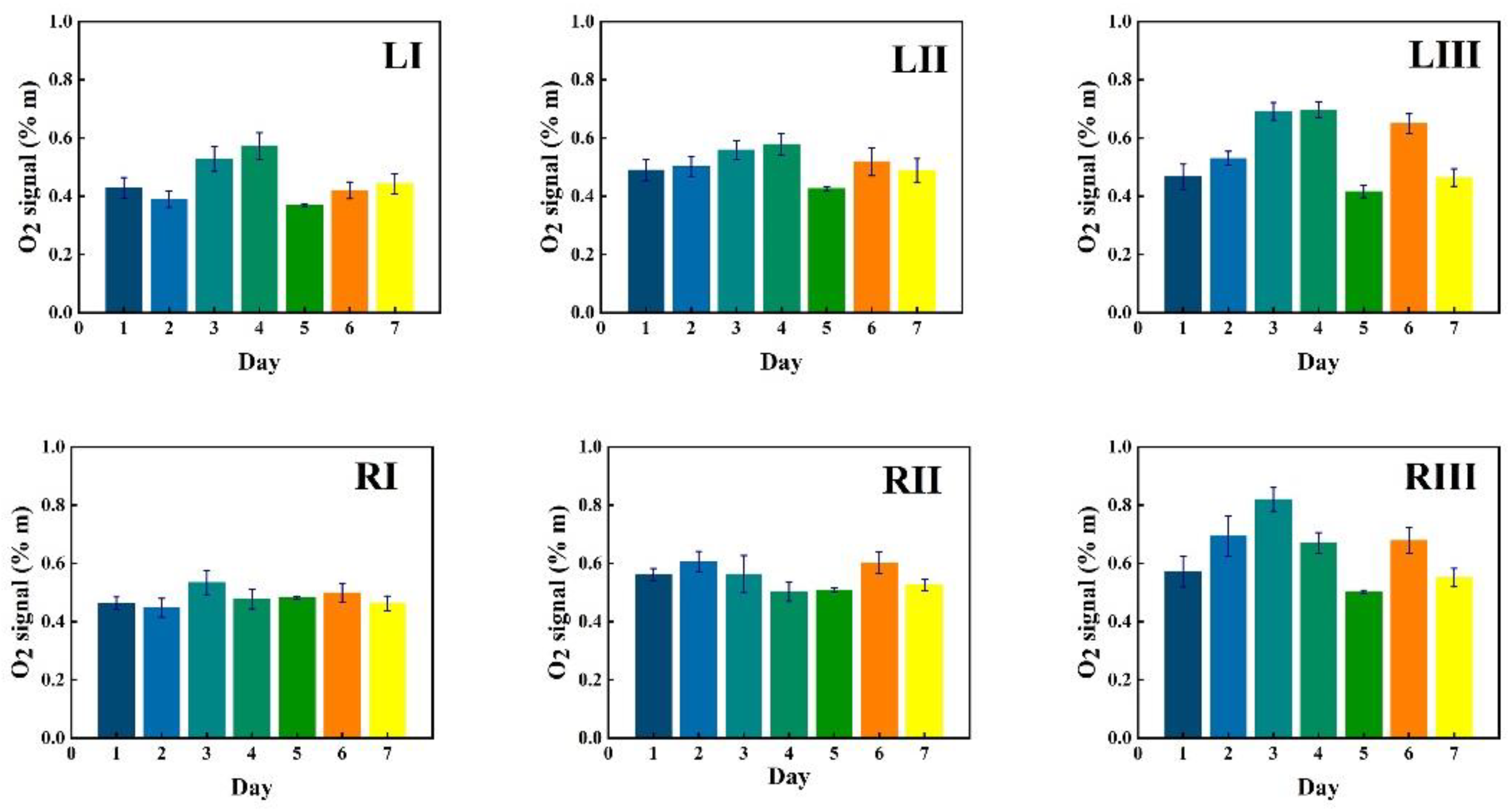
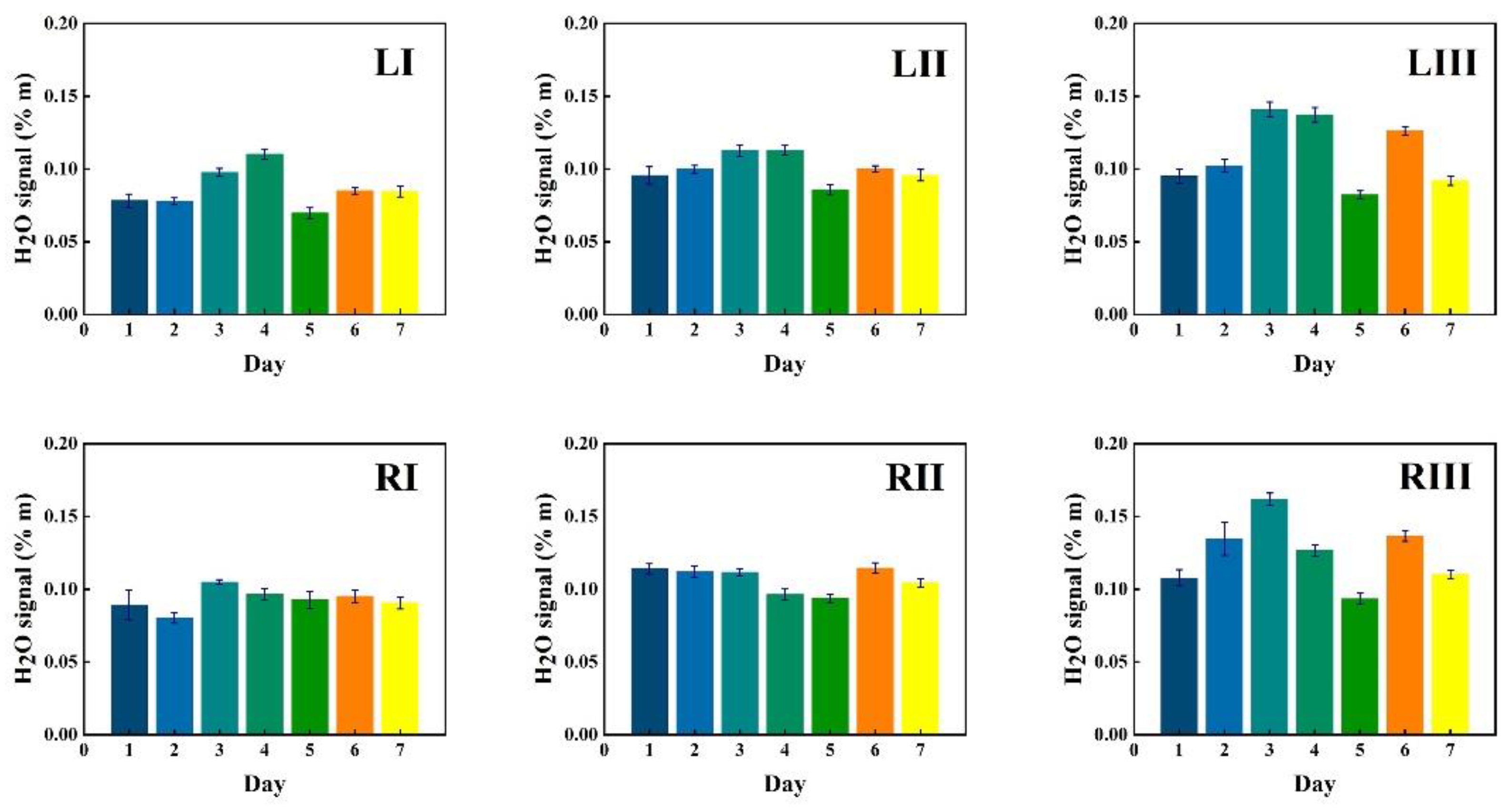
3.2.1. Monitoring during One Week
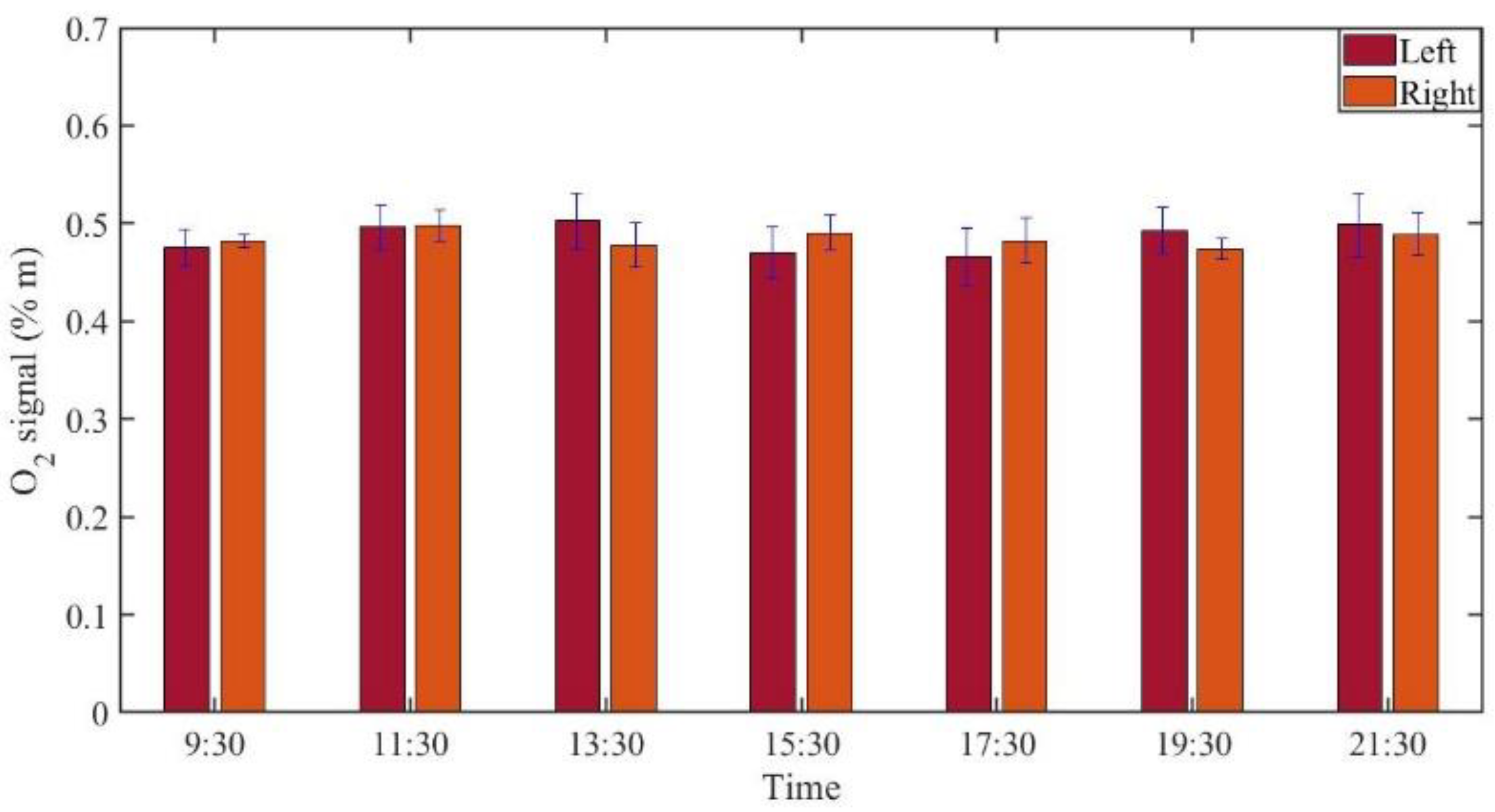
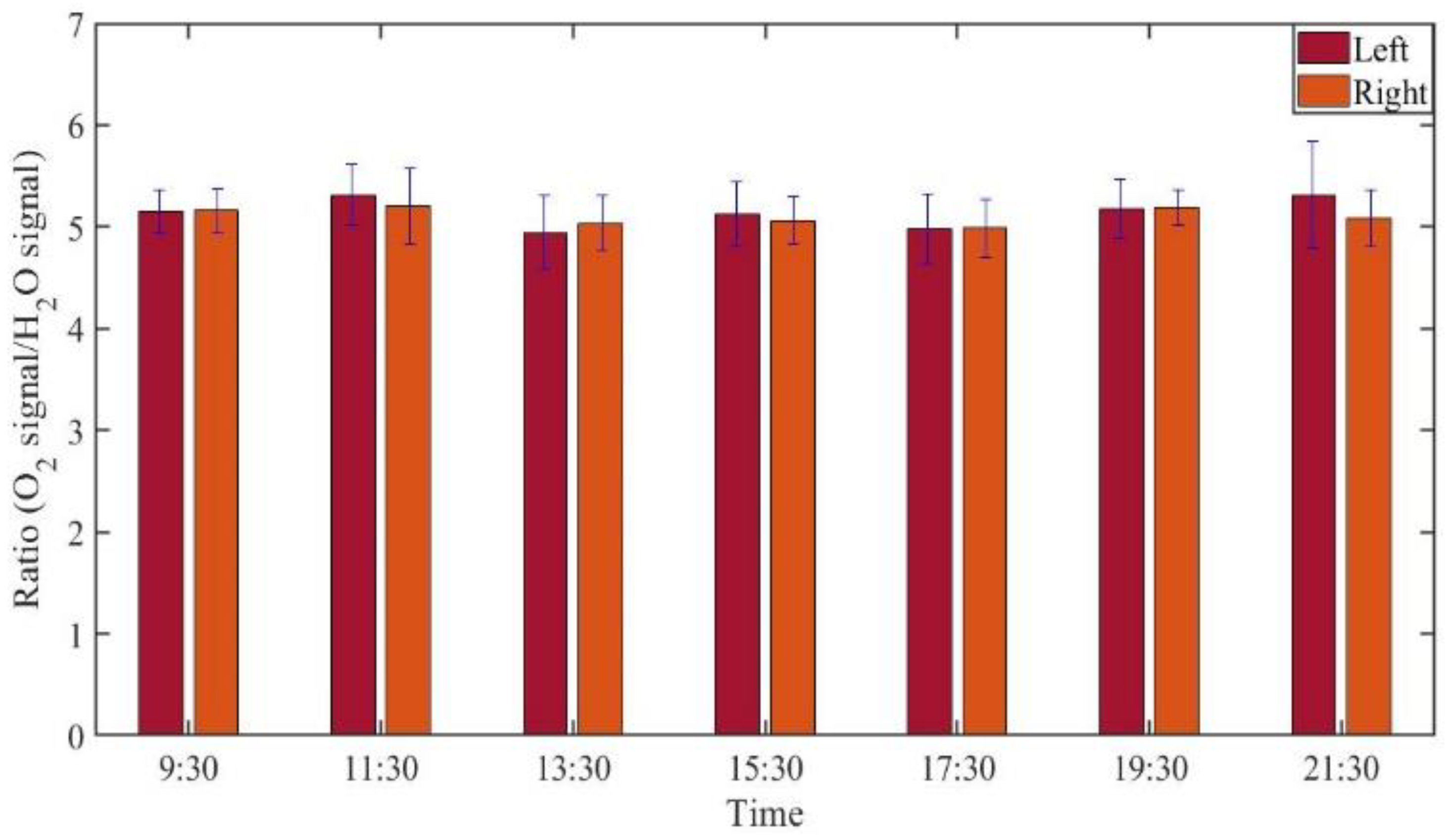
3.2.2. Monitoring during One Day
3.3. Oxygen Concentration Retrieval
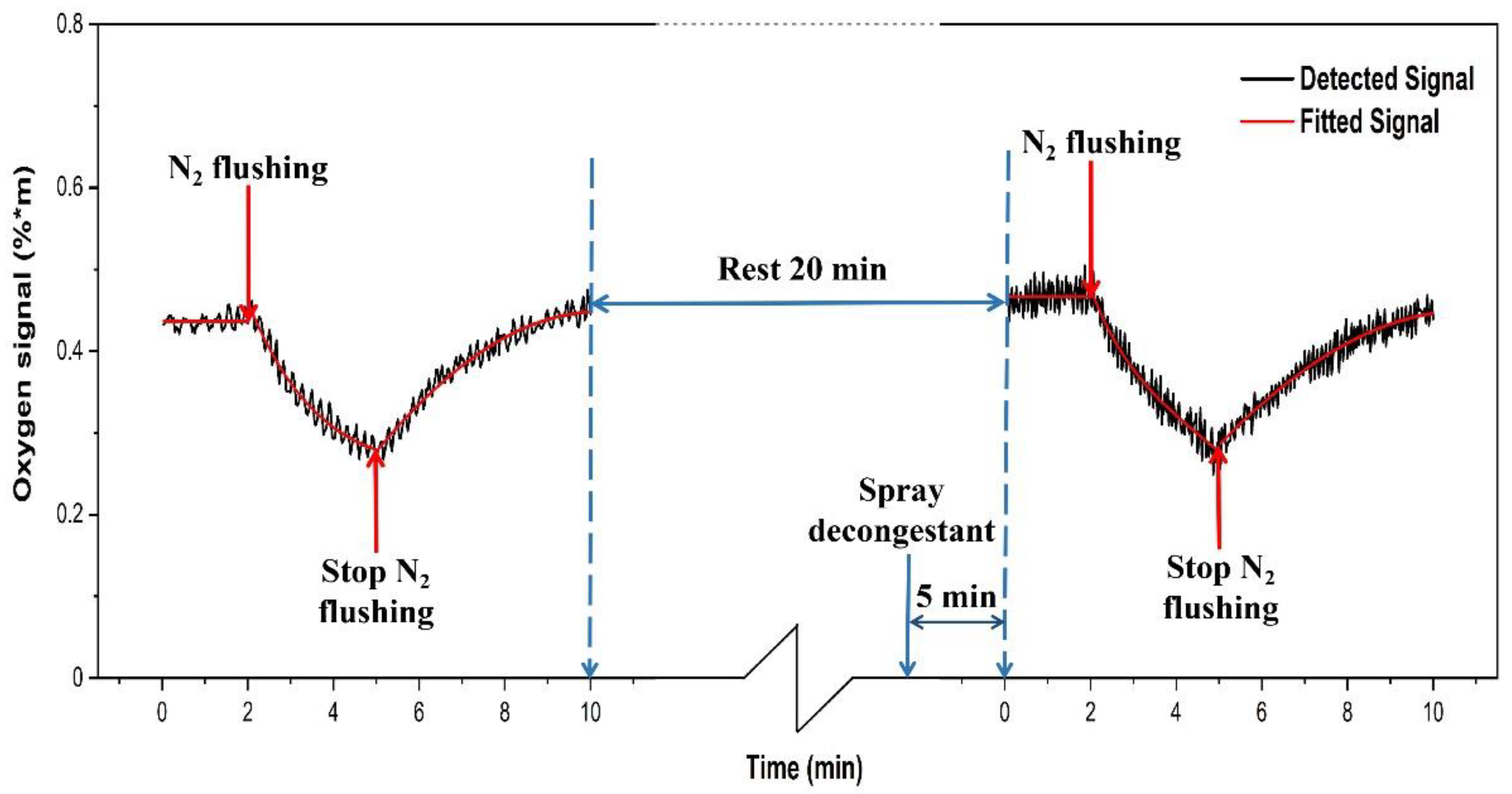
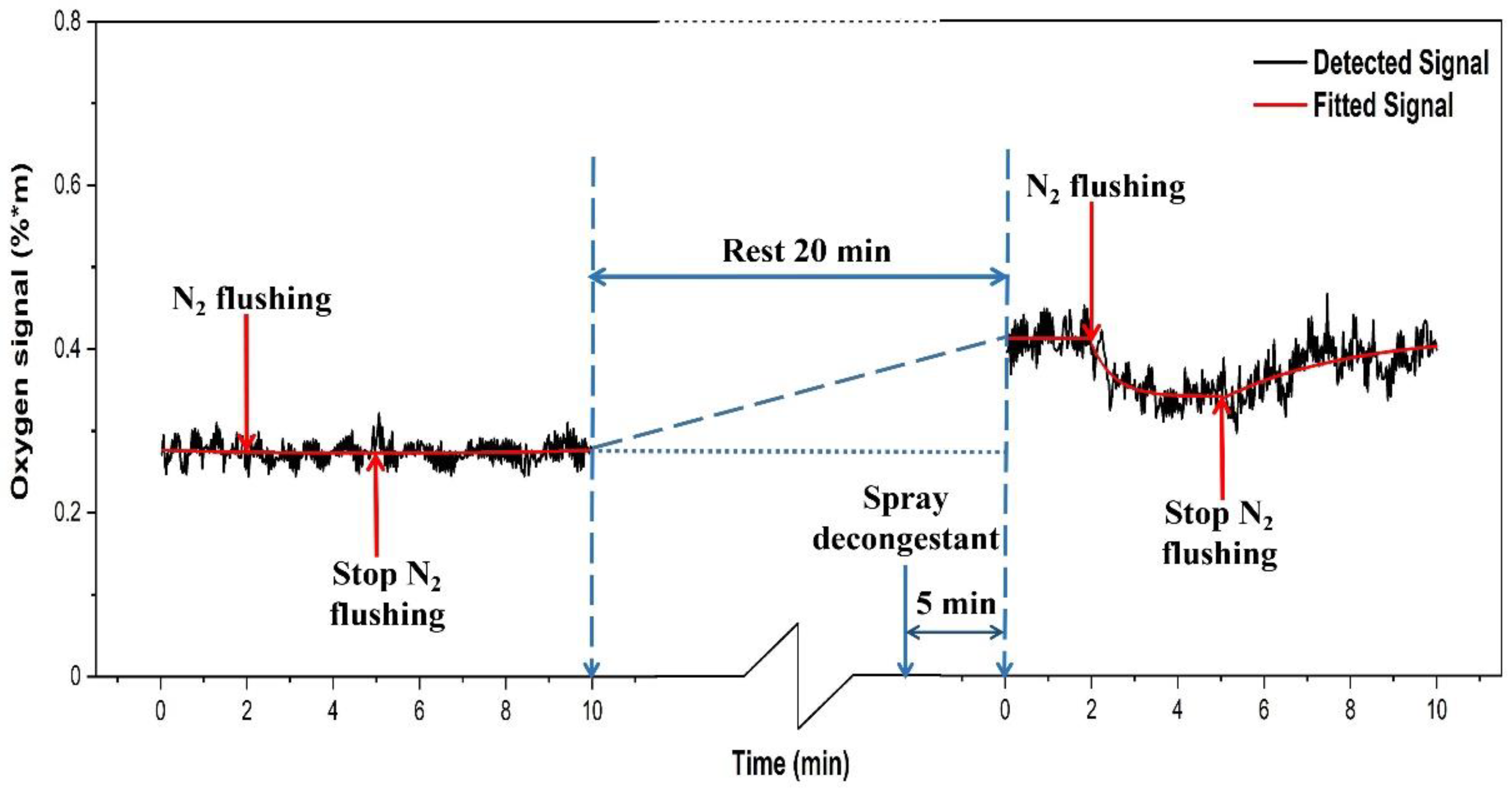
3.4. Gas Transport through Ostia
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snow, V.; Mottur-Pilson, C.; Hickner, J.M. Principles of appropriate antibiotic use for acute sinusitis in adults. Ann. Intern. Med. 2001, 134, 495–497. [Google Scholar] [CrossRef]
- Anzai, Y.; Yueh, B. Imaging evaluation of sinusitis: Diagnostic performance and impact on health outcome. Neuroimag. Clin. N. Am. 2003, 13, 251–263. [Google Scholar] [CrossRef]
- Leo, G.; Mori, F.; Incorvaia, C.; Barni, S.; Novembre, E. Diagnosis and management of acute rhinosinusitis in children. Curr. Allergy Asthma Rep. 2009, 9, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.A.; Orlandi, R.; Rudmik, L. Cost of adult chronic rhinosinusitis: A systematic review. Laryngoscope 2015, 125, 1547–1556. [Google Scholar] [CrossRef]
- Rudmik, L. Economics of chronic rhinosinusitis. Curr. Allergy Asthma Rep. 2017, 17, 20. [Google Scholar] [CrossRef]
- Brook, I. Current issues in the management of acute bacterial sinusitis in children. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Anand, V.K. Epidemiology and economic impact of rhinosinusitis. Ann. Otol. Rhinol. Laryngol. 2004, 113, 3–5. [Google Scholar] [CrossRef]
- Jankowski, R.; Nguyen, D.T.; Poussel, M.; Chenuel, B.; Gallet, P.; Rumeau, C. Sinusology. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 263–268. [Google Scholar] [CrossRef] [PubMed]
- The 6 Major Hazards of Sinusitis Should not Be Underestimated! The Difference of Rhinitis and Cold. Available online: https://zhuanlan.zhihu.com/p/32055363 (accessed on 27 June 2021).
- Whyte, A.; Boeddinghaus, R. The maxillary sinus: Physiology, development and imaging anatomy. Dentomaxillofac. Radiol. 2019, 48, 20190205. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.Y.O. The formation of the human paranasal sinuses. Acta Otolaryngol. 1984, 97, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, F.A. An integrated approach to frontal sinus surgery. Otolaryngol. Clin. N. Am. 2006, 39, 437–461. [Google Scholar] [CrossRef]
- Ebell, M.H.; McKay, B.; Dale, A.; Guilbault, R.; Ermias, Y. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann. Fam. Med. 2019, 17, 164–172. [Google Scholar] [CrossRef]
- Rosenfeld, R.M. Acute sinusitis in adults. N. Engl. J. Med. 2016, 375, 962–970. [Google Scholar] [CrossRef]
- Smith, S.S.; Ference, E.H.; Evans, C.T.; Tan, B.K.; Kern, R.C.; Chandra, R.K. The prevalence of bacterial infection in acute rhinosinusitis: A systematic review and meta-analysis. Laryngoscope 2015, 125, 57–69. [Google Scholar] [CrossRef]
- Autio, T.J.; Koskenkorva, T.; Leino, T.K.; Koivunen, P.; Alho, O.P. Longitudinal analysis of inflammatory biomarkers during acute rhinosinusitis. Laryngoscope 2017, 127, E55–E61. [Google Scholar] [CrossRef]
- Patel, H.; Zhang, W.; Kuo, Y.; Sharma, G. Impact of choosing wisely on antibiotic prescription and CT scan use for uncomplicated acute rhinosinusitis (ARS). J. Allergy Clin. Immunol. 2018, 141, AB166. [Google Scholar] [CrossRef]
- Melanie, D.; Desrosiers, M. Guidelines for management of acute bacterial rhinosinusitis: Impact on Quebec physicians’ prescriptions for antibiotics. Otolaryngol. Head Neck Surg. 2007, 136, 258–260. [Google Scholar] [CrossRef]
- Benninger, M.S.; Holzer, S.E.S.; Lau, J. Diagnosis and treatment of uncomplicated acute bacterial rhinosinusitis: Summary of the Agency for Health Care Policy and Research evidence-based report. Otolaryngol. Head Neck Surg. 2000, 122, 1–7. [Google Scholar] [CrossRef]
- Lux, C.; Johnston, J.; Zoing, M.; Biswas, K.; Taylor, M.; Douglas, R. Antibiotic treatment for chronic rhinosinusitis: Prescription patterns and associations with patient outcome and the sinus microbiota. Front. Microbiol. 2020, 11, 595555. [Google Scholar] [CrossRef]
- Lemiengre, M.B.; Driel, M.L.; Merenstein, D.; Liira, H.; Mäkelä, M.; De Sutter, A.I. Antibiotics for acute rhinosinusitis in adults. Cochrane. Database Syst. Rev. 2018, 9, CD006089. [Google Scholar] [CrossRef]
- Stefani, S.; Chung, D.R.; Lindsay, J.A.; Friedrich, A.W.; Kearns, A.M.; Westh, H.; MacKenzie, F.M. Meticillin-resistant Staphylococcus aureus (MRSA): Global epidemiology and harmonisation of typing methods. Int. J. Antimicrob. Agents. 2012, 39, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef]
- Raut, A.; Jankharia, B. Paranasal Sinuses in Health and Disease. In Rhinology and Facial Plastic Surgery; Stucker, F., Kenyon, G., Lian, T., Draf, W., Schick, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 35–62. [Google Scholar]
- Head, K.; Chong, L.Y.; Piromchai, P.; Hopkins, C.; Philpott, C.; Schilder, A.G.; Burton, M.J. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane. Database Syst. Rev. 2016, 4, CD011994. [Google Scholar] [CrossRef]
- Ebell, M.H.; Hansen, J.G. Proposed clinical decision rules to diagnose acute rhinosinusitis among adults in primary care. Ann. Fam. Med. 2017, 15, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ebell, M.H.; McKay, B.; Guilbault, R.; Ermias, Y. Diagnosis of acute rhinosinusitis in primary care: A systematic review of test accuracy. Br. J. Gen. Pract. 2016, 66, e612–e632. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, M.; Somesfalean, G.; Alnis, J.; Andersson-Engels, S.; Svanberg, S. Analysis of gas dispersed in scattering solids and liquids. Opt. Lett. 2001, 26, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Svanberg, S. Gas in scattering media absorption spectroscopy—From basic studies to biomedical applications. Lasers Photonics Rev. 2013, 7, 779–796. [Google Scholar] [CrossRef]
- Svanberg, K.; Svanberg, S. Monitoring free gas in situ for medical diagnostics using laser spectroscopic techniques. In Frontiers in Biophotonics for Translational Medicine; Olivo, M., Dinish, U.S., Eds.; Springer Science and Business Media: Singapore, 2016. [Google Scholar]
- Buck, A.L. New equations for computing vapor pressure and enhancement factor. J. Appl. Meteorol. Climatol. 1981, 20, 1527–1532. [Google Scholar] [CrossRef]
- Mei, L.; Somesfalean, G.; Svanberg, S. Pathlength determination for gas in scattering media absorption spectroscopy. Sensors 2014, 14, 3871–3890. [Google Scholar] [CrossRef]
- Persson, L.; Svanberg, K.; Svanberg, S. On the potential of human sinus cavity diagnostics using diode laser gas spectroscopy. Appl. Phys. B 2006, 82, 313–317. [Google Scholar] [CrossRef]
- Persson, L.; Andersson, M.; Cassel-Engquist, M.; Svanberg, K.; Svanberg, S. Gas monitoring in human sinuses using tunable diode laser spectroscopy. J. Biomed. Opt. 2007, 12, 054001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lewander, M.; Guan, Z.G.; Svanberg, K.; Svanberg, S.; Svensson, T. Clinical system for non-invasive in situ monitoring of gases in the human paranasal sinuses. Opt. Express 2009, 13, 10849–10863. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Svanberg, S. Wavelength modulation spectroscopy-digital detection of gas absorption harmonics based on Fourier analysis. Appl. Opt. 2015, 54, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Lewander, M.; Lindberg, S.; Svensson, T.; Siemund, R.; Svanberg, K.; Svanberg, S. Noninvasive diagnostics of the maxillary and frontal sinuses based on diode laser gas spectroscopy. Rhinology 2012, 50, 26–32. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.; Li, T.; Lin, H.; Svanberg, K.; Svanberg, S. Assessment of human sinus cavity air volume using tunable diode laser spectroscopy, with application to sinusitis diagnostics. J. Biophotonics 2015, 8, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Mekamy, S. In-Vivo Study on the Stability of the Use of Gas Absorption Spectroscopy as a Technology for Accessing Sinus Cavity Size and Oxygen Gas Concentration. Master’s Thesis, Department at Biomedical Engineering, Lund University, Lund, Sweden, 2020. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Han, N.; Lin, Y.; Huang, J.; Svanberg, S.; Svanberg, K. Gas Monitoring in Human Frontal Sinuses–Stability Considerations and Gas Exchange Studies. Sensors 2021, 21, 4413. https://doi.org/10.3390/s21134413
Zhang H, Han N, Lin Y, Huang J, Svanberg S, Svanberg K. Gas Monitoring in Human Frontal Sinuses–Stability Considerations and Gas Exchange Studies. Sensors. 2021; 21(13):4413. https://doi.org/10.3390/s21134413
Chicago/Turabian StyleZhang, Han, Ning Han, Yueyu Lin, Jiawen Huang, Sune Svanberg, and Katarina Svanberg. 2021. "Gas Monitoring in Human Frontal Sinuses–Stability Considerations and Gas Exchange Studies" Sensors 21, no. 13: 4413. https://doi.org/10.3390/s21134413
APA StyleZhang, H., Han, N., Lin, Y., Huang, J., Svanberg, S., & Svanberg, K. (2021). Gas Monitoring in Human Frontal Sinuses–Stability Considerations and Gas Exchange Studies. Sensors, 21(13), 4413. https://doi.org/10.3390/s21134413






